#Utility Parameters Report Development
Explore tagged Tumblr posts
Text

industrial reports with E signature
#Purified Water Distribution System report development#Report development for fermenters#Utility Parameters Report Development#Industrial Reports Development by Configurator#Best Industrial Reporting Solution#Best Reporting Software for Manufacturing Industry#Pressure Hold Test Report Development#PREPARATION Report Development
1 note
·
View note
Text
The Role of Scheme Management Software in Business

The corporate world has become more competitive recently, and with it, scheme management. If schemes are effectively managed, they play a crucial role in enhancing sales, customer satisfaction, and profits. The question then arises: Do sales executives need help coordinating their campaigns effectively, which poses challenges for maintaining customer satisfaction and profitability maximization? Does your company struggle with growth due to errors, delays, and inefficiencies caused by manual operations?
For those looking to streamline promotions and enhance efficiency, it's worth exploring scheme management software. This application is designed to streamline the process of managing offers and schemes for national distributors or manufacturers. It allows you to enter scheme parameters like validity period, scheme concept, terms and conditions, price involved, etc., into the system and share the information. Let's explore how it can drive business expansion.
What Makes Efficient Scheme Management So Important?
The scheme management platform helps manage promotional plans that involve developing, launching, and monitoring strategies to boost sales and revenue. Previously, errors, paperwork, and complex calculations were common while implementing schemes manually. Anticipated outcomes included inefficiencies and challenges in measuring program success.
Companies have shifted their approach to scheme administration by utilizing scheme management software. It streamlines various aspects of administration so businesses can effortlessly create, execute, and oversee schemes. It is recognized as workflow efficiency software because it enhances business productivity. Let’s explore how implementing this software can revolutionize your business operations and drive growth.
1. Adaptability in Defining Schemes
Scheme management software integrates supplier loyalty programs with defined regulations. Its dynamic system assists end-users in effectively planning, creating, and developing innovative strategies. Regarding channel sales management, field force automation, or sales tracking, software for managing schemes can be customized to fit specific client requirements.
2. Optimized Dealer Satisfaction
Supervisors can track project advancement through the scheme management app's enterprise resource planning (ERP) integration. It is structured and operates methodically, expediting the implementation of new projects. Additionally, it efficiently manages credit notes, boosting sales, customer trust, and overall profits.
3. Boosts Productivity
Once in auto mode, the scheme assessment speeds up and requires minimal human involvement. Scheme management software greatly enhances reclaiming schemes, which are known for their challenges and setbacks, resulting in lost opportunities. It improves productivity, generates leads, increases brand visibility, and fosters dealer confidence.
4. Emphasizing a Strong Sense of Responsibility
Operating scheme automation software involves significant responsibility, as it documents and displays all credit transactions, records, and deals. In addition, the software's report production feature simplifies generating reports on sales, events/promotions, costs, and costings.
Scheme management software enhances corporate operations, scheme performance, and sales. Streamlining design, validation, and monitoring processes enhances productivity and drives revenue growth.
Nural Schemes enables you to share schemes and evaluate their performance. It has benefited a wide range of sales professionals and retail outlets. Clients have experienced significant growth in human capital, revenue, and operational expense savings with various scheme combinations. Rely on Nural for optimizing business solutions and top-notch workflow efficiency software. Schedule a demo today.
3 notes
·
View notes
Text
Grading Guidelines: Dirt Work Industry Evaluation
In the realm of construction and land development, the grading of terrain is a fundamental yet intricate aspect that determines the success and stability of a project. Grading for building foundations, landscaping, or road construction, the manipulation and levelling of earth – commonly known as dirt work – demand precise evaluation criteria to ensure safety, efficiency, and quality. The following guidelines outline key parameters for evaluating dirt work within the construction industry.

1. Precision in Earth Manipulation:
The grading process necessitates accuracy in cutting, filling, and leveling the terrain. Evaluating dirt work involves scrutinizing the precision of earth-moving machinery, adherence to proposed grades, and the uniformity of finished surfaces. The grading should align precisely with architectural and engineering plans to guarantee structural integrity and optimal functionality.
2. Drainage and Erosion Control:
Effective grading must incorporate provisions for proper drainage and erosion control. Assessment criteria encompass the creation of slopes that facilitate water runoff, the installation of retention basins, and erosion prevention measures. A thorough evaluation ensures that the grading design effectively mitigates water-related issues, preserving the stability of the landscape.
3. Compliance with Environmental Regulations:
Evaluation criteria extend beyond functional aspects to encompass adherence to environmental standards. Assessors examine the implementation of erosion control measures, prevention of soil contamination, and compliance with local regulations governing land disturbance. Environmental considerations are integral to responsible and sustainable dirt work practices.
4. Safety Measures and Compliance:
Safety protocols and compliance with occupational standards are paramount in evaluating dirt work. Assessing safety includes scrutinizing the utilization of safety equipment, worker training, and adherence to established safety guidelines. A comprehensive evaluation ensures a secure working environment for personnel involved in the grading process.
5. Material Quality and Utilization:
Evaluation criteria encompass the quality of materials used in the grading process. Assessors examine the appropriateness of soil types, the quality of fill materials, and the utilization of approved resources. The efficient use of materials without compromising structural integrity is a key aspect of evaluating dirt work.
6. Documentation and Reporting:
Accurate documentation of the grading process is crucial for evaluation purposes. This includes maintaining records of initial site conditions, progress reports, material usage, and any deviations from the proposed grading plan. Detailed and comprehensive reporting aids in assessing the adherence to project specifications.
The "Grading Guidelines: Dirt Work Industry Evaluation" serve as a framework to assess and ensure the quality, safety, and compliance of earth-moving activities in construction and land development. Adhering to these guidelines fosters not only successful project outcomes but also responsible environmental practices, ultimately contributing to sustainable and safe infrastructure development.
2 notes
·
View notes
Text
2/3AUG2023
Post 1
Hello everyone current or future. I made this blog with a bunch of intentions but the main one being a time capsule/live progress feed. I'm going by Acura (no inspiration I just like the name) and I have a decent amount of aspirations.
I want to be an artist-turned vtuber. I've been in undesirable places to ask for advice and haven't really utilized them but I will eventually.
I want to get out of my current home situation so I can properly live on my own and enjoy myself
I want to grow to understanding myself more
The parameters of number 3 are kinda strange but in short, I feel like my soul left me some time ago. I still feel emotions but many of them are locked in my mind (e.g lots of recent suicidal ideation, do not worry I don't ever really commit) Which leads to a lot of conflicts.
But enough of that! More about me:
I do not like being around many of my peers.
I dislike celebrities of any and every caliber (ironic since I want to engage in what is online celebrityhood.)
I'm learning japanese but I'm elementary level and extremely rusty.
I have an extensive family, a good portion of which I love, but my condition will worsen and worsen if I interact with them more.
My favorite publisher, developer, whatever title you want to give for that accolade is Square.
I love FFX.
I am ?? years old and am in year ?? of university.
I also lie a lot but I will try to be much more honest on here for the sake of maintaining accountability.
Regardless, if this is used for a deep dive into my past if I'm successful or a police report if I've truly axed myself, welcome!
That being said, I'll draw tomorrow.

2 notes
·
View notes
Text
Sodium Formate Market Size, Share, Key Drivers, Trends, Challenges and Competitive Analysis
Executive Summary Sodium Formate Market :
Global sodium formate market size was valued at USD 495.73 million in 2023 and is projected to reach USD 820.42 million by 2031, with a CAGR of 6.5% during the forecast period of 2024 to 2031.
Sodium Formate Market report makes available all the details about historic data about the industry, present market trends, future product environment, marketing strategies, technological innovation, upcoming technologies, emerging trends or opportunities, and the technical progress in the related industry. Businesses have started adopting a market research report solution for sound decision making and superior management of goods and services. The Sodium Formate Market report makes available market potential for each geographical region based on the growth rate, macroeconomic parameters, consumer buying patterns, their preferences for particular product and market demand and supply scenarios.
The competitive landscape part of the report provides a clear insight into the market share analysis of key industry players. PDF form or spreadsheets have been used for the delivery of this Sodium Formate Market report to the users. Nonetheless, upon client’s specific requirement, PPT format can also be offered. CAGR values for the market for an estimated forecast period are mentioned in the report which helps determine costing and investment values or strategies. In addition, this Sodium Formate Market report also offers top to bottom assessment of the market as far as income and developing business sector is concerned. Lot of efforts have been taken to leave no stone unturned while forming this Sodium Formate Market report.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Sodium Formate Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-sodium-formate-market
Sodium Formate Market Overview
**Segments**
- **By Grade**: The global sodium formate market can be segmented based on the grade into industrial grade and pharmaceutical grade. Industrial grade sodium formate is commonly used in industries for various applications such as leather tanning, de-icing, and as a buffering agent in the production of formic acid. On the other hand, pharmaceutical grade sodium formate is utilized in the pharmaceutical industry for specific applications where high purity standards are required.
- **By Application**: Sodium formate finds applications across various industries including leather industry, chemical industry, oil and gas industry, and others. In the leather industry, sodium formate is used in the process of chrome tanning as a reducing agent. In the chemical industry, it is used in the production of formic acid, sodium hydrosulfite, and other chemicals. The oil and gas industry utilizes sodium formate as a drilling fluid additive and in well completion operations.
- **By Region**: Geographically, the global sodium formate market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Asia Pacific region is expected to dominate the sodium formate market due to the presence of key manufacturing industries such as leather, chemical, and oil and gas in countries like China and India. North America and Europe are also significant markets for sodium formate owing to the increasing demand for the product in various industrial applications.
**Market Players**
- **Perstorp Holding AB**: A leading player in the sodium formate market, Perstorp Holding AB offers high-quality sodium formate products for various industrial applications. The company focuses on innovation and sustainability in its product offerings, catering to the diverse needs of customers in different industries.
- **Helm AG**: Helm AG is another key player in the sodium formate market, known for its wide range of sodium formate products that meet the highest quality standards. The company has a strong global presence and strategic partnerships in place to ensure a competitive edge in the market.
- **Shaanxi Debon Chemical Co., Ltd.**: Shaanxi Debon Chemical Co., Ltd. is a prominent supplier of sodium formate, providing customized solutions to customers in different industries. The company's focus on research and development allows it to offer innovative sodium formate products that meet evolving market requirements.
The global sodium formate market is characterized by intense competition among key players striving to enhance their product offerings and expand their market presence. With the increasing demand for sodium formate in diverse industries, market players are focusing on strategic collaborations, product innovations, and geographical expansions to gain a competitive advantage in the market.
The global sodium formate market is poised for significant growth driven by several key factors. One of the key drivers is the increasing demand for sodium formate in the leather industry, where it is used in processes such as chrome tanning. The leather industry is experiencing growth due to rising consumer demand for leather products globally, especially in regions like Asia Pacific and Europe. This is expected to drive the demand for sodium formate as a key ingredient in the tanning process. Additionally, the chemical industry is witnessing steady growth, particularly in the production of formic acid, which further fuels the demand for sodium formate as a raw material.
Moreover, the oil and gas industry's continuous exploration and drilling activities are creating a robust demand for sodium formate as a drilling fluid additive and in well completion operations. The need for efficient and effective drilling fluids to enhance drilling operations is propelling the demand for sodium formate in the oil and gas sector. This trend is expected to continue driving market growth in the coming years, especially in regions with thriving oil and gas sectors like North America and the Middle East.
Furthermore, the pharmaceutical industry's stringent quality standards and increasing focus on research and development are expected to drive the demand for pharmaceutical-grade sodium formate. As pharmaceutical companies aim to enhance the purity and efficacy of their products, the demand for high-quality sodium formate is likely to increase. This presents an opportunity for market players to cater to the pharmaceutical industry's specific requirements and expand their product offerings to capitalize on this growing segment.
In terms of regional dynamics, Asia Pacific is anticipated to lead the global sodium formate market due to its strong presence of key manufacturing industries such as leather, chemical, and oil and gas. Countries like China and India are major contributors to the market growth in the region, driven by industrialization, urbanization, and infrastructure development. North America and Europe are also key markets for sodium formate, supported by the presence of established industries and the adoption of advanced technologies in manufacturing processes.
Overall, the global sodium formate market is poised for substantial growth fueled by diverse end-user industries and regional dynamics. Market players are focusing on product innovation, sustainability, and strategic partnerships to stay competitive and capture emerging opportunities in the market. With the increasing demand for sodium formate across various sectors, the market is expected to witness continued expansion and evolution in the coming years.The global sodium formate market is experiencing significant growth driven by a range of factors. One of the key drivers is the rising demand for sodium formate in the leather industry, particularly in processes like chrome tanning. With the growing consumer demand for leather products globally, especially in regions like Asia Pacific and Europe, the demand for sodium formate as a crucial component in the tanning process is expected to increase. Similarly, the chemical industry is also witnessing steady growth, especially in the production of formic acid, which further boosts the demand for sodium formate as a raw material.
Furthermore, the oil and gas industry's continuous exploration and drilling activities are creating robust demand for sodium formate as a drilling fluid additive and in well completion operations. The need for efficient drilling fluids to improve drilling operations is boosting the demand for sodium formate in the oil and gas sector. This trend is likely to continue to drive market growth in regions with thriving oil and gas sectors like North America and the Middle East.
Additionally, the pharmaceutical industry's stringent quality standards and focus on research and development are anticipated to increase the demand for pharmaceutical-grade sodium formate. As pharmaceutical companies aim to enhance the purity and effectiveness of their products, the demand for high-quality sodium formate is expected to rise. This presents an opportunity for market players to meet the specific requirements of the pharmaceutical industry and expand their product portfolios to cater to this growing segment.
In terms of regional dynamics, Asia Pacific is projected to lead the global sodium formate market due to its strong presence of key manufacturing industries such as leather, chemical, and oil and gas. Countries like China and India are significant contributors to market growth in the region, driven by industrialization, urbanization, and infrastructure development. North America and Europe are also key markets for sodium formate, supported by established industries and the adoption of advanced technologies in manufacturing processes.
Overall, the global sodium formate market is set for substantial growth propelled by diverse end-user industries and regional variations. Market players are concentrating on product innovation, sustainability, and strategic partnerships to stay competitive and capitalize on emerging opportunities in the market. With the increasing demand for sodium formate across various sectors, the market is anticipated to witness continuous expansion and evolution in the foreseeable future.
The Sodium Formate Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-sodium-formate-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
The report can answer the following questions:
Global major manufacturers' operating situation (sales, revenue, growth rate and gross margin) of Sodium Formate Market
Global major countries (United States, Canada, Germany, France, UK, Italy, Russia, Spain, China, Japan, Korea, India, Australia, New Zealand, Southeast Asia, Middle East, Africa, Mexico, Brazil, C. America, Chile, Peru, Colombia) market size (sales, revenue and growth rate) of Sodium Formate Market
Different types and applications of Sodium Formate Market share of each type and application by revenue.
Global of Sodium Formate Market size (sales, revenue) forecast by regions and countries from 2022 to 2028 of Sodium Formate Market
Upstream raw materials and manufacturing equipment, industry chain analysis of Sodium Formate Market
SWOT analysis of Sodium Formate Market
New Project Investment Feasibility Analysis of Sodium Formate Market
Browse More Reports:
Global Organometallic Market Global Dome Security Market Global Rabies Prophylaxis Market Middle East and Africa Trauma Devices Market Global Neurological Biomarkers Market Global Automotive Rain Sensor Market Asia-Pacific Maintenance Repair and Operations (MRO) Market Global Automotive Battery Thermal Management System Market Global Subscription and Billing Management Market Global Osteosarcoma Drug Market Global Specialty PACS Market Global Gardening Pots Market Asia-Pacific Specialty Oilfield Chemicals Market North America Sweet Potato Powder Market Global Minimal Residual Disease Market Global Endometrial Resection Devices Market Europe Contrast Injector Market Global Discrete Diodes Market Global Composite Packaging Market Global Vascular Grafts and Peripheral Stents Market Global Colored Gemstones Market Global Web Analytics Market Global Tumor Necrosis Factor (TNF) Inhibitor Drugs Market Global Dental Adhesive Market California Biostimulants Market Global Thrombotic Thrombocytopenic Purpura (Moschcowitz Disease) Market Global Coagulation Testing Market North America Network Packet Broker Market Asia-Pacific Inherited Retinal Diseases Market Global Quicklime Market Global Carbon Fiber Tape Market Global Frozen Drinks Market Global User Experience (UX) Research Software Market Europe Augmented Reality (AR) and Mixed Reality (MR) Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag: Sodium Formate Market, Sodium Formate Market Size, Sodium Formate Market Share, Sodium Formate Market Growth
0 notes
Text
Dry Ice Production Cost Report by Procurement Resource

Procurement Resource, a trusted provider of procurement intelligence and market research, presents the latest Dry Ice Production Cost Report. This comprehensive study offers detailed insights into the cost structure of setting up and operating a dry ice manufacturing unit, assisting businesses, investors, and stakeholders in making informed financial and strategic decisions.
Overview of Dry Ice
Dry ice is the solid form of carbon dioxide (CO₂), widely utilized for its cooling properties and sublimation characteristics. It is essential in cold chain logistics, food preservation, pharmaceutical transportation, and industrial cleaning processes. Its increasing adoption across logistics, healthcare, and food industries is driving market growth and demand for cost-effective production.
Dry Ice Production Cost Analysis
The report delivers an in-depth breakdown of all critical cost components involved in the dry ice manufacturing process. It outlines capital and operational expenditure, offering a clear view of the financial outlay required to establish a viable production facility.
Key Cost Components Covered:
1. Raw Material Costs
Procurement of liquid carbon dioxide (LCO₂) as the primary input
Sourcing, transportation, and storage costs
2. Utility and Energy Costs
Electricity consumption for compression, cooling, and pelletizing
Water and other utility usage
Energy efficiency parameters
3. Equipment and Machinery Costs
CO₂ storage tanks and refrigeration systems
Dry ice pelletizers or block machines
Compressors, purifiers, and auxiliary systems
Cost variations based on capacity and automation level
4. Labor and Overhead
Skilled/unskilled workforce wages
Maintenance and quality control staffing
Administrative and operational overheads
5. Packaging and Logistics
Insulated containers and specialized packaging
Transport and cold chain management for distribution
6. Capital Investment
Land, plant setup, and infrastructure development
Installation and commissioning charges
Cost Structure and Financial Indicators
The report provides detailed financial modeling and projections:
Total Production Cost per Ton/Kilogram
Break-even Analysis
Profit Margins and Return on Investment (ROI)
Sensitivity Analysis based on Input Price Fluctuations
Cost Trends Over Time
Regional and Market Insights
The report also highlights regional cost variations, influenced by factors such as:
Local CO₂ availability and prices
Utility rates and labor costs
Regulatory compliance and environmental standards
The Asia-Pacific region, particularly India and China, exhibits competitive production cost advantages due to abundant feedstock and low labor costs.
Why Choose Procurement Resource?
Procurement Resource offers expert-driven, data-backed production cost analysis reports that empower decision-makers with the tools needed to optimize manufacturing operations and control costs. Services include:
Accurate and detailed cost modeling
Price trend forecasts for raw materials and utilities
Customized economic feasibility reports
Benchmarking against global cost standards
Get a Free Sample Report
Interested in exploring the Dry Ice Production Cost Report in more detail?
Request your free sample copy here: https://www.procurementresource.com/production-cost-report-store/dry-ice/request-sample
Contact Us
Company Name: Procurement Resource Contact Person: Ashish Sharma (Sales Representative) Email: [email protected] Location: 30 North Gould Street, Sheridan, WY 82801, USA Phone: UK: +44 7537171117 USA: +1 307 363 1045 Asia-Pacific (APAC): +91 8850629517
#DryIce#manufacturing units#procurement intelligence#market research#food industries#healthcare#cleaning processes
0 notes
Text
Guide to Understanding, Building, and Optimizing API-Calling Agents
New Post has been published on https://thedigitalinsider.com/guide-to-understanding-building-and-optimizing-api-calling-agents/
Guide to Understanding, Building, and Optimizing API-Calling Agents


The role of Artificial Intelligence in technology companies is rapidly evolving; AI use cases have evolved from passive information processing to proactive agents capable of executing tasks. According to a March 2025 survey on global AI adoption conducted by Georgian and NewtonX, 91% of technical executives in growth stage and enterprise companies are reportedly using or planning to use agentic AI.
API-calling agents are a primary example of this shift to agents. API-calling agents leverage Large Language Models (LLMs) to interact with software systems via their Application Programming Interfaces (APIs).
For example, by translating natural language commands into precise API calls, agents can retrieve real-time data, automate routine tasks, or even control other software systems. This capability transforms AI agents into useful intermediaries between human intent and software functionality.
Companies are currently using API-calling agents in various domains including:
Consumer Applications: Assistants like Apple’s Siri or Amazon’s Alexa have been designed to simplify daily tasks, such as controlling smart home devices and making reservations.
Enterprise Workflows: Enterprises have deployed API agents to automate repetitive tasks like retrieving data from CRMs, generating reports, or consolidating information from internal systems.
Data Retrieval and Analysis: Enterprises are using API agents to simplify access to proprietary datasets, subscription-based resources, and public APIs in order to generate insights.
In this article I will use an engineering-centric approach to understanding, building, and optimizing API-calling agents. The material in this article is based in part on the practical research and development conducted by Georgian’s AI Lab. The motivating question for much of the AI Lab’s research in the area of API-calling agents has been: “If an organization has an API, what is the most effective way to build an agent that can interface with that API using natural language?”
I will explain how API-calling agents work and how to successfully architect and engineer these agents for performance. Finally, I will provide a systematic workflow that engineering teams can use to implement API-calling agents.
I. Key Definitions:
API or Application Programming Interface : A set of rules and protocols enabling different software applications to communicate and exchange information.
Agent: An AI system designed to perceive its environment, make decisions, and take actions to achieve specific goals.
API-Calling Agent: A specialized AI agent that translates natural language instructions into precise API calls.
Code Generating Agent: An AI system that assists in software development by writing, modifying, and debugging code. While related, my focus here is primarily on agents that call APIs, though AI can also help build these agents.
MCP (Model Context Protocol): A protocol, notably developed by Anthropic, defining how LLMs can connect to and utilize external tools and data sources.
II. Core Task: Translating Natural Language into API Actions
The fundamental function of an API-calling agent is to interpret a user’s natural language request and convert it into one or more precise API calls. This process typically involves:
Intent Recognition: Understanding the user’s goal, even if expressed ambiguously.
Tool Selection: Identifying the appropriate API endpoint(s)—or “tools”—from a set of available options that can fulfill the intent.
Parameter Extraction: Identifying and extracting the necessary parameters for the selected API call(s) from the user’s query.
Execution and Response Generation: Making the API call(s), receiving the response(s), and then synthesizing this information into a coherent answer or performing a subsequent action.
Consider a request like, “Hey Siri, what’s the weather like today?” The agent must identify the need to call a weather API, determine the user’s current location (or allow specification of a location), and then formulate the API call to retrieve the weather information.
For the request “Hey Siri, what’s the weather like today?”, a sample API call might look like:
GET /v1/weather?location=New%20York&units=metric
Initial high-level challenges are inherent in this translation process, including the ambiguity of natural language and the need for the agent to maintain context across multi-step interactions.
For example, the agent must often “remember” previous parts of a conversation or earlier API call results to inform current actions. Context loss is a common failure mode if not explicitly managed.
III. Architecting the Solution: Key Components and Protocols
Building effective API-calling agents requires a structured architectural approach.
1. Defining “Tools” for the Agent
For an LLM to use an API, that API’s capabilities must be described to it in a way it can understand. Each API endpoint or function is often represented as a “tool.” A robust tool definition includes:
A clear, natural language description of the tool’s purpose and functionality.
A precise specification of its input parameters (name, type, whether it’s required or optional, and a description).
A description of the output or data the tool returns.
2. The Role of Model Context Protocol (MCP)
MCP is a critical enabler for more standardized and robust tool use by LLMs. It provides a structured format for defining how models can connect to external tools and data sources.
MCP standardization is beneficial because it allows for easier integration of diverse tools, it promotes reusability of tool definitions across different agents or models. Further, it is a best practice for engineering teams, starting with well-defined API specifications, such as an OpenAPI spec. Tools like Stainless.ai are designed to help convert these OpenAPI specs into MCP configurations, streamlining the process of making APIs “agent-ready.”
3. Agent Frameworks & Implementation Choices
Several frameworks can aid in building the agent itself. These include:
Pydantic: While not exclusively an agent framework, Pydantic is useful for defining data structures and ensuring type safety for tool inputs and outputs, which is important for reliability. Many custom agent implementations leverage Pydantic for this structural integrity.
LastMile’s mcp_agent: This framework is specifically designed to work with MCPs, offering a more opinionated structure that aligns with practices for building effective agents as described in research from places like Anthropic.
Internal Framework: It’s also increasingly common to use AI code-generating agents (using tools like Cursor or Cline) to help write the boilerplate code for the agent, its tools, and the surrounding logic. Georgian’s AI Lab experience working with companies on agentic implementations shows this can be great for creating very minimal, custom frameworks.
IV. Engineering for Reliability and Performance
Ensuring that an agent makes API calls reliably and performs well requires focused engineering effort. Two ways to do this are (1) dataset creation and validation and (2) prompt engineering and optimization.
1. Dataset Creation & Validation
Training (if applicable), testing, and optimizing an agent requires a high-quality dataset. This dataset should consist of representative natural language queries and their corresponding desired API call sequences or outcomes.
Manual Creation: Manually curating a dataset ensures high precision and relevance but can be labor-intensive.
Synthetic Generation: Generating data programmatically or using LLMs can scale dataset creation, but this approach presents significant challenges. The Georgian AI Lab’s research found that ensuring the correctness and realistic complexity of synthetically generated API calls and queries is very difficult. Often, generated questions were either too trivial or impossibly complex, making it hard to measure nuanced agent performance. Careful validation of synthetic data is absolutely critical.
For critical evaluation, a smaller, high-quality, manually verified dataset often provides more reliable insights than a large, noisy synthetic one.
2. Prompt Engineering & Optimization
The performance of an LLM-based agent is heavily influenced by the prompts used to guide its reasoning and tool selection.
Effective prompting involves clearly defining the agent’s task, providing descriptions of available tools and structuring the prompt to encourage accurate parameter extraction.
Systematic optimization using frameworks like DSPy can significantly enhance performance. DSPy allows you to define your agent’s components (e.g., modules for thought generation, tool selection, parameter formatting) and then uses a compiler-like approach with few-shot examples from your dataset to find optimized prompts or configurations for these components.
V. A Recommended Path to Effective API Agents
Developing robust API-calling AI agents is an iterative engineering discipline. Based on the findings of Georgian AI Lab’s research, outcomes may be significantly improved using a systematic workflow such as the following:
Start with Clear API Definitions: Begin with well-structured OpenAPI Specifications for the APIs your agent will interact with.
Standardize Tool Access: Convert your OpenAPI specs into MCP Tools like Stainless.ai can facilitate this, creating a standardized way for your agent to understand and use your APIs.
Implement the Agent: Choose an appropriate framework or approach. This might involve using Pydantic for data modeling within a custom agent structure or leveraging a framework like LastMile’s mcp_agent that is built around MCP.
Before doing this, consider connecting the MCP to a tool like Claude Desktop or Cline, and manually using this interface to get a feel for how well a generic agent can use it, how many iterations it usually takes to use the MCP correctly and any other details that might save you time during implementation.
Curate a Quality Evaluation Dataset: Manually create or meticulously validate a dataset of queries and expected API interactions. This is critical for reliable testing and optimization.
Optimize Agent Prompts and Logic: Employ frameworks like DSPy to refine your agent’s prompts and internal logic, using your dataset to drive improvements in accuracy and reliability.
VI. An Illustrative Example of the Workflow
Here’s a simplified example illustrating the recommended workflow for building an API-calling agent:
Step 1: Start with Clear API Definitions
Imagine an API for managing a simple To-Do list, defined in OpenAPI:
openapi: 3.0.0
info:
title: To-Do List API
version: 1.0.0
paths:
/tasks:
post:
summary: Add a new task
requestBody:
required: true
content:
application/json:
schema:
type: object
properties:
description:
type: string
responses:
‘201′:
description: Task created successfully
get:
summary: Get all tasks
responses:
‘200′:
description: List of tasks
Step 2: Standardize Tool Access
Convert the OpenAPI spec into Model Context Protocol (MCP) configurations. Using a tool like Stainless.ai, this might yield:
Tool Name Description Input Parameters Output Description Add Task Adds a new task to the To-Do list. `description` (string, required): The task’s description. Task creation confirmation. Get Tasks Retrieves all tasks from the To-Do list. None A list of tasks with their descriptions.
Step 3: Implement the Agent
Using Pydantic for data modeling, create functions corresponding to the MCP tools. Then, use an LLM to interpret natural language queries and select the appropriate tool and parameters.
Step 4: Curate a Quality Evaluation Dataset
Create a dataset:
Query Expected API Call Expected Outcome “Add ‘Buy groceries’ to my list.” `Add Task` with `description` = “Buy groceries” Task creation confirmation “What’s on my list?” `Get Tasks` List of tasks, including “Buy groceries”
Step 5: Optimize Agent Prompts and Logic
Use DSPy to refine the prompts, focusing on clear instructions, tool selection, and parameter extraction using the curated dataset for evaluation and improvement.
By integrating these building blocks—from structured API definitions and standardized tool protocols to rigorous data practices and systematic optimization—engineering teams can build more capable, reliable, and maintainable API-calling AI agents.
#2025#ADD#add task#adoption#agent#Agentic AI#agents#ai#AI adoption#ai agent#AI AGENTS#ai use cases#alexa#Amazon#amp#Analysis#anthropic#API#API agents#API caling agents#APIs#apple#applications#approach#Article#artificial#Artificial Intelligence#assistants#Building#claude
0 notes
Text
Extend Component Life: How Laser Cladding is Revolutionizing Industries
Laser Cladding Market Growth & Trends
The global Laser Cladding Market size is projected to reach an impressive USD 1,042.1 million by 2030. This growth is anticipated at a compound annual growth rate (CAGR) of 9.3% from 2024 to 2030, as detailed in a new report by Grand View Research, Inc.
The increasing focus on lightweight materials and advanced engineering solutions across vital industries such as aerospace and automotive has significantly bolstered the demand for laser cladding. This technology is crucial for a range of applications, from repairing and refurbishing worn-out components to adding specialized functional coatings for purposes like creating thermal barriers or enhancing electrical conductivity.
Advancements in Laser Technology Fueling Expansion
Ongoing advancements in laser technology are a primary driver of market growth. These innovations include the development of higher power lasers, improved beam delivery systems, and enhanced process monitoring and control capabilities. Such technological progress has broadened the spectrum of materials that can be effectively processed and increased the complexity of geometries that can be coated. This, in turn, has expanded the potential applications of laser cladding, accelerating its adoption across a diverse array of industries.
Enhancing Infrastructure Lifespan and Operational Efficiency
Laser cladding plays a critical role in extending the lifespan of essential infrastructure and equipment in sectors such as energy, mining, and heavy machinery. By applying robust protective coatings to vulnerable areas prone to wear, corrosion, or erosion, laser cladding helps mitigate operational risks, minimize downtime, and significantly enhance asset reliability. This translates into substantial cost savings and improved operational efficiencies for asset-intensive industries.
Market Structure: Consolidation and Vertical Integration
The laser cladding market demonstrates a degree of consolidation and vertical integration, particularly among larger players with extensive capabilities and global reach. Equipment manufacturers in this sector often provide a comprehensive suite of solutions to their customers, which may include material supply, engineering services, and aftermarket support. This strategic integration helps streamline the supply chain, enhance customer service, and allows companies to capture a larger share of the overall value chain.
Curious about the Laser Cladding Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Laser Cladding Market Report Highlights
Based on type, the diode lasers segment led the market with the largest revenue share of 43% in 2023. The adoption of diode lasers is driven by their flexibility in beam shaping and modulation, enabling fine-tuning of process parameters to achieve desired coating properties
Based on type, the fiber lasers segment is expected to grow at a significant CAGR over the forecast period, due to their superior beam quality, high power density, and reliability. Fiber lasers utilize optical fibers as the gain medium, offering excellent beam stability and delivery characteristics
Based on material, the cobal based alloys segment led the market with the largest revenue share of 35% in 2023, due to their excellent wear resistance, corrosion resistance, and high-temperature strength. These alloys are widely used in applications such as aerospace, oil and gas, and power generation industries
Based on end-use, the aerospace and defense segment led the market with a revenue share of 31% in 2023. The aerospace and defense sector represents a significant end-user segment for global market. Laser cladding is used for repairing and enhancing critical components such as turbine blades, engine parts, and aircraft structures
Asia Pacific led the market in 2023, owing to expanding manufacturing sectors and increasing investments in infrastructure and technology driving the demand for laser cladding solutions
Laser Cladding Market Segmentation
Grand View Research has segmented the global laser cladding market report based on type, material, end-use, and region:
Laser Cladding Type Outlook (Revenue, USD Million, 2018 - 2030)
Diode Lasers
Fiber Lasers
CO2 Lasers
YAG Lasers
Laser Cladding Material Outlook (Revenue, USD Million, 2018 - 2030)
Cobalt Based Alloys
Nickel Based Alloys
Iron Based Alloys
Carbide & Carbide Blends
Others
Laser Cladding End-use Outlook (Revenue, USD Million, 2018 - 2030)
Aerospace & Defense
Oil & gas
Automotive
Power Generation
Medical
Others
Download your FREE sample PDF copy of the Laser Cladding Market today and explore key data and trends.
0 notes
Text
Essential Mobile Application Features for 2025: What Users Expect Today

Mobile applications are no more an added advantage but have become essential in people’s lives. As the mobile platform continues to permeate into all aspects, including shopping, entertainment, learning, and working, the utility app users demand better app performances, innovations, and customized services.
This is because every stakeholder today is planning to have their business online to explore the benefits that come with it, hence the need to prepare and design your mobile application with the following features to meet the needs of 2025 and beyond.
1. Personalization Powered by AI

Personalization has become the cornerstone of great user experiences. Apps equipped with artificial intelligence (AI) analyze user behavior, preferences, and patterns to offer tailored recommendations, content, and notifications.
For example, e-commerce apps recommend products based on past purchases, while streaming platforms suggest content aligned with users’ tastes.
Integrating AI ensures a dynamic and engaging experience that keeps users coming back for more.
2. Cross-Platform Compatibility

When there are more devices on the market, and these devices use different operating systems, the users expect the applications to be as fluid as possible. For it to be as effective as a smartphone application or software, it should have compatibility with Android, iOS, and any web platform.
This saves developmental costs and affords users a similar experience regardless of the device they choose to stick with.
Organizations can use other mobile app development software tools like Flutter or React Native to accomplish this goal effectively.
3. Voice and Gesture Control

With voice operations like Siri, Alexa, and Google Assistant, among others, now going mainstream, the apps have to have voice and even gesture support.
Users expect to perform tasks hands-free, making voice commands a must-have feature.
The intelligent movements make the app navigation easy and accessibility friendly to all the users across different age brackets.
4. Advanced Security Features

With cyber threats on the rise, security is a top priority for app users.
Features like fingerprint/face unlock, encryption of data across the application, and multi-factor authentication are no longer luxuries.
Consumers require confidence that their data is safe, particularly in the apps that require information of such nature as is found in banking or health applications.
5. Offline Functionality

The users themselves often cannot always be connected to the internet, although they are very particular about some functionalities of the app not requiring an internet connection.
Offline functionality is important for note-taking, navigation, and entertainment apps.
This way an app is guaranteed to keep its users engaged in spite of connectivity, as most data would have been cached locally.
6. Seamless Payment Integration

Electronic commerce has later on become a trend, so ensuring that payments require easy and streamlined processes is a necessity.
Apps have to support a variety of payment choices such as credit cards, digital wallet services, Apple Pay, Google Pay, cryptocurrency, and the like.
Minimizing the steps of completing the order and enhancing the security parameters increases user satisfaction and sales.
7. Social Media Integration

In the world of sharing, the apps that can integrate the users with their significant social accounts, including Instagram, Twitter, or LinkedIn, turn out to be beneficial.
The use of social media accounts streamlines the sign-up process; reporting of achievements for games or even recommendations for a product increases the circulation of the app and engagements.
8. Real-Time Updates and Push Notifications

Keeping users informed in real-time is essential.
Push notifications ensure users don’t miss out on updates, offers, or alerts.
However, they must be used judiciously to avoid overwhelming the user. Tailored notifications based on user behavior perform better than generic ones.
READ MORE- https://www.precisio.tech/essential-mobile-application-features-for-2025-what-users-expect-today/
#technology#Marketing#Business#Digital Marketing#Artificial Intelligence#Essential Mobile Application#Information technology#IT services
0 notes
Text
Agentic AI vs GPT: What’s best for Your Business?

The AI revolution is in full swing, and two powerful paradigms are leading the charge: Generative AI (like GPT models) and Agentic AI. While both are built on cutting-edge AI foundations, they serve fundamentally different purposes. Understanding these distinctions is crucial for businesses looking to strategically deploy AI and extract maximum value. It's not about which is "better" overall, but which is "best" for a specific business need.
Let's break down their core differences and help you decide.
Generative AI (GPT-style Models): The Master of Content and Conversation
Generative AI, exemplified by models like OpenAI's GPT series, Google's Gemini, or Anthropic's Claude, excels at creating new content based on patterns learned from vast datasets. They are phenomenal at understanding and generating human-like text, images, code, audio, and even video.
Core Function: Creation, Transformation, and Retrieval of Information.
Best for Your Business If You Need:
Content Generation at Scale:
Marketing: Drafting blog posts, social media captions, ad copy, email newsletters.
Customer Service: Generating detailed FAQ answers, script templates, or personalized customer responses.
Internal Communications: Summarizing meetings, drafting internal memos, creating training materials.
Information Synthesis and Explanation:
Research: Quickly summarizing lengthy reports, academic papers, or market analyses.
Knowledge Management: Creating concise explanations of complex topics, building interactive knowledge bases.
Q&A and Chatbots: Powering conversational interfaces that provide comprehensive answers to user queries.
Code Assistance:
Generating code snippets, debugging existing code, refactoring, or translating code between languages.
Creative Brainstorming:
Generating new product ideas, marketing campaign concepts, or design variations.
Think of GPT-style AI as your ultimate creative assistant, content factory, and conversational knowledge base.
Agentic AI: The Autonomous Task Executor
Agentic AI, or AI Agents, takes AI a significant step further. It's not just about generating information; it's about autonomously understanding a goal, planning a sequence of actions, executing those actions, interacting with external tools and environments, and iterating until the goal is achieved.
Core Function: Autonomous Goal Achievement and Task Automation.
Best for Your Business If You Need:
Automated Multi-Step Workflows:
Sales & Lead Nurturing: An agent could identify potential leads, research their company, draft personalized outreach emails, schedule follow-ups, and update the CRM – all autonomously.
Customer Support: Beyond answering questions, an agent could troubleshoot issues, access customer accounts, initiate refunds, or escalate complex cases by integrating with internal systems.
Complex Problem Solving:
Financial Analysis: An agent could research market trends, analyze company financials, identify investment opportunities, and execute trades based on defined parameters.
Supply Chain Management: An agent could monitor real-time disruptions, dynamically reroute shipments, re-order stock from alternative suppliers, and update inventory systems.
Dynamic Interaction with Tools & APIs:
Agents can connect to and utilize a wide array of existing software (CRM, ERP, ticketing systems, databases, web browsers) to perform tasks that span multiple applications.
Autonomous Research & Development:
An agent could conduct literature reviews, design experiments, run simulations, analyze results, and even propose new hypotheses in scientific research.
Think of Agentic AI as your autonomous project manager, intelligent personal assistant, or automated problem-solver.
The Powerful Synergy: Not Either/Or, But Both
The most transformative AI solutions will increasingly combine both paradigms. An Agentic AI often uses Generative AI as a powerful tool within its workflow:
An agent performing market research might use a GPT model to summarize articles it found via web search.
An agent writing code for a new feature might ask a GPT model to generate a specific function or debug an error.
An agent managing customer support might use a GPT model to draft a empathetic and accurate response before sending it.
Conclusion:
Choosing between Generative AI and Agentic AI isn't about picking a winner, but about understanding your specific business challenge. Do you need to create content, communicate effectively, and synthesize information? GPT-style Generative AI is your powerhouse. Do you need to automate complex, multi-step tasks, interact autonomously with systems, and achieve defined goals? Agentic AI is your strategic solution.
The future of business intelligence and automation lies in leveraging the unique strengths of both, building sophisticated systems where the creative power of generative models fuels the autonomous execution of intelligent agents, delivering unprecedented value.
0 notes
Text
Global Distributed Fiber Optic Sensor Market to Hit $1.98 Billion by 2032

Distributed Fiber Optic Sensor (DFOS) Market Analysis:
The global Distributed Fibre Optic Sensing (DFOS) Market size was valued at US$ 1.42 billion in 2024 and is projected to reach US$ 2.71 billion by 2032, at a CAGR of 9.6% during the forecast period 2025-2032
Distributed Fiber Optic Sensor (DFOS) Market Overview
Distributed sensing technology enables continuous, real-time measurements of the entire length of the fiber optic cable.Unlike traditional sensors, which rely on discrete sensors measured at predetermined points, distributed sensing does not rely on manufactured sensors but USES optical fibers. Distributed fiber optic sensor (DFOS) is an ideal choice for monitoring critical infrastructure or facilities.These sensors are small in size, low in cost, impervious to electromagnetic interference, and mechanically and chemically compatible with most building materials, making them ideal for building very large monitoring networks.Distributed optical fiber sensor (DFOS) is commonly used to measure parameters in real time and is difficult to obtain at high resolution over long distances.This limitation can be overcome by using distributed sensors. Distributed optical fiber sensing system usually consists of laser light source, sensing optical fiber (cable) and detection unit. It is an automatic monitoring system.The measurement is based on the principle of backscattering of light transmitted in the optical fiber.Into a certain energy in the optical fiber and the width of laser pulse, it while they are in the optical fiber transmission to the scattering light, after the creation of the state of light scattering in optical fiber damage and the influence of the change, will not be back scattering of light after WDM, detection, demodulation, into the signal is real-time signal processing system can be displayed, and consists of light waves in optical fiber transmission speed and back light echo time for these information.The distributed optical fiber sensing (DFOS) counted in this report only includes the host system, not the sensing optical fiber (cable) and display equipment.
Distributed Fiber Optic Sensors (DFOS) are advanced sensing systems that utilize fiber optic cables to detect changes in temperature, strain, acoustics, and other environmental variables along the entire length of the fiber. These sensors are widely used in industries like oil & gas, civil engineering, power, and security monitoring due to their high sensitivity, real-time monitoring capabilities, and durability in harsh environments.
Key Players in the Global DFOS Market
The global DFOS market is moderately concentrated, with the top players holding significant shares. Leading companies include:
Schlumberger
Halliburton
Baker Hughes
These top three players together account for over 30% of the global market share, showcasing strong technological leadership and industry experience.
Regional Production Insights
North America: Dominates the market with over 40% share, attributed to a strong presence in the oil & gas industry and advanced technological infrastructure.
China: Holds approximately 30% of the global production, driven by rapid industrialization and infrastructure development.
Europe: Accounts for about 15% of global output, supported by growth in energy and construction sectors.
Market Segmentation by Type
DTS (Distributed Temperature Sensing): Holds the largest market share at approximately 55%, driven by its wide adoption in temperature-critical applications such as downhole monitoring and pipeline leak detection.
Other types include DAS (Distributed Acoustic Sensing) and DSS (Distributed Strain Sensing).
Market Segmentation by Application
Petroleum and Petrochemical: Dominates the application segment with a market share of over 45%, owing to its crucial role in wellbore monitoring, reservoir characterization, and pipeline management.
Other application areas:
Power & utility
Civil engineering (bridges, tunnels)
Safety and security (perimeter and intrusion detection)
Transportation infrastructure
We have surveyed the Distributed Fiber Optic Sensor (DFOS) manufacturers, suppliers, distributors, and industry experts on this industry, involving the sales, revenue, demand, price change, product type, recent development and plan, industry trends, drivers, challenges, obstacles, and potential risks This report aims to provide a comprehensive presentation of the global market for Distributed Fiber Optic Sensor (DFOS), with both quantitative and qualitative analysis, to help readers develop business/growth strategies, assess the market competitive situation, analyze their position in the current marketplace, and make informed business decisions regarding Distributed Fiber Optic Sensor (DFOS). This report contains market size and forecasts of Distributed Fiber Optic Sensor (DFOS) in global, including the following market information:
Global Distributed Fiber Optic Sensor (DFOS) market revenue, 2020-2025, 2026-2031, ($ millions)
Global Distributed Fiber Optic Sensor (DFOS) market sales, 2020-2025, 2026-2031, (Units)
Global top five Distributed Fiber Optic Sensor (DFOS) companies in 2024 (%)
Distributed Fiber Optic Sensor (DFOS) Key Market Trends :
Growing Adoption in Oil & Gas Industry DFOS is increasingly used in petroleum and petrochemical sectors for real-time well monitoring and pipeline inspection due to its reliability and long-distance sensing capabilities.
Shift Towards Smart Infrastructure Monitoring The rise in smart city projects and infrastructure upgrades has boosted the demand for DFOS for monitoring bridges, tunnels, and buildings.
Integration with AI and Data Analytics DFOS systems are being enhanced with AI algorithms for predictive maintenance and improved data interpretation in complex environments.
Technological Advancements in DTS and DAS Innovations in Distributed Temperature Sensing (DTS) and Distributed Acoustic Sensing (DAS) are driving efficiency and expanding DFOS applications.
Expansion in Public Safety and Security There’s increasing use of DFOS for perimeter and intrusion detection in high-security areas like airports, government buildings, and industrial zones.
Distributed Fiber Optic Sensor (DFOS) Market Regional Analysis :
https://semiconductorinsight.com/wp-content/uploads/2025/01/download-34_11zon-1.png
North America:Strong demand driven by EVs, 5G infrastructure, and renewable energy, with the U.S. leading the market.
Europe:Growth fueled by automotive electrification, renewable energy, and strong regulatory support, with Germany as a key player.
Asia-Pacific:Dominates the market due to large-scale manufacturing in China and Japan, with growing demand from EVs, 5G, and semiconductors.
South America:Emerging market, driven by renewable energy and EV adoption, with Brazil leading growth.
Middle East & Africa:Gradual growth, mainly due to investments in renewable energy and EV infrastructure, with Saudi Arabia and UAE as key contributors.
Total Market by Segment:
Global Distributed Fiber Optic Sensor (DFOS) market, by Type, 2020-2025, 2026-2031 ($ millions) & (Units) Global Distributed Fiber Optic Sensor (DFOS) market segment percentages, by Type, 2024 (%)
DTS
DAS
Others (DSS, etc.)
Global Distributed Fiber Optic Sensor (DFOS) market, by Application, 2020-2025, 2026-2031 ($ Millions) & (Units) Global Distributed Fiber Optic Sensor (DFOS) market segment percentages, by Application, 2024 (%)
Petroleum and Petrochemical
Electricity
Traffic Tunnel Bridge
Metallurgy
Security Monitoring
Fire and Public Safety
Colleges and Scientific Research Institutions
Others
Competitor Analysis The report also provides analysis of leading market participants including:
Key companies Distributed Fiber Optic Sensor (DFOS) revenues in global market, 2020-2025 (estimated), ($ millions)
Key companies Distributed Fiber Optic Sensor (DFOS) revenues share in global market, 2024 (%)
Key companies Distributed Fiber Optic Sensor (DFOS) sales in global market, 2020-2025 (estimated), (Units)
Key companies Distributed Fiber Optic Sensor (DFOS) sales share in global market, 2024 (%)
Further, the report presents profiles of competitors in the market, key players include:
Schlumberger
Halliburton
Baker Hughes
Fotech Solutions
Silixa
OptaSense (QinetiQ)
AP Sensing
OZ Optics
LIOS (NKT Photonics)
Omnisens
Hifi Engineering
Future Fibre Technologies (Ava Group)
Bandweaver
Shanghai Huawei Technology
AGIOE
Hunan Guangsheng Optical Fiber Sensing Company
Wuhan Ligong Guangke Company Limited
CNPC Aobo (Chengdu) Technology
Guoxing Huijin (Shenzhen) Technology
Zhuhai Xunwei Oil and Gas Well Information Technology
Anton Oilfield Services (Group) Ltd
Chengdu Well Plus Oilfield Service
Beijing Perception Technology
Xiamen Fengxing Optoelec-tek
Jiangsu Huaneng Cable
Jiangsu Zhongtian Technology
Jiangsu Hengtong Power Cable
Market Drivers
Increasing Demand for Real-Time Monitoring The ability of DFOS to provide continuous, real-time data across long distances makes it ideal for critical applications such as oil wells, tunnels, and electrical grids.
Rising Investment in Smart Infrastructure Governments and private sectors are investing heavily in infrastructure development, boosting the adoption of DFOS for structural health monitoring.
Superior Performance in Harsh Environments DFOS is resistant to electromagnetic interference and extreme environmental conditions, making it a preferred choice over traditional sensors.
Market Restraints
High Initial Setup Cost The deployment of DFOS systems requires significant capital investment in laser sources, detection units, and integration, limiting its adoption for small-scale use.
Complex Installation and Calibration Installing and calibrating DFOS systems can be technically challenging, often requiring specialized personnel and tools.
Limited Awareness in Emerging Markets In several developing regions, the awareness and understanding of DFOS technology are still limited, slowing down market growth.
Market Opportunities
Expansion in Renewable Energy Sector DFOS can play a crucial role in monitoring wind turbines, solar farms, and other renewable infrastructure, opening new market opportunities.
Advancements in Fiber Optic Technology Continued R&D in fiber optics and sensing technology is likely to enhance DFOS capabilities, making it more efficient and cost-effective.
Increased Focus on Industrial Safety With growing concerns about workplace safety, industries are turning to DFOS systems for real-time hazard detection and accident prevention.
Market Challenges
Data Management and Interpretation Handling and analyzing the vast amount of data generated by DFOS systems remains a technical hurdle, especially in complex environments.
Integration with Existing Systems Integrating DFOS with legacy systems and infrastructure can be difficult, requiring additional time and investment.
#Global Distributed Fiber Optic Sensor Market#Global Distributed Fiber Optic Sensor Market Share#Global Distributed Fiber Optic Sensor Market Size
0 notes
Text
Can AI Predict Your Disease? Discover SGP’s VPK42™ Fingerprint Technology with Docture-Poly™
youtube

In today’s fast-evolving health landscape, early detection and personalized care are no longer futuristic dreams — they’re today’s reality. At Sai Ganga Panakeia (SGP), we’ve brought ancient Ayurvedic intelligence and modern AI together in one game-changing solution: VPK42™ Fingerprint Technology, delivered through our cutting-edge health device — Docture-Poly™.
🌿 What is VPK42™?
VPK stands for Vata, Pitta, and Kapha — the core energies that determine your body type and health in Ayurveda. VPK42™ is SGP’s proprietary diagnostic system that decodes your unique doshic signature using 42+ parameters, giving us your Metabolic Fingerprint.

✨ Enter Docture-Poly™

Dr. Ravishankar Polisetty
Dr Ravishankar Polisetty’s Innovation The World’s FirstVPK 42 Fingerprinting(Ayurvedic Metabolic Fingerprint)Powerful AI & ML Based iOT Technology
Dr. Ravishankar Polisetty is a distinguished Indian physician renowned for his integrative approach to healthcare, blending modern medicine with ancient Ayurvedic wisdom. He is the founder and Director of Research & Development at Sai Ganga Panakeia Ltd (SGP), a Hyderabad-based company specializing in personalized medicine and biotechnology .saigangapanakeia.inLinkedIn
Educational and Professional Background
Dr. Polisetty’s academic credentials are extensive, encompassing:
MD in General Medicine
Specializations in Laparoscopic, Laser, and Cardiovascular Surgery
PhD in Cardiovascular Surgery
Doctorates in Naturopathic and Alternative Medicine from India, Canada, and the USA
Doctorate in Humanitarian Sciences
Certified Watson Data Scientist with expertise in Python, C++, Artificial Intelligence, and Machine LearningLog in or sign up to view+5saigangapanakeia.in+5About.me+5
His multidisciplinary expertise enables him to integrate traditional Ayurvedic principles with contemporary medical practices and advanced data science techniques .saigangapanakeia.in+3saigangapanakeia.in+3Pharmabiz+3
Innovations in Healthcare
Dr. Polisetty is the pioneer of “Poly Scientific Ayurveda” (PSA), a methodology that combines Ayurvedic concepts with modern scientific research. He has developed “Docture-Poly,” an IoT-based digital doctor assistant that analyzes an individual’s Vata, Pitta, and Kapha (VPK) fingerprint to provide personalized health recommendations. This non-invasive tool utilizes AI and machine learning algorithms to offer tailored dietary, herbal, and lifestyle guidance .saigangapanakeia.in+5Pharmabiz+5panacea-cro.academia.edu+5saigangapanakeia.in+2saigangapanakeia.in+2panacea-cro.academia.edu+2panacea-cro.academia.edu
Global Recognition and Impact
Dr. Polisetty’s contributions have garnered international acclaim. He has been invited twice to address the British Parliament’s House of Commons and has received the “Lead India Bharat Ratna” memento from former President Dr. APJ Abdul Kalam for his research in ancient Indian sciences .saigangapanakeia.in+1panacea-cro.academia.edu+1
Under his leadership, SGP has reported significant improvements in the quality of life for patients with end-stage diseases, claiming a 95% improvement rate and over 63% of patients becoming medication-free .LinkedIn+2saigangapanakeia.in+2Pharmabiz+2
Publications and Research

🤖 AI + Ayurveda: How the Technology Works
Docture-Poly™ uses sensors, AI algorithms, and centuries-old Ayurvedic principles to:
Monitor your vital functions
Assess your VPK balance
Detect early signs of disease
Suggest natural interventions through food, supplements, and lifestyle corrections

🔍 Why It’s Revolutionary
Unlike routine medical tests that offer one-size-fits-all results, Docture-Poly™ with VPK42™:
Understands your body type and tendencies
Reveals the “why” behind the “what”
Offers individualized plans for recovery and prevention
Avoids harsh interventions by supporting the body naturally
💡 What Can It Predict?
With the Docture-Poly™ + VPK42™ system, we can assess the risk or presence of:
Lifestyle and metabolic disorders
Liver and kidney imbalances
Hormonal issues
Stress-related dysfunctions
Gut health problems
Chronic fatigue, skin issues, and more
And it does this before they turn into full-blown diseases.
🩺 What Comes Next?
Once you get your VPK42™ report via Docture-Poly™, the SGP expert panel provides:
🧪 Targeted Ayurvedic Supplements
🥗 VPK-specific diet and hydration plans
🧸 Yoga, movement, and breathing routines
📊 Progress tracking and follow-up assessments
🛡️ Lifestyle guidance to prevent relapse
🚀 Preventive Healthcare, Redefined
At Sai Ganga Panakeia, we believe prediction alone isn’t enough — prevention is key. And with Docture-Poly™, we’ve made it possible to achieve both through a single device.
Whether you’re battling chronic symptoms or simply want to stay ahead of disease, Docture-Poly™ gives you control over your wellness journey — at your home, on your time.
🙌 Experience the Future Today
Book your Docture-Poly™ doorstep demo now and uncover your VPK42™ fingerprint with SGP’s integrative care team.
🔗 [ Make an Appointment ] ✨ Because your body already knows what it needs — Docture-Poly™ just helps you listen.
#ayurvedaindia#healthinnovation#madeinindia#naturebasedhealing#sgpproducts#sgpwellness#trustedayurveda#artists on tumblr#branding#entrepreneur#Youtube
1 note
·
View note
Text
Experience Smarter Trading with UCFX Markets AI Technology

UCFX Markets: Your Shortcut to Crypto Success
UCFX Markets is proud to announce the launch of its cutting-edge AI-driven crypto strategies, designed to empower investors to achieve unparalleled financial mastery in the volatile world of cryptocurrency. This innovative platform leverages advanced artificial intelligence to provide users with actionable insights, optimized trading strategies, and robust risk management tools, setting a new standard in the crypto investment landscape.
In an era where digital currencies dominate financial discussions, UCFX Markets stands out by offering a sophisticated AI-powered solution that caters to both novice and seasoned investors. The platform's AI algorithms analyze vast amounts of market data in real-time, identifying trends, predicting market movements, and executing trades with precision and speed that surpass human capabilities.
Relevance in Today's Market
The cryptocurrency market is renowned for its rapid fluctuations and high volatility, which can be daunting for investors seeking consistent returns. UCFX Markets addresses this challenge by utilizing AI to mitigate risks and enhance decision-making processes. By automating trading strategies, the platform reduces the emotional biases that often lead to poor investment choices, ensuring that users can achieve financial mastery with confidence.
"Financial markets are evolving at an unprecedented pace, and the integration of AI into trading strategies is not just a trend but a necessity," said the CEO of UCFX Markets. "Our platform is designed to democratize access to sophisticated trading tools, enabling investors to maximize their potential in the crypto space."
Key Features of AI-Driven Strategies
Real-Time Market Analysis: UCFX Markets employs AI to continuously monitor and analyze market conditions, providing users with up-to-the-minute insights that inform their trading decisions.
Automated Trading Execution: The platform's AI algorithms execute trades automatically based on predefined parameters, ensuring optimal timing and minimizing the impact of human error.
Personalized Investment Plans: Users can customize their investment strategies according to their risk tolerance and financial goals, with AI offering tailored recommendations to enhance portfolio performance.
Comprehensive Risk Management: UCFX Markets integrates advanced risk management tools that help users safeguard their investments against market volatility and unforeseen downturns.
User Testimonials
Investors who have adopted UCFX Markets have reported significant improvements in their trading outcomes. "Since I started using UCFX Markets, my investment returns have become more consistent and reliable," shared a satisfied user. "The AI-driven strategies have taken the guesswork out of trading, allowing me to focus on my financial goals with peace of mind."
Future Developments
Looking ahead, UCFX Markets is committed to continuous innovation, with plans to incorporate machine learning advancements and expand its range of supported cryptocurrencies. The platform aims to stay at the forefront of AI-driven trading technology, ensuring that users always have access to the most effective tools for financial mastery.
Engage with UCFX Markets
Investors interested in elevating their crypto trading experience are encouraged to explore UCFX Markets. By harnessing the power of AI, the platform offers a transformative approach to cryptocurrency investment, paving the way for sustained financial growth and success.
Take the Next Step
Discover how UCFX Markets can revolutionize your investment strategy. Visit https://ucfxmbot.com/ today and start your journey towards financial mastery with AI-driven crypto strategies.
0 notes
Text
Global Critical Care Diagnostics Market driven by innovations and 6% CAGR growth until 2030
The global critical care diagnostics market is set to witness a growth rate of 6% in the next 5 years. Increasing prevalence of chronic and acute diseases; technological advancements in diagnostics; growing demand for point-of-care testing; increasing healthcare expenditures; and pandemics and public health emergencies are some of the key factors driving the critical care diagnostics market.
Critical care diagnostics refers to a specialized segment of medical diagnostics focused on identifying, monitoring, and managing life-threatening conditions in critically ill patients. These diagnostics provide rapid, accurate results to aid clinical decision-making in emergency rooms, intensive care units (ICUs), and other critical care settings. Key applications include detecting sepsis, acute respiratory distress syndrome (ARDS), trauma, and cardiovascular emergencies. Critical care diagnostics utilize advanced technologies such as point-of-care testing, molecular diagnostics, and imaging systems to ensure timely interventions. Their primary goal is to improve patient outcomes by enabling prompt diagnosis and treatment of severe, time-sensitive medical conditions.
Download a free sample report now 👉 https://meditechinsights.com/critical-care-diagnostics-market/request-sample/
Increasing prevalence of chronic and acute diseases to propel market demand
The increasing prevalence of chronic and acute diseases, such as cardiovascular disorders, diabetes, respiratory infections, and sepsis, is a major driver of the critical care diagnostics market. These conditions often require rapid and accurate diagnostic tools to enable timely interventions and improve patient outcomes in critical care settings. The growing global burden of aging population, lifestyle-related disorders, and infectious disease outbreaks like COVID-19 has heightened the demand for advanced diagnostic technologies. Hospitals and intensive care units increasingly rely on point-of-care testing, biomarker assays, and molecular diagnostics to detect and monitor these life-threatening conditions, fueling market growth.
Technological advancements in diagnostics are driving the market growth
Technological advancements in diagnostics are significantly driving the growth of the critical care diagnostics market by improving the speed, accuracy, and efficiency of disease detection. Innovations such as point-of-care testing (POCT), molecular diagnostics, and AI-powered tools enable rapid and precise identification of critical conditions like sepsis, cardiac disorders, and respiratory infections. Integration of advanced biomarkers and multiplex testing platforms allows simultaneous detection of multiple parameters, reducing diagnostic time in emergency settings. Additionally, automation and digital connectivity in diagnostic systems streamline workflows and enhance decision-making in critical care units, meeting the rising demand for faster and more reliable diagnostic solutions.
Competitive Landscape Analysis
The global critical care diagnostics market is marked by the presence of established and emerging market players such as Abbott; F. Hoffmann-La Roche Ltd.; Becton, Dickinson and Company; bioMérieux; Danaher Corporation; EKF Diagnostics Holdings plc; QuidelOrtho Corporation; Chembio Diagnostics, Inc.; Bio-Rad Laboratories, Inc.; and Bayer AG; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Download a sample report for in-depth competitive insights https://meditechinsights.com/critical-care-diagnostics-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global critical care diagnostics market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, test type, application, and end user.
Market Size & Forecast (2023-2030), By Test Type, USD Million
Routine & Special Chemistry
Flow Cytometry
Hematology
Immunoproteins
Microbial & Infectious Disease
Coagulation Testing
Others
Market Size & Forecast (2023-2030), By Application, USD Million
Cardiovascular Diseases
Respiratory Diseases
Sepsis and Infectious Diseases
Trauma and Injury
Neurological Conditions
Others
Market Size & Forecast (2023-2030), By End User, USD Million
Hospital Operating Rooms and ICUs
Ambulatory Surgical Centers
Point-of-Care Settings
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
Chinese Hamster Ovary Cells Market Size, Share, Trends, Demand, Growth and Competitive Outlook
Executive Summary Chinese Hamster Ovary (CHO) Cells Market :
The global chinese hamster ovary (CHO) Cells market was valued at USD 416.79 million in 2024 and is expected to reach USD 800.49 million by 2032
During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 8.50%, primarily driven by rising demand for biologics and biosimilar
Comprehensive data and brilliant forecasting techniques used in Chinese Hamster Ovary (CHO) Cells Market report coincide with precision and correctness. Furthermore, it endows with historic data, present market trends, environment, technological innovation, upcoming technologies and the technical progress in the related industry. With this market report it becomes simpler for customers to understand the various drivers and restraints impacting the market during the forecast period. The report also displays the analysis and estimation of important industry trends, market size, and market share. Chinese Hamster Ovary (CHO) Cells Market analysis report is valuable for both regular and emerging market player in the industry and provides in-depth market insights.
The winning Chinese Hamster Ovary (CHO) Cells Market research report is generated with the best and advanced tools of collecting, recording, estimating and analysing market data. With the precise and high-tech information, about industry, businesses can know about the types of consumers, consumer’s demands and preferences, their perspectives about the product, their buying intentions, their response to particular product, and their varying tastes about the specific product already existing in the market through this report. The market insights covered in the report simplifies managing Market of goods and services effectively. For in depth understanding of market and competitive landscape, Chinese Hamster Ovary (CHO) Cells Market report serves a lot of parameters and detailed data about industry.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Chinese Hamster Ovary (CHO) Cells Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-chinese-hamster-ovary-cells-cho-market
Chinese Hamster Ovary (CHO) Cells Market Overview
**Segments**
- By Product (Equipments, Media, Reagents, Services) - By Application (Bioproduction, Drug Discovery, Toxicity Testing, Others) - By End-User (Biopharmaceutical Companies, Contract Research Organizations, Academic Institutes)
The global Chinese Hamster Ovary (CHO) cells market is segmented based on product, application, and end-user. In terms of product, the market is categorized into equipments, media, reagents, and services. The media segment is expected to witness significant growth due to the increasing demand for specialized cell culture media for CHO cells in bioproduction and drug discovery applications. With advancements in technology, there is a rising need for specific reagents tailored for CHO cell lines, which is also contributing to the growth of this segment. Additionally, services such as cell line development and cell banking are becoming essential in the biopharmaceutical industry, further driving the services segment of the market.
When analyzing the market by application, the segments include bioproduction, drug discovery, toxicity testing, and others. Bioproduction is the dominant segment in the global CHO cells market, owing to the widespread use of CHO cells in manufacturing therapeutic proteins and antibodies. The drug discovery segment is anticipated to grow at a significant rate as CHO cells are valuable tools in screening assays and target validation studies. Toxicity testing utilizing CHO cells is gaining traction due to its high sensitivity and reliability, propelling the growth of the toxicity testing segment. Other applications such as disease modeling and regenerative medicine are also fueling the market expansion.
Moreover, the market is segmented by end-user into biopharmaceutical companies, contract research organizations, and academic institutes. Biopharmaceutical companies hold a substantial market share as they extensively utilize CHO cells for the large-scale production of biologics. The contract research organizations segment is witnessing rapid growth as these entities provide outsourcing services for cell line development and characterization. Academic institutes are increasingly adopting CHO cells in research activities, creating a steady demand for cell culture products and services in educational settings.
**Market Players**
- Lonza - Thermo Fisher Scientific - Merck KGaA - Selexis SA - Catalent, Inc. - WuXi Biologics - Sartorius AG - JSR Corporation - Horizon Discovery Group plc - Boehringer Ingelheim International GmbH
Key players in the global Chinese Hamster Ovary (CHO) cells market include Lonza, Thermo Fisher Scientific, Merck KGaA, Selexis SA, Catalent, Inc., WuXi Biologics, Sartorius AG, JSR Corporation, Horizon Discovery Group plc, and Boehringer Ingelheim International GmbH. These companies are actively engaged in strategic collaborations, product developments, and acquisitions to strengthen their market presence and expand their product portfolios in the CHO cells segment.
The global Chinese Hamster Ovary (CHO) cells market is experiencing steady growth driven by factors such as the increasing demand for specialized cell culture media, advancements in technology leading to tailored reagents for CHO cell lines, and the essentiality of services like cell line development and banking in the biopharmaceutical industry. With the media segment expected to witness significant growth, companies are focusing on developing innovative and specialized culture media that cater to the specific needs of CHO cells in bioproduction and drug discovery applications. Additionally, the rising need for specific reagents tailored for CHO cell lines is fueling growth in this segment, as researchers and biopharmaceutical companies seek high-quality products to support their cell culture requirements. The services segment, including offerings like cell line development and banking, is becoming increasingly crucial in the biopharmaceutical sector, further boosting market growth.
In terms of applications, the dominance of the bioproduction segment in the CHO cells market is evident, owing to the widespread use of CHO cells in manufacturing therapeutic proteins and antibodies. The drug discovery segment is poised for significant growth as CHO cells continue to be valued tools in screening assays and target validation studies. Moreover, the toxicity testing segment is gaining traction due to the high sensitivity and reliability of CHO cells, making them ideal for assessing the safety of compounds in drug development processes. Other applications such as disease modeling and regenerative medicine are also playing a role in expanding the market by showcasing the versatility and adaptability of CHO cells in various research areas.
When considering end-users, biopharmaceutical companies are at the forefront of the CHO cells market due to their extensive utilization of CHO cells for large-scale biologics production. Contract research organizations are rapidly growing as they offer outsourcing services for cell line development and characterization, providing valuable support to companies in need of specialized expertise. Academic institutes are increasingly incorporating CHO cells into their research activities, creating a steady demand for cell culture products and services within educational settings. This trend is indicative of the broader adoption of CHO cells across different industry sectors, highlighting the versatility and significance of these cells in various applications.
Overall, the global CHO cells market is characterized by intense competition among key players such as Lonza, Thermo Fisher Scientific, Merck KGaA, Selexis SA, Catalent, Inc., WuXi Biologics, Sartorius AG, JSR Corporation, Horizon Discovery Group plc, and Boehringer Ingelheim International GmbH. These companies are actively engaging in strategic initiatives like collaborations, product developments, and acquisitions to enhance their market position and broaden their product offerings in the CHO cells segment. The market is poised for further growth as advancements in biotechnology and increasing research activities continue to drive demand for CHO cells and related products and services.The global Chinese Hamster Ovary (CHO) cells market is a dynamic and rapidly evolving industry driven by the increasing demand for specialized cell culture products and services. Companies operating in this market are continuously focusing on developing innovative solutions to cater to the specific needs of biopharmaceutical companies, contract research organizations, and academic institutes. The advancements in technology have paved the way for tailored reagents and specialized culture media, which are essential components for the growth and proliferation of CHO cells in various applications such as bioproduction, drug discovery, and toxicity testing.
In recent years, the market has witnessed a surge in strategic collaborations among key players to enhance their product portfolios and expand their market presence. Companies like Lonza, Thermo Fisher Scientific, and Merck KGaA have been at the forefront of these initiatives, aligning themselves with industry partners to leverage complementary strengths and resources. Such collaborations not only facilitate the development of cutting-edge products but also enable companies to tap into new market segments and geographical regions.
Moreover, acquisitions have played a crucial role in shaping the competitive landscape of the CHO cells market. Companies like Sartorius AG and WuXi Biologics have made strategic acquisitions to broaden their service offerings and strengthen their position in the market. These acquisitions often provide companies with access to proprietary technologies, novel research platforms, and a skilled workforce, thereby enhancing their competitive advantage.
Another key trend in the market is the increasing focus on sustainability and environmental responsibility. As the biopharmaceutical industry continues to grow, there is a growing awareness of the environmental impact of cell culture processes. Companies are investing in sustainable practices and green technologies to reduce their carbon footprint and minimize waste generation. This shift towards sustainability not only aligns with regulatory requirements but also resonates with a growing number of environmentally conscious consumers.
In conclusion, the global CHO cells market is poised for substantial growth in the coming years, driven by technological advancements, strategic collaborations, and a focus on sustainability. Companies that can adapt to these market trends, innovate their product offerings, and forge strategic partnerships are likely to emerge as key players in this competitive landscape. As the demand for CHO cells and related products continues to rise across various end-user segments, the market presents lucrative opportunities for growth and expansion.
The Chinese Hamster Ovary (CHO) Cells Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-chinese-hamster-ovary-cells-cho-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
This Comprehensive Report Provides:
Improve strategic decision making
Research, presentation and business plan support
Show emerging Chinese Hamster Ovary (CHO) Cells Marketopportunities to focus on
Industry knowledge improvement
It provides the latest information on important market developments.
Develop an informed growth strategy.
Build technical insight
Description of trends to exploit
Strengthen competitor analysis
By providing a risk analysis, you can avoid pitfalls that other companies may create.
Ultimately, you can maximize your company's profitability.
Browse More Reports:
Europe Transradial Access Market Global Fruit and Herbal Tea Market Global Security Assertion Markup Language (SAML) Authentication Market Global Metal Fabrication Market Europe Soy Protein Concentrate Market Global Dental Intraoral Radiology Equipment Market Global Metal Packaging Market Global Copper Sulphate Market Global Breast Ultrasound Market North America Biostimulants Market Global Instant Noodles Market Asia-Pacific Radiopharmaceuticals Market Global Agrochemical Intermediates Market Global Flight Simulator Market Global Polyurethane (PU) Microspheres Market Global Biostimulants Market Global Virtual Pipeline Systems Market Global Stand-Up Paddleboard Market Asia-Pacific Bladder Disorders Market North America Augmented Reality (AR) and Mixed Reality (MR) Market Guatemala Specific Pollen Allergies Market Global Cardiovascular Digital Solutions Market Middle East and Africa Automotive Battery Thermal Management System Market Asia- Pacific Plasma Fractionation Market Global Ribonucleic Acid (RNA) Interference Market Global Canned Tuna Market Global Walnut Oil Market Global Digital Shipyard Market Global Guar Gum Market Global Shoe Rack Market Global Needle Free Blood Drawing Devices Market Global Free Range Eggs Market Global Acrylic Bathtub Market Global Vegan Footwear Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
0 notes
Text
Low Voltage Battery Management Market Enters High-Growth Phase Through 2035
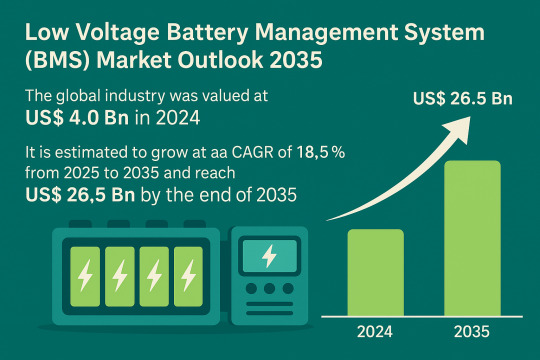
The global Low Voltage Battery Management System (BMS) market, valued at approximately US$ 4.0 billion in 2024, is projected to surge to an estimated US$ 26.5 billion by 2035, exhibiting a robust compound annual growth rate (CAGR) of 18.5%. This growth is primarily driven by the increasing adoption of electric vehicles (EVs), rapid integration of renewable energy storage solutions, and the expansion of consumer electronics utilizing lithium-ion battery technology.
Low voltage BMS are critical for the safe, efficient, and long-lasting operation of lithium-ion and other battery types, ensuring optimized performance by managing charging cycles, battery health, and safety parameters. The rising global emphasis on electrification and carbon footprint reduction underscores the market’s rapid growth trajectory.
Market Drivers & Trends
Surging EV Adoption: The automotive sector leads demand for low voltage BMS as manufacturers accelerate EV development to meet stringent emission regulations and rising consumer demand for sustainable transportation. BMS technology optimizes battery efficiency, safety, and longevity—crucial to EV performance.
Renewable Energy Storage Expansion: Increasing deployment of solar, wind, and other renewable energy sources requires reliable energy storage. Low voltage BMS systems play a pivotal role in maximizing battery life and grid stability by regulating charge/discharge cycles and preventing hazards like overheating.
Advancements in Smart BMS: Innovations such as predictive maintenance, energy management software, and enhanced communication interfaces are improving BMS functionalities, reducing costs, and mitigating safety risks, further boosting market adoption.
Latest Market Trends
Compact and High-Capacity BMS Solutions: Products like Sensata Technologies’ c-BMS24X introduced in May 2023 exemplify the trend toward compact, scalable systems tailored for low voltage EV applications including scooters, three-wheelers, forklifts, and automated guided vehicles (AGVs).
Integration of Sodium-Ion Batteries: Companies like Clarios are investing in sodium-ion battery tech to produce low-voltage BMS that enhance battery sustainability and performance for automotive applications.
Strategic Collaborations and Mergers: Market leaders are focusing on partnerships and acquisitions to expand technology portfolios and geographic reach, driving innovation and competitiveness.
Key Players and Industry Leaders
The low voltage BMS market is moderately consolidated with key players holding significant market shares. Major companies driving innovation and growth include:
Continental AG
Eberspaecher Vecture Inc.
Elithion Inc.
EMUS UAB
Ewert Energy Systems, Inc.
Honeywell International Inc.
Infineon Technologies AG
Johnson Matthey PLC
KPM Power Inc.
Leclanché SA
Lithium Balance A/S
Nuvation Engineering
Renesas Electronics Corporation
Stafl Systems, LLC
Victron Energy B.V.
These industry leaders invest heavily in R&D to enhance BMS functionalities such as cell balancing, predictive analytics, and state-of-health monitoring, enabling safer and more efficient battery operation.
Gain a preview of important insights from our Report in this sample – https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86446
Recent Developments
In October 2024, Clarios invested in Altris to develop low-voltage sodium-ion batteries for automotive BMS systems, aiming to enhance battery longevity and sustainability.
In May 2023, Sensata Technologies launched the c-BMS24X, a compact BMS solution supporting up to 24 cells in series and 2000 amps, optimizing performance for low voltage EV applications including scooters, three-wheelers, and forklifts.
Continuous advancements in software-driven BMS systems, integrating predictive maintenance and energy optimization algorithms, are gaining traction industry-wide.
Market Opportunities
The low voltage BMS market presents significant opportunities across multiple sectors:
Automotive: Growing EV penetration creates demand for sophisticated BMS that improve range, safety, and battery lifespan.
Renewable Energy: Energy storage systems require reliable BMS for efficient charge/discharge cycles and thermal management, crucial for grid stability.
Consumer Electronics: Increasing use of lithium-ion batteries in portable devices necessitates advanced BMS for safety and durability.
Industrial Equipment: AGVs, forklifts, and other battery-powered machinery depend on BMS to optimize operational uptime and reduce maintenance.
Emerging Battery Technologies: Adoption of sodium-ion, flow, and lithium polymer batteries opens new avenues for specialized low voltage BMS solutions.
Future Outlook
The low voltage battery management system market is poised for strong growth over the next decade, with forecasted expansion driven by the electrification of transportation, accelerated renewable energy adoption, and continuous technological innovations. The CAGR of 18.5% from 2025 to 2035 highlights the sector’s vibrant potential.
Emerging markets in Asia Pacific are expected to lead growth, driven by government incentives, industrialization, and a strategic focus on sustainable energy and transportation.
Smart BMS solutions that integrate AI, IoT, and cloud analytics are anticipated to revolutionize battery management, offering predictive maintenance and improved system resilience.
Market Segmentation
By Offering: Hardware (Battery Monitoring Unit, Battery Control Unit, Communication Interface, Power Module), Software (State of Charge/Health Monitoring, Predictive Analytics, Energy Management, Cell Balancing), and Services.
By Type: Motive Battery, Stationary Battery.
By Battery Type: Lithium-ion-based (82.1% share in 2024), Lead-acid-based, Lithium Polymer, Nickel-based, Flow Batteries, Others (Sodium ion, LFP cells).
By Topology: Centralized, Distributed, Modular.
By Voltage Range: Below 20V, 21-40V, 41-60V.
By Battery Pack Design: Prismatic Cell, Pouch Cell, Cylindrical Battery Packs.
By Application: Automotive (Two-Wheeler, Passenger Cars, Commercial Vehicles, Buses), Industrial (AGVs, Emergency Storage, Portable Tools), Energy & Utilities (Renewable and Non-renewable Systems), Consumer Electronics (Drones, Computing Devices, Appliances), IT and Telecom, Aerospace and Defense, Healthcare.
Regional Insights
Asia Pacific accounted for 48.2% of the market in 2024 and is expected to grow at a CAGR of 20.8% through 2035. The region benefits from:
Rapid industrialization and urbanization
Strong governmental push toward EV adoption and renewable energy
Leading automotive manufacturers investing in advanced BMS technologies
Rising consumer electronics demand
North America and Europe follow as key markets due to mature automotive industries, renewable energy projects, and advanced technology adoption.
Why Buy This Report?
Comprehensive analysis of the global low voltage BMS market with historic and forecast data through 2035
In-depth coverage of market drivers, restraints, opportunities, and technological trends
Strategic insights into competitive landscape, key players, and their developments
Detailed market segmentation and regional analysis
Valuable for manufacturers, suppliers, investors, and stakeholders to make informed business decisions
Access to proprietary market share data and Porter’s Five Forces analysis
Identification of emerging opportunities and risk factors in this rapidly evolving market
Frequently Asked Questions
Q1: What is a low voltage battery management system (BMS)? A low voltage BMS is a system that monitors and manages battery packs operating below 60V to ensure safety, efficiency, and longevity, primarily used in electric vehicles, renewable energy storage, and consumer electronics.
Q2: What is driving the growth of the low voltage BMS market? Key drivers include the global shift to electric vehicles, increasing deployment of renewable energy storage systems, technological advancements in smart BMS, and government regulations favoring electrification.
Q3: Which battery types are dominant in the market? Lithium-ion-based battery management systems dominate the market with over 82% share, due to their superior energy density, efficiency, and widespread adoption.
Q4: Which regions are leading in low voltage BMS adoption? Asia Pacific leads the market, supported by rapid industrial growth, large automotive sector investments, and robust renewable energy initiatives.
Q5: What applications benefit most from low voltage BMS? Automotive (EVs), renewable energy storage, consumer electronics, industrial equipment, and IT & telecom sectors are major application areas.
About Transparency Market Research Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information. Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports. Contact: Transparency Market Research Inc. CORPORATE HEADQUARTER DOWNTOWN, 1000 N. West Street, Suite 1200, Wilmington, Delaware 19801 USA Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com Email: [email protected]
0 notes