#electronegativity
Explore tagged Tumblr posts
Text
Polarization of the Carbonyl Group
-- Carbonyl group:
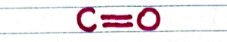
-- The oxygen has two lone pairs
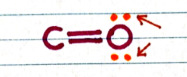
-- The oxygen is more electronegative than the carbon
-- The polarization arrow will show electrons being pulled toward the oxygen

-- The carbon is partially positive
-- Partially positive = δ+
-- The oxygen is partially negative
-- Partially negative = δ-
-- These symbols go by the arrow
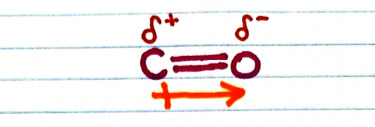
-- The polarization makes the boiling points of aldehydes and ketones higher than hydrocarbons
-- The larger the carbonyl group, the less soluble it is in water
-- If it has more than six carbons, it is insoluble
.
Patreon
#studyblr#notes#my notes#functional groups#polarization#electronegativity#carbonyl group#aldehydes#ketones#polarity#organic chemistry#ochem#orgo#orgo notes#organic chemistry notes#organic chem#orgo chem#study guides#mcat#mcat chemistry#mcat orgo#mcat ochem#mcat organic chemistry#mcat studyblr#premed studyblr#organic chemicals#organic reactions#chemical reactions#advanced chemistry#life science
9 notes
·
View notes
Text
For example, the electron density around the hydrogen atoms in fluoromethane (figure 20.23) is less than that around the hydrogen atoms in chloromethane (figure 20.24), due to the greater electronegativity of fluorine relative to chlorine.


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#electron density#hydrogen#fluoromethane#fluorine#chloromethane#chlorine#methane#electronegativity
2 notes
·
View notes
Text
Okay but it's obviously fluorine, right? Most electronegative element. Fluorine's out there getting slutty, serving cunt. Fluorine's reactive, it fucks.
Tumblr staff: ten options is enough for polls, right? No one needs more than that on a regular basis. The average tumblr user: Hey guys which element of the periodic table do you think is the most fuckable?
#I'm ace so I guess what do I know about fuckability#but y'all see my vison right?#right?#Fluorine#electronegativity
279K notes
·
View notes
Text
Giải mã "ĐỘ ÂM ĐIỆN" - Quyền Lực Ngầm Trong Liên Kết Hóa Học!

Độ âm điện là gì mà "quyền lực" vậy? Nói một cách dễ hình dung, độ âm điện chính là "khả năng hút electron" của một nguyên tử khi nó "bắt cặp" (tức là tạo liên kết hóa học) với một nguyên tử khác. Nguyên tử nào có độ âm điện lớn hơn thì sẽ hút electron trong liên kết về phía mình mạnh hơn.
🌟 Ý nghĩa của độ âm điện:
Giúp chúng ta hiểu rõ hơn về bản chất của các liên kết hóa học (liên kết cộng hóa trị phân cực hay không phân cực, liên kết ion).
Dự đoán được tính chất hóa học của các phân tử.
📜 Quy luật biến đổi "quyền lực" này trong bảng tuần hoàn: Nhớ mấy "bí kíp" này là hiểu ngay nè:
Trong một chu kỳ (đi từ trái sang phải): Độ âm điện có xu hướng TĂNG DẦN. Càng về cuối chu kỳ (phía phi kim), các nguyên tố càng "hút" electron mạnh hơn.
Trong một nhóm (đi từ trên xuống dưới): Độ âm điện lại có xu hướng GIẢM DẦN. Các nguyên tố ở dưới "hiền" hơn, ít tranh giành electron hơn.
Mẹo nhỏ: Các bạn phi kim (như Oxi, Flo, Clo...) thường có độ âm điện cao ngất ngưởng, còn các bạn kim loại (như Natri, Kali...) thì độ âm điện lại khá thấp. Flo (F) chính là "trùm cuối" với độ âm điện cao nhất đó!
Hiểu về độ âm điện giúp chúng ta giải thích được rất nhiều hiện tượng hóa học thú vị xung quanh. Mong là chia sẻ này giúp cả nhà thấy hóa học gần gũi và dễ hiểu hơn nha!
Xem thêm bài viết chi tiết tại: https://vietchem.com.vn/tin-tuc/bang-do-am-dien-cua-cac-nguyen-to-hoa-hoc-co-ban.html
1 note
·
View note
Text
VSEPR and Molecular Geometry Explained!
youtube
Being able to draw Lewis Structures with the correct geometry is the first step in chemistry to being able to understand and explain different properties and behaviors of various molecules. In this video we will cover Valence Shell Electron Pair Repulsion, also known as VSEPR, and how that affects the shapes of different molecules!
Molecular Geometry Chart: Email us and we will send you a FREE copy!
Tadashi Science
https://www.youtube.com/channel/UCXrKyd6XS4oyhjKppE4ZZvw/videos
https://www.youtube.com/channel/UCXrKyd6XS4oyhjKppE4ZZvw/about
#tadashiscience#chemistry#science#physics#physicalscience#tutoring#bonddipoles#atoms#molecules#polarity#cations#anions#electronegativity#Youtube
1 note
·
View note
Text
What is Electron Affinity?
Electron affinity refers to the amount of energy released or absorbed when an electron is added to a neutral atom in the gas phase to form a negative ion. Essentially, it measures the tendency of an atom to gain an electron. This property is critical for understanding how atoms become ions and participate in ionic bonding. Enroll now at Tutoroot.
0 notes
Text
Very important addition to my chemistry notes

63 notes
·
View notes
Text
god i love the idea that bill and the other weirdo monsters couldnt leave gravity falls is because theyre TOO weird yk? like gravity falls attracts weird and theyre so weird that they physically cant leave? love it. eating that shit up
#gravity falls#headcanon#side note:#also reminds me of atomic radius and how how electronegative an atom is affects how close electrons are pulled to the nucleus#can you tell i love chemistry#btw!!! in university!!?? shits crazy man
4 notes
·
View notes
Text

justification for the terse 'false' I wrote on previous img; the above from chatgpt as I did not know precise proportions just that the quantities of anaerobes (from aerotolerant to obligatory, basically either using fermentation to generate atp or having a proper electron transport chain only without an oxygen at the terminus) are significant and not outnumbered by obligate aerobes
#microbiology#I think a lot of the pathogens that cause illness in humans are facultative anaerobes (staph strep e.coli salmonella hib etc)#these aren't necessarily responsible for decomposition of corpses but many are held at bay by the immune system#which obviously ceases its efforts post mortem. one might imagine a sort of broken-dam effect#reading further it looks like aerobic respiration produces more atp than anaerobic and both produce more than fermentation#this seems to make a sort of sense as oxygen is the second most electronegative element#so efficiency of facultative anaerobes may be improved in presence of oxygen which would explain increased bacterial activity in oxygen-ric#oxygen-rich environments.
0 notes
Text
ptable.com save me save me ptable.com
#rare woman in stem post????#lignin nmr is killing me rn#just nmr spectra in general#theyre very useful but GOD#why is it NOBODY CAN TEACH THEM PROPERLY?????#electronegativity table seems to haunt me
0 notes
Text
Hydrogen bonding (Fig. 12.5). A variety of hydrogen-bonding reactions are possible, involving oxygen- and nitrogen-containing functional groups of both the HM and the organic pesticide molecule. In the example shown, the insecticide carbaryl is hydrogen-bonded to HM using the two electronegative atoms, nitrogen and oxygen, in the carbamate structure.

"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#hydrogen#chemical bonding#chemical reactions#oxygen#nitrogen#humic matetial#pesticide#insecticide#carbaryl#electronegativity#carbamate
0 notes
Text
me trying to tell if a molecule is polar or non polar based on vibes alone
#chem class did not make it explicitly clear how to tell the difference without using electronegativity#not allowed periodic tables in the quiz#so i must use the vibe check#my posts
0 notes
Text
════ ⋆★⋆ ════
☆˚₊‧ ɴᴏᴡ ᴘʟᴀʏɪɴɢ ... ╰┈➤ 𝚜𝚘𝚏𝚝𝚎𝚛, 𝚑𝚊𝚛𝚍𝚎𝚛, 𝚒𝚗-𝚋𝚎𝚝𝚠𝚎𝚎𝚗. 𝚙𝚝.𝟹/𝟺 𐙚₊˚
⋆★⋆ kiss me and tell me that you'll stay. ⋆★⋆ Part 1 & Part 2.



♫ ᴘʟᴀʏɪɴɢ: pushing it down and praying by lizzie mcalpine (3:54) // ༉‧₊˚ " he gives what he can, but now i don't know what he's giving for. " ᝰ.ᐟ

✰ pairing: calvin evans x fem!lab tech!reader
✰ cw: (no use of y/n & not proofread) smut, oral (f!recieving), calvin is a munch, revelation of feelings, kissing, exhibitionism if you squint, spit kink if you squint, swearing, some major yearning, angsty at the end, cliff hanger.. sorry
✰ word count: 3.1k+
✰ summary: you and calvin develop feelings for each other over the span of months and get to know each other better, but the entire thing with fran and her friends detests you from getting with him. until calvin takes you from your lab and brings you into his own and feelings among other things are exchanged.
(IMPORTANT: collaborated with @sammygidd with writing process + planning)
════ ⋆★⋆ ════
༺colour chart༻ reader ❀ calvin ⚛︎ fran ✿ dr. donatti ☔︎
The stars glistened outside of the window of the lab the both of you were in, working late once more, added to the tally of the days spent in his own lab. Calvin was sitting at one of the counters, looking over his notes, jotting down corrections every so often. You were sitting, more like lying down on his battered couch that has been victim to too many chemical spills and burns throughout his time at Hastings. His head perked up from his notebook as he heard you softly snoring, the moon emitting a light overtop of your sleeping face.
His eyes scanning over you for a moment too long, taking in your features.. Making little mental notes on how you looked while sleeping. “Wow ..” He quickly caught himself muttering something so simple under his breath, clearing his throat, but something that meant so much — he was catching feelings for you, strong ones. He rested his head on his hand, just watching you sleep. Your hair framing your face, a strand falling overtop one of your eyes. Calvin hesitated before his hand reached out, tucking the loose strand behind your ear, his thumb resting against your cheek for a little too long. He revoked his hand almost immediately as he saw you stir, pretending as if he were writing his notes.
Your eyes slowly flutter open as you wake up, taking in the dimly lit lab around you – lifting your head, and stretching in the process. You yawned softly, looking over your shoulder to see Calvin taking some notes. “What have you written so far?” Speaking softly, a soft rasp to your voice, you smiled. “Uhm--” Calvin looked down at his notes, noticing that there were smudges on the page from when you took his attention. “Just on trends with electronegativity in the periodic table.. But– it’s all smudged." Calvin tsked as he realised that the ink got onto the sleeves of his button up and over the sides of his hands, his hands pushed up his sleeves to his elbows.
In your sleep-induced state, you couldn’t help but stare - your lips parted slightly as your gaze trailed over his arms, like the first time. You then grabbed his notes off the table, trying to deduce his smudged writing and drawings, standing up you walked over to the sink. The uneven tiling caused you to stumble a bit, but then arms circled your waist. “Hey– hey, whoa. Careful.” Calvin helped you stand up straight, you then became very aware of the proximity or lack thereof between the two of you. “I don’t need my science partner falling on me.” A small smirk graced his face as he tucked a strand of hair that was disturbed from your near fall, his touch lingered just for a moment. Then an inch closer, you could feel the heat of his breath - your nerves on fire as his fingers grazed your waist. But something told you to tear away, told you that this was wrong - that you were feeding into the rumours Fran and her friends developed about you and Calvin, you didn’t want to prove them right. “I should go home– it’s late.” You nodded to yourself, avoiding his eye contact as you stepped away. Calvin’s hands slipped from your waist as you stepped away, grabbing your things. “Wait– I.. I’m sorry.” Calvin started up,
“No, Calvin. You’re fine.” You grabbed your necessities from your desk, shoving them haphazardly into your bag. “I didn’t mean for it to be like that—” “I’m not saying you did.” You finally met his gaze, his blue eyes boring into your own - full of confusion, sadness and something you couldn’t quite note. Calvin grew silent at your remark, leaning back against the sink. “Goodnight, Dr. Evans.”
Calvin was going to respond when he heard you call him by his title, reminding him of the separation between the two of your roles in the lab - a reversal of everything the two of you have built in a way too, all because he got a little lovestruck. “Goodnight.” Calvin looked to the ground, not looking up till you left - not wanting to cement the image of you leaving him all alone in his mind. As he heard the click of your heels retreating and the familiar close of his lab door, it felt quiet. The quiet that he typically enjoyed, the solitude and the isolation from everyone else. But now it just felt empty, saddening.
You two never talked about that late night in the lab, never talked about the light touches, the closeness, the intimacy - you both preferred not to have that conversation. Regardless, over the span of a couple more weeks, you and Calvin continued to grow closer learning the little things, like how he hated the rain but loved water like lakes and rivers, how he got into chemistry in the first place. Calvin showed you a side you didn’t know existed in people, tranquility, kindness, acceptance. He listened to you and seemed The scientists and secretaries gossiped about it, continuously. Earning you weird looks in the hallway whenever you were beside him, or walking to his lab. But you didn’t mind it, at least you thought you didn’t.
You were in your designated lab, keeping to yourself - looking over some notes Calvin gave you a couple hours ago. Speak of the Devil, Calvin just walked in, looking over the place quickly, practically radiating nervous energy until his gaze landed on you. Without a single word, Calvin walked over to you, a hand placed to your lower back as he directed you out of the room. “Calvin? What’s going on?” He didn’t say a thing, walking you into his lab a couple doors down. You were looking around, confused. “If this is about the other night, I really don't–” He then pulled you into a kiss, you immediately pulled back. “Calvin, I–” You placed a hand to your lips, looking up at him. You detested these thoughts for so long, but now it seems like nothing could be anymore right. You looked at him, just for a beat, before pulling him into another kiss. This one more heated, his hands moved to your hips - his touch tentative at first, fingers lightly grazing your hip bone. “Tell me that you want this– please.” Calvin mumbled against your lips, the words spilling out before he could process them. “I want this, Calvin.” “I know– I know that this isn’t professional..” “I don’t care about being professional right now, not with you.” Then something shifted in him, you felt it in the air - like your words just broke all of the restraints. His arms wrapped around your waist, moving to sit you on a clearing on one of the lab tables.
“Don’t touch or break anything.” His tone authoritative, he looked over you - as if, if he didn’t keep you in his sights you’d disappear. Silence fell over the two of you, the sound of heaving breaths filling the space - you tentatively bit your lip looking up at him.
Calvin’s tongue flicked over his lips, staring you down as he raised a hand to pull your kissed bottom lip from your teeth - dragging it down until his thumb let go of it. “I want to go down on you.” His blatant request took you by surprise, but the way he was looking at you like an experiment made your stomach flip.
“I– I want that too, but you dont need–” You cut yourself off as he moved to kneel on his knees in front of you. He was eager, and he wanted this too, almost more than you. You watched as he rolled up his sleeves, unbuttoning the cuffs - revealing the toned arms you’ve grown to love so much. He looked up at you, his eyes boring into yours as he opened a couple of the top buttons on his shirt. “If you make a mess you clean it.” Calvin looked dead serious as he moved closer to you - hands pushing up your skirt. His fingers gently tracing the patterns of the tights you wore, he muttered a compliment under his breath you couldn’t hear over the beating of your own heart. He made quick work of the buckles on your shoes placing them down neatly beside him, his gaze returning to the tights.
“As much as I love these, I’d much rather have them off." “Mhm–” “Lift your hips.” You complied, lifting your hips off the counter a bit as he started to take off the stretchy fabric. Your discarded tights joined your shoes beside him.
Calvin’s hands then moved over your legs, feeling the soft skin beneath his palms without the covering cloth, his hands were big against your legs, the veins defined as his hands gripped onto the underneath of your thighs - pulling you closer to the edge of the counter.
Calvin then bunched your skirt to your hips, placing a kiss to the top of your knee, then one closer to the inside of your leg, then your thigh - placing a soft bite to the inside of the smooth skin, easing it over with an apology kiss.
The sensation was good on its own, but it was the way his eyes were watching your face the entire time, watching your reactions and what felt good. His large hands returned to your legs, parting your thighs so he could nestle himself further between them. His pointer finger trailed down the edge of your panties, taking note of the way the lace felt under his touch and how your breath hitched in response.
Your hand moved down to cup the side of his face, he moved his head to kiss your palm - his kisses trailing up to the tip of your fingers, taking two of them into his mouth. As the warm heat of his mouth enveloped your fingers, your jaw dropped a bit - lips parted as you watched him suckle them. His eyes hooded and staring into yours made a soft whimper come out of you.
Calvin retracted, your fingers leaving his mouth - he placed a soft kiss to the wet digits before resuming his previous state. He hooked your legs over his shoulders, moving impossibly closer to your core. He placed a kiss to your inner thigh next to your soaked cunt, then another over your clothed slit - earning yet another moan from you.
Long fingers moved aside your laced panties, leaning back onto his calves as he admired the state of you. Kissed lips, the blush creeping up your neck, the way your pussy fluttered around nothing as he examined you like he was about to take you apart, which in a way he was.
Calvin then dove in, not wanting to waste another moment. He licked a stripe all the way from your entrance to your clit, eyes trained on yours the entire time. He took your clit into your mouth, noting how your hands scrambled into his hair - encouraging him further. He rolled the swollen nub between his teeth and tongue, a bit of spit and mixture of your arousal dripping down the side of his mouth.
His mouth then moved down to your entrance, lapping at your arousal like a dog - tongue poking into your heat a bit. Calvin’s nose bumped against your clit, earning a moan from you - your head fell back, colliding with a couple beakers, making them clatter against each other from the force.
Calvin’s voice was muffled against your pussy but you could still hear him and feel the vibrations, “What did I say?” “S-Sorry..” You placed a hand over your mouth being reminded that you were in a public space, and as Calvin continued his rhythm without faltering, your hands tightened in his neatly combed hair but he didn’t care, not in the slightest.
Calvin continued to lap at your cunt, tongue picking up any arousal that poured out of you - his mouth then placed a couple kisses to your inner thigh, moving back onto his calves again. Then you noticed the sheer amount of slick and spit that coated his mouth, chin and tip of his nose, a small whimper extracted from you. His tongue darted out to wet his lips, tasting you on him - earning a small groan from him.
His fingers moved to your pussy, the coldness of them a stark contrast to your aching, hot core. Calvin’s middle and pointer fingers pulled back your folds, revealing you completely to him - watching as your core clenched around nothing. “Don’t worry, sweet girl. I’ll let you cum.” He moved back in, his pace increasing tenfold, eager to make you cum on his tongue. Your hands weaved themselves back into his hair, tugging at the roots - making him groan softly against you. His hands moved to the undersides of your thighs, moving them further apart.
You felt that coil tighten in your stomach, “Calvin– I- I’m gonna..” But Calvin didn't say a word, his eyes trained on your face eager for your reaction as he moved his tongue faster against your weeping cunt.
Your heels dug into his back as your orgasm overcame you, your moans bouncing off the walls. You didn’t care if people heard you at this point. Calvin held you through it - not letting up with his tongue until you were pushing at his shoulders from overstimulation.
Calvin moved back, the only sound in the room being both of your heaving breaths, Calvin wiped your slick from his face - gently pulling your panties back overtop your core, placing a kiss to your thigh before pulling your skirt back down your body. He eventually rose from his spot, running a hand through his tugged tugged hair.
Calvin then let out a frustrated sigh as he looked down at himself. “Now my pants are all scuffed--"Calvin mumbled, wiping off some of the dust from the floor that made its way onto the knees of his pants. You were watching him the entire time, breath still heaving, then you grew bored. You placed a hand to the side of Calvin’s face, tilting his head back to yours. Your other hand moved to his arm, pulling him into another heated kiss. You tasted yourself on his lips but you didn’t care, and he didn’t seem to care about his pants all that much anymore.
– – – – – – – –
The next day, you came into work earlier than you usually did and took more time that morning doing your hair and makeup, not really thinking that Calvin would notice that much, but he was observant and it doesn’t hurt to feel prettier than usual for a day. You walked into your lab first, setting down your things - noticing how the scientists just stared at you, sharing laughs and whispers. “More dolled up than you usually are Lab Tech, any reason why?” “Any scientists you’re trying to impress?” “Anyone need supplies from Evans’ office?”
And you just stood there, staring at them all - and you wondered if Calvin told them about what you did, but Calvin wouldn’t do that. He wasn’t the type and he openly says that he doesn’t talk to these idiots anyways, but you still worry and you still spiral. Maybe someone heard something? Or maybe people just started talking again because both you and Calvin walked out of this very lab you were in, in a hurry. Or maybe people heard you, that would be the most obvious one.
You needed to find Calvin, and fast. Ask him to explain everything and tell you that it was all a misunderstanding and it was all under control, give you some peace of mind. You walked out of the lab, ignoring the hoots and whistles as you did. You beelined for Calvin’s lab, but as you were walking down the hallway - you saw Calvin walking down the same one from the opposite way, then he caught sight of you, stepping back for a moment before returning the same way he came from. You were lost and confused, was he ignoring you? Was this all a ploy to get you fired? What was he doing? You had so many questions yet no answers, you then felt a hand on your shoulder - you quickly turned around, shrugging it off. It was Fran, her voice quiet and almost mocking as you saw the slight smirk on her face and the enjoyment radiating off of her, “Dr. Donatti needs to see you right now, hun. He says it’s important.” “Yeah… I’ll be there in a minute, I just need to-” “He said now, not in a minute.”
You let you a sigh, walking down the hallway to Dr. Donatti’s office. People stared at you and whispered, and you just had to stay there and take it.
“We’ve heard things of a relationship that is evolving between you and Dr. Calvin, a romantic relationship between a lab tech and a chemist is against our policy and promotes an uncomfortable workspace.” You shifted in your seat a bit, “We’re not together, Dr. Donatti. I can promise you that, it’s just rumours.” It was true for the most part, you and Calvin never actually confirmed anything, just had a hookup, whether that hookup was meaningless or not was still up in the air.
“Even if they’re just rumours, we cannot risk our reputation here. Fran will help you pack your things, I’ll give you a few days to say your goodbyes.” “What?-- n-no, you can’t do that. I’ve worked so hard for this job, for you.” “You’re just a lab tech, you can be easily replaced, sweetheart.” That pet name made your blood boil, you stood up. “Is Calvin getting fired?” “No, he’s a chemist.” “If you’re firing me for committing what you propose, you should fire him too, he committed to it as much as I did.” “So you admit, there is something going on between you and Dr. Evans?” “That’s– that’s besides the point, you’re being unfair. You’re being subjective and sexist.”
Donatti also stood up, “What gives you the authority to speak to me with such a tone, proclaiming I’m sexist for wanting to protect Hasting’s reputation.” “And you’re proclaiming that rumours about me that for the majority aren’t true are justifiable enough to fire me.”
Dr. Donatti hesitated, “Y..You–” He fell silent for a moment, “I’ll talk to the board about.. reconsidering your termination.” You can tell he was apprehensive saying that. You didn’t say anything in return, you didn’t say thank you - grabbing your things and leaving his office. You were going to find Calvin and you were going to speak to him, if it was the last thing you did at this job. Stalking towards his lab, your eyes stung and it felt like the world was staring at you, you made your way to the familiar door, hand grabbing the handle.
════ ⋆★⋆ ════
taglist; @gryffindorquid-ditchcap-blog, @hushhhs09 @justsomerandompersonintheworld, @yagurlannastasia, @cultish-corner, @lynn7nunez, @emma8895eb, @the-lynnie-the-pooh @itzbowiepullman
#spaceycat#x reader#smut#lessons in chemistry#lessons in chemistry smut#lessons in chemistry x reader#lessons in chemistry calvin#lessons in chemistry calvin evans#calvin evans lessons in chemistry#calvin lessons in chemistry#calvin evans smut#calvin evans x reader#calvin evans angst#lessons in chemistry angst#calvin evans#calvin evans imagine#calvin evans fanfic#lessons in chemistry tv show#lewis pullman#lewis pullman characters
223 notes
·
View notes
Text
What is the Understanding the Concept of Electronegativity?
Electronegativity is a fundamental concept in chemistry that refers to the ability of an atom to attract shared electrons to a chemical bond. Electronegativity is crucial in predicting the behavior of different elements and compounds. In this blog post, we will delve into the definition of electronegativity, explore its periodic trends, provide examples, and explain how to calculate it effectively along with the Electronegativity table.
Understanding the Concept of Electronegativity
Now let’s delve into the concept of Electronegativity, and the definition of Electronegativity.
What is Electronegativity?
Electronegativity refers to the ability of an atom to attract shared electrons in a chemical bond. This property is not an intrinsic characteristic of an atom but rather depends on the atom’s environment in a molecule. The concept was first introduced by Linus Pauling in 1932, who developed a scale to quantify electronegativity values. The Pauling scale is the most commonly used today, with values ranging from 0.7 for francium to 4.0 for fluorine.
Electronegativity is pivotal in determining the nature of chemical bonds. When two atoms with different electronegativities form a bond, the shared electrons are more attracted to the atom with higher electronegativity. This creates a polar bond with a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom. If the difference in electronegativity is significant, the bond can become ionic, with electrons being transferred entirely from one atom to another.
Electronegativity Definition
Electronegativity can be defined as the measure of an atom’s tendency to attract and bind with electrons. It is a dimensionless quantity, typically measured on the Pauling scale, and reflects the atom’s ability to attract electrons in a chemical bond. Higher electronegativity indicates a stronger ability to attract electrons.
Concept of Electronegativity Table of Elements
The periodic table offers valuable insights into the electronegativity of each element. The trend of electronegativity in the periodic table showcases intriguing patterns that help us understand the behavior of different elements in chemical reactions.
Comprehension of Electronegativity Chart
Electronegativity charts visually represent the electronegativity values of elements. These charts typically use a color gradient or numerical scale to highlight differences in electronegativity across the periodic table. By examining an electronegativity chart, students can quickly identify trends and compare the relative electronegativity of different elements.
For example, fluorine, with the highest electronegativity value of 4.0, is at the top right of the periodic table. This high value indicates fluorine’s strong ability to attract electrons. On the other hand, elements like francium and cesium, located at the bottom left, have much lower electronegativity values, reflecting their weaker attraction for electrons.
Understanding these trends is crucial for predicting chemical behavior. Electronegativity differences between bonding atoms can explain why certain reactions occur and how molecules are structured. For instance, the significant electronegativity difference between sodium (Na) and chlorine (Cl) leads to the formation of an ionic bond in sodium chloride (NaCl), while the smaller difference between hydrogen (H) and chlorine (Cl) results in a polar covalent bond in hydrogen chloride (HCl).
Electronegativity Trend in the Periodic Table
Electronegativity trends in the periodic table follow a predictable pattern. Generally, electronegativity increases across a period (from left to right) and decreases down a group (from top to bottom). This trend is due to atomic structure and the effective nuclear charge experienced by valence electrons.
Across a period, the number of protons in the nucleus increases, leading to a stronger attraction for bonding electrons. This results in higher electronegativity values. For instance, moving from lithium (Li) to fluorine (F) in the second period, electronegativity increases steadily.
Down a group, the increase in atomic radius reduces the effective nuclear charge experienced by the outer electrons. Consequently, these electrons are less strongly attracted to the nucleus, resulting in lower electronegativity. For example, in group 17 (halogens), electronegativity decreases from fluorine (F) to iodine (I).
Understanding these trends helps predict the nature of chemical bonds. For instance, elements with similar electronegativity values are likely to form covalent bonds, while those with significant differences tend to form ionic bonds. These trends also explain reactivity patterns, such as why fluorine is highly reactive due to its high electronegativity.
What is the Electronegativity Series?
The electronegativity series is a list of elements arranged based on their electronegativity values. This series provides a helpful guide for predicting the behavior of components in different chemical reactions and bond formations.
Electronegativity is a critical concept that influences the interactions between atoms in chemical compounds. By understanding electronegativity, scientists and researchers can predict the outcomes of chemical reactions and design new molecules with specific properties. Similarly learn complex concepts like Electronegativity with the help of Tutoroot Chemistry Online Tuition. Click here to book a FREE DEMO session from expert faculty.
0 notes
Text
The periodic table shown in figure 5.5 presents commonly used values, developed by the US scientist Linus Pauling (figure 5.6), based on bond energies.


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#periodic table#electronegativity#pauling scale#american#scientist#linus pauling
1 note
·
View note
Note
what actually is fire? like physically? i know it's from a combustion reaction, but that doesn't explain what it physically is. my guess would be rapid vibration of molecules, or the breaking of chemical bonds?
Good question!
Fire is indeed a chemical reaction (the breaking of chemical bonds), often called a combustion reaction, that generates enough heat (the vibration of atoms and molecules) to sustain itself, so long as it has fuel. Most of the time, combustion reactions require an oxidant as well. (Oxidants are a class of chemical that are very accepting of electrons and electron bonds…so-called because oxygen is the most common here on Earth. We’ll get to how they work in a sec.)
Let’s take wood as a basic example.
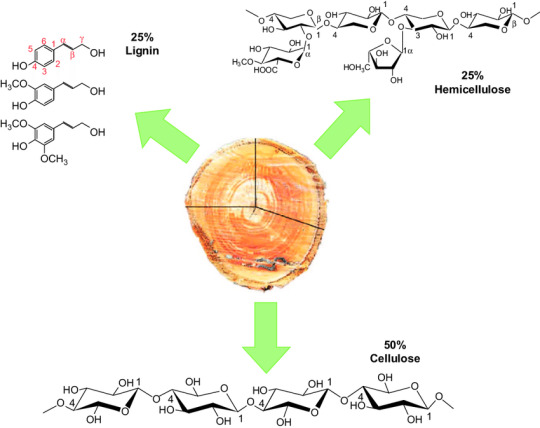
As you can see, wood is primarily composed of chains of carbon and hydrogen (called hydrocarbons)
(Biochemists are weird and don’t always label the carbons, just because they’re so common in biology—basically any “unlabeled corner” in the above diagrams represents a carbon.)
When a particular section of the wood is exposed to a point of high heat and oxygen, the agitation of all that heat energy will allow the oxygen (already very electronegative, or accepting of chemical bonds) to literally tear the hydrogen and the carbon away from each other in order to bond with them instead (like a jealous dance partner), creating water vapor and carbon dioxide, respectively.
But, when hydrocarbons are torn apart, they release some of the energy contained in the electrons that form their bonds, thus creating heat. This heat happens to be enough to cause nearby sections of the wood to also undergo combustion, and the process thus can spread through a matchstick, campfire, or forest.
A similar process occurs, albeit at differing rates, in pretty much every combustion reaction. (The gas in your car, for instance, combusts much faster.) All you need is a fuel that contains energy in its bonds in a format that a nearby oxidant can release, and a spark to get the party started.
#chemistry#you might note that this reaction seems similar to the energy reaction used to create ATP in the body: if so—good eye!#oxidation is a supremely useful tool in chemistry for the release of energy. in the body the source of that energy is glucose#and oxygen serves as a catalyst and place for the excess electrons to go. but the purpose of the reaction is ultimately to create ATP#but it’s interesting that we’re all technically powered by a very slow combustion reaction of the food that we eat!
75 notes
·
View notes