#hypervitaminosis A syndrome
Text
Hypervitaminosis A: The Most Of Its Causes And Symptoms
Hypervitaminosis A: The Most Of Its Causes And Symptoms
Commonly, vitamins and minerals are essential and necessary for health, but over-consuming them may cause side effects, especially when we over-consume vitamin A, (Hypervitaminosis A).
What is hypervitaminosis A?
Hypervitaminosis A occurs when large amounts of vitamin A enter the body exceeding its need. This condition is temporary because of consuming large amounts of vitamin A in a short…

View On WordPress
#foods with vitamin A#hypervitaminosis A#hypervitaminosis A liver#hypervitaminosis A symptoms#hypervitaminosis A syndrome#hypervitaminosis A treatment#is vitamin A water soluble#sources of vitamin A#vitamin A#vitamin A benefits#vitamin A for eyes#vitamin A for hair#vitamin A function#vitamin A good for#vitamin A high foods#vitamin A in carrots#vitamin A in foods#vitamin A is good for#vitamin A on skin#vitamin A rich foods#vitamin A side effects#vitamin A side effects too much#vitamin A toxicity
0 notes
Text
CELLULAR INJURY
Types of calcification
Calcium deposits appear deeply basophilic (arrow in A ) on H&E stain.

Dystrophic calcification
Ca2+ Deposition: In abnormal (Diseased) tissues.
Extent: Tends to be localized (eg, calcific aortic stenosis).
Associated Conditions: TB (lung and pericardium) and other granulomatous infections, liquefactive necrosis of chronic abscesses, fat necrosis, infarcts, thrombi, schistosomiasis, congenital CMV, toxoplasmosis, rubella, psammoma bodies, CREST syndrome, atherosclerotic plaques can become calcified.
Etiology: 2° to injury or necrosis.
Serum Ca2+ levels: Normal.
Metastatic calcification
Ca2+ Deposition: In normal tissues.
Extent: Widespread (ie, diffuse, metastatic).
Associated Conditions: Predominantly in interstitial tissues of kidney, lung, and gastric mucosa (these tissues lose acid quickly; ↑ pH favors Ca2+ deposition).
Nephrocalcinosis of collecting ducts may lead to nephrogenic diabetes insipidus and renal failure.
Etiology: 2° to hypercalcemia (eg, 1° hyperparathyroidism, sarcoidosis, hypervitaminosis D) or high calcium-phosphate product levels (eg, chronic kidney disease with 2° hyperparathyroidism, long-term dialysis, calciphylaxis, multiple myeloma).
Serum Ca2+ levels: Usually abnormal.
2 notes
·
View notes
Text
So many dumb ways to die
I’ve seen so many humans are weird posts where humans are seen as the invincible warrior species, but one thing that I’ve yet to see mentioned is how ridiculously easy it is for humans to die in some cases. For that reason, I will list a few here.
Humans can die by ...
eating a larger meal after a long fast (refeeding syndrome)
their own immune system overreacting to a peanut (allergies)
accidentally inhaling a small amount of water that causes vocal chords to spasm and close up... hours after exiting the pool (dry drowning)
Speaking of drowning, people can also drown in inches deep water if the water is cold and they fall face first
consuming too much of certain nutrients (hypervitaminosis)
food accidentally going down the wrong pipe (choking)
(what is mentioned above can also happen with a human’s own vomit)
blood getting into the wrong parts of the body, especially the brain (aneurysm)
Feel free to add to this list
#HUMANS ARE WEIRD#humans are space oddities#humans are space orcs#humans are space australians#space oddity#space australia#space orcs
5K notes
·
View notes
Text
Vitamin D, Ergocalciferol


Brand Name: Drisdol
Generic Available
Common Dosage Forms:
Capsules: Each softgel contains Ergocalciferol, USP 1.25 mg (equivalent to 50,000 IU of vitamin D), in vegetable oil.
Drops: 200 IU of ergocalciferol/drop (8,000 IU/mL)
*Also available in lower strength OTC forms
FDA Indications/Dosages:
For the treatment of refractory rickets, also known as vitamin D resistant rickets: 12,000-500,000 IU/day
For the treatment of hypoparathyroidism: 50,000-200,000 IU/day concomitantly with calcium lactate 4 grams, six times a day.
Non-FDA Indications/Dosages:
For the treatment of vitamin D deficiency in adults (25-hydroxyvitamin D3 <20 ng/mL): 50,000 IU weekly for 8 weeks given with supplemental calcium followed by 1,000 IU daily with supplemental calcium.
Pharmacology/Pharmacokinetics:
Ultraviolet light converts 7-dehydrocholesterol in the skin to cholecalciferol (vitamin D3). Both cholecalciferol and ergocalciferol (vitamin D2) are converted in the liver to 25-hydroxyvitamin D forms and then in the kidney to 1,25-dihydroxyvitamin D forms. Vitamin D (D2 or D3) is essential for the proper regulation of calcium in plasma and the activity of parathyroid hormone. Vitamin D is involved in the absorption of calcium and phosphorous from the small intestine thereby providing the necessary levels of calcium and phosphate in the plasma to promote bone mineralization. Ergocalciferol is effective in treating the hypocalcemia and hyperphosphatemia seen in hypoparathyroidism.
Drug Interactions:
Mineral oil interferes with the absorption of fat-soluble vitamins, including vitamin D. Addition of thiazide diuretics to hypoparathyroid patients concurrently taking ergocalciferol may cause hypercalcemia.
Contraindications/Precautions:
Contraindicated in patients with hypercalcemia, malabsorption syndrome, and hypervitaminosis D. Idiopathic hypercalcemia may be caused by hypersensitivity to vitamin D. In these cases, vitamin D must be strictly restricted. The range between therapeutic and toxic doses is narrow. Dosage levels must be individualized to prevent toxic effects. Frequent blood calcium levels should be taken. Adequate dietary calcium is necessary for clinical response to vitamin D therapy.
Adverse Effects:
The range between therapeutic and toxic doses is narrow. Toxicity may result in impairment of renal function, mental retardation, bone demineralization, calcification of the soft tissues, nausea, anorexia, constipation, anemia, and weight loss.
Patient Consultation:
May be taken without regard to meals.
Closely follow prescribed calcium supplementation.
Do not exceed prescribed dosage.
Contact a physician if the above side effects are severe or persistent.
Store in a cool, dry place away from light and children.
If a dose is missed, skip it and return to dosing schedule.
0 notes
Text
Biomed Grid | Vitamin A and its Derivatives- Retinoic Acid and Retinoid Pharmacology
Introduction
In vivo, the fat soluble Vitamin A (retinol) can be reversibly metabolised to the aldehyde (retinal) which can in turn, be further oxidised in a non-reversible manner to retinoic acid (RA). Enzymes that oxidize retinol to retinaldehyde belong to two classes: the cytosolic alcohol dehydrogenases (ADHs) belonging to the mediumchain dehydrogenases/ reductase family; and microsomal shortchain dehydrogenases/reductases (retinol dehydrogenases, RDHs [1]. The next step in RA synthesis is the oxidation of retinaldehyde to RA, which is carried out by three retinaldehyde dehydrogenases (RALDHs): RALDH1, RALDH4 and RALDH3 [1,2]. The orange pigment of carrots (beta-carotene) can be represented as two connected retinyl groups, which are used in the body to contribute to vitamin A levels [3]. The physiological and biological actions of this class of substances centre on vision, embryonic development and production, cellular growth and differentiation, skin health, and maintenance of immune function.
Initial studies had focused on vitamin A deficiency and its major consequences: night blindness and Xerophtalmia. Fridericia and Holm [4] investigated the influence of dietary A in the rhodopsin of the retina. Clearly, the rats lacking the fat-soluble vitamin A had a defect in the function of visual purple. Yudkin [5] achieved one of the earliest identifications of vitamin A as a component of the retina. Subsequently, Wald [6] determined the amount of vitamin A present in pig retinas. Wald G [7,8] was well established the visual cycle: light decomposed rhodopsin to retinal and opsin. Retinal could either recombine with opsin to reform rhodopsin or it converted to free retinol. Retinol could reform rhodopsin, but only in the presence of the RPE(Kuhne). The further structure and metabolism of retinoids implicated that retinaldehyde was the visual pigment.
Biochemistry of Vitamin A
There is now a well-developed medicinal chemistry of RA (Figure 1 & 3) [9]. The group of pharmacologically used retinoids include vitamin A (all-trans retinol), tretinoin (all-trans retinoic acid), isotretinoin (13-cis retinoic acid) and alitretinoin (9-cis retinoic acid). The monoaromatic retinoids include acitretin and etretinate. The third generation polyaromatic retinoids include bexarotene and tazarotene. In view of this broad spectrum of pharmacological activity, these substances provide useful to treat multifactorial dermatological disorders and other hematological disorders such as acute promyelocytic leukemias (APL) (Figure 1 & Figure 2) [8,10-12].
Figure 1
Figure 1 & 2: Discovery of the molecular basis of vitamin A derivative retinoic acid action (Figure data adapted from Zhu G,January1991,2013,at the top);and vitamin A in vision cycle (Figure data adapted from Wald G,1935;Wolf G,2001,at the bottom).
Function of Vitamin A and its Physiological Role Vision
Vitamin A is needed by the eye retina,11-cis-retinal (a derivative of vitamin A) is bound to the protein “opsin” to form rhodopsin (visual purple) in rods cells [8], the molecule necessary for both low light (scotopic vision). As light enters the eye, the 11-cis-retinal is isomerized to all-trans retinal in photoreceptor cells of the retina. This isomerization induces a nervous signal (a type of G regulatory protein) along the optic nerve to the visual center of the brain. After separating from opsin, the all-transretinal is recycled and converted back to the 11-cis-retinal form via a series of enzymatic reactions. The all-trans- retinal dissociates from opsin in a series of steps called photo-bleaching. The final stage is conversion of 11-cis-retinal rebind to opsin to reform rhodopsin in the retina [6-8] (Figure 2) vision cycle. Kuhne showed that rhodopsin in the retina is only regenerated when the retina is attached to retinal pimented epithelium (RPE) [8]. As the retinal component of rhodopsin is derived from vitamin A, a deficiency of vitamin A inhibit the reformation of rhodopsin and lead to night blindness. Within this cycle, all-trans retinal is reduced to all-trans retinol in photoreceptors via RDH8 and possible RDH12 in rods and transported to RPE. In the RPE, all-trans retinol is converted to 11- cis retinol, then 11-cis retinol is oxidized to 11-cis-retinal via RDH5 with possible RDH11 and RDH11 [1]. This represent each RDH for the roles in the visual cycle (Figure 2) .
Figure 3: A well developed medical chemistry of retinoic acid (RA). The all-trans and 13-cis forms of retinoic acid,two isomers of RA,are equally effective inhibiting proliferation. Retinyl acetate,and retinal(Vitamin A) are less potent inhibitor. Am80(Tamibarotene) is more potent inhibitor. The chemical structures of more potent analogues involved from labile flexible polyene structures to aromatic stable moieties are shown[9,10].
Embryonic Development
More recent, vitamin A and its metabolites play a key importance in embryo morphogenesis, development and differentiation in normal tissues. Retinoic acid (RA) is lipophilic molecule that act as ligand for nuclear RA receptors (RARs), converting them from transcriptional repressor to activators [2,11,12] in RA signaling pathway. It has been demonstrated that retinoic acid was identified as a morphogen(teratogen) responsible for the determination of the orientation of the limb outgrowth in chicken [13,14] and its retinoic acid receptors (RARs) appear at early stage of human embryonic development in certain types of tissues [15]. Vitamin A play a role in the differentiation of this cerebral nerve system in Xenopus laevi. The other molecules that interact with RA are FGF-8, Cdx and Hox genes, all participating in the development of various structures within fetus. For instance, this molecule plays an important role in hindbrain development. Both too little or too much vitamin A results in the embryo:defect in the central nervous system, various abnormalities in head and neck, the heart, the limb, and the urogenital system [15]. With an accumulation of these malformations, an individual can be diagnosed with DeGeorge syndrome [2].
Dermatology
Vitamin A, in the retinoic acid form, plays an important role in maintaining normal skin health through differentiating keratinocytes (immature skin cells) into immature epidermal cells. In earlier studies, Frazier and Hu (1931) [16] made the observation that both hypovitaminosis A and hypervitaminosis A provokes epithelial alterations together with decreased keratinization and hair loss. At present,13-cis retinoic acid (Isotretinoin) in clinical used to acne treatment. The mechanism was shown to reducing secretion of the sebaceous glands, triggering NGAL (neutrophil gelatinase-associated lipocalin) and other gene expression and selectively inducing apoptosis [17]. But precise action of retinoid therapeutic agents in dermatological diseases are being researched.
Hematopoiesis
vitamin A is important for the regulation of hematopoietic stem cell dormancy [18]. Mice maintained on a vitamin A-free diet loss HSCs (hematopoietic stem cells), showing a disrupted re-entry into dormancy after exposure to inflammatory stress stimuli. This condition highlight the impact of dietary vitamin A on the regulation of cell-cycle mediated stem cell plasticity [19]. In vitro, all-trans retinoic acid (ATRA) stimulates at least two-fold the clonal growth of normal human CFU-GM and early erythroid precursor BFU-E [20]. Cis-RA stimulates clonal growth of some myeloid leukemia cells. In suspension culture, there was an increase in cell number at day 5 in the presence of RA in half of 31 samples, which suggest that RA may play a role in the proliferation and survival of certain leukemia clones in vitro [21,22].
In contrast to the enhancement of normal hematopoietic proliferation, RA (10-6 - 10-9 mol/l) is capable of inducing differentiation of the F9 mouse teratocarcinoma, HL-60 cells [23,24] and some blasts from patients with promyelocytic leukemia [23]. Maximum HL-60 differentiation (90% of cells) occurs after a 6 day exposure to 10-6mol/l retinoic acid. Further in vitro studies found that retinoic acid induced differentiation of leukemic blast cells in only 2 of 21 patients with AML, both of these patients had promyelocytic variant [24]. These data suggest that retinoids may induce maturation of promyelocytes. Retinoic acid also inhibits the proliferation of other dermatological malignant cells (Myger,1975; Peck,1975).
Maintenance of Immune Homeostasis
There is a link between retinoid and immune homeostasis. RA is crucial for maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. de Mendonca Oliveira LM and colleagues [25] have in detail illustrated the impact of retinoic acid on immune cells and inflammatory diseases. After the absorption and metabolism of vitamin A and its precursor(β-carotene) into RA by alcohol dehydrogenase(ADH) and retinal dehydrogenase(RALDH) in CD103+ DC cells in gut, RA plays an important roles in mucosal immune response by promoting differentiation of Foxp3+ inducible regulatory T (Treg) cell and immunoglobulin(Ig) A production. In this process, RA promote dendritic cells to express CD103 and to produce RA. Vitamin A and zinc deficiency (VAD) lead to a decrease of serum IgA. Oral administration of RA in VAD mice can efficiently be reestablishing IgA production. These effects are mediated by an increase of the early B cell factor 1(EBF1) and paired box protein-5(pan-5) transcription factors, which are critical for B cell development. RA accelerates the maturation of human B cells and their differentiation into antibody-secreting plasma cells.
In addition, RA induces the homing of innate immune cells, such as innate lymphoid cells (ILCs) besides regulatory and effector T and B cells, to the gut. Among three ILCs, ILC3 depend on the transcription factor retinoic acid receptor-related orphan nuclear receptor gamma (RORrt) and secrete IL-17 and IL-22. During infections, RA can induce the production of proinflammatory cytokines by dendritic cells (DCs), promoting the generation of effector T cells and restoring the balance of Th17/Treg cells in the GALT (gut-associated lymphoid tissue), and the protection of the mucosa. Moreover, vitamin A is capable of inducing the IL-6- driven induction of proinflammatory T(H) 17 cells, promoting antiinflammatory T reg cells differentiation, regulating the balance between pro- and anti-inflammatory immunity [26,27].
Retinoid Acids in MDS Treatment
The geometric isomer of the naturally occurring retinoic acid is 13-cis retinoic acid (13-CRA). Based on in vitro and in vivo antineoplastic activity, this agent has entered clinical trials for a variety of neoplasms including MDS. Retinoic acid is one of the biological inducers of differentiation that has been preliminarily tested in patients with preleukemia. Myelodysplastic syndrome (MDS) are a group of hematopoietic disorders characterized by ui- or multilineage maturation defects of the bone marrow [28]. Differentiation induction therapy is used in MDS to improve this maturation defects and induce a multilineage clinical response in a subgroup of MDS patients.13-CRA may have moderate effect on 20-30% of patients with MDS [29]. A various of combination therapy with 13-cis RA and growth factors G-CSF or erythropoietin (EPO) improve impaired cytokine secretion (IL-1beta, IL-6, IL-8) from monocytes [30]. In a prospective multicenter study, EPO-beta- ATRA [31] or EPO-13-cis RA [32] combination appears to erythroid response reaching about 36%-60% of therapeutic efficacy in anemia of low/intermediate risk MDS(LDMDS) (marrow blasts < 10% or excluding RARBt). More data analysis, erythroid response maintained an independent positive impact on survival, particularly in non-RARE patients in the first 3 years from diagnosis (90% survival in EPO responders compared to 50% of non-responders) [33]. Zhu [34] successfully conducted a CR patient with refractory anemia with multilineage megaloblastic dysplasia following traditional medicine and erythropoiesis-stimulating agent vitamin B12 and folate growth factor. His peripheral parameters presented pancytopenia (hemoglobin 59g/l, red blood cell count 1.9x1012/l, leukocyte count 2.6x109/l, platelet value 11.8x109/l).
He remained well over 10 years. While another MDS had its unequivocal evidence of disease progression in response to phytohemagglutinin (PHA), inducing the generation of interleukin-2, accelerating the number recovery of CFU-S and initiating DNA synthesis of cells. She had 2.5% blast plus promyelocytes in ~70% cellular marrow before beginning PHA, and 20.7% blast plus promyelocytes in a 90% cellular marrow after ten days (total dosage 250mg) of PHA. Venditt etal [35] conduct that 23 patients with high-risk myelodysplastic syndrome (HRMDS) were treated with a 10 days course of oral ATRA (45mg/m2) and subcutaneous low-dose cytosine arabinoside (LDARAc) given at the dose of 20mg twice a day. In all cases (RAEB9, RAEBt9 and CMML4) [36] bone marrow blasts infiltration was greater than 10% (12-30%). Overall, 5(23%) of 22 patients achieved complete responder and 2(9%) as partial responders. The overall median survival was 8 months (range 1-27months), whereas the median survival of responders was 16months(8-27months), the median duration of response was 11months(2-21months). It seems that the combination of ATRA and LDARA-c may be effective in approximately 30% of HRMDS patients [35].
Valproic acid (VPA) has been used as an anticovulsant for decades. VPA is a potent inhibitor of histone deacetylases (HDAc). It can modify the structure of chromatin allowing recruitment of transcription factors to restore epigenetically suppressed genes. VPA has been shown to posses antiproliferative activity and to overcome the differentiation block in leukemia blast cells [37]. Some clinical trials with VPA monotherapy or in combination with ATRA have been reported in MDS. In a piloty study of Kuendgen and colleagues [38- 40] patients with MDS or AML secondary to MDS were treated with VPA monotherapy or with ATRA later resulting in a 44% of response rate. In the follow-up study of 43 patients, an even higher response rate of 52% was observed in those low-risk MDS patients, while for the patients with excess blasts (RAEB) and CMML response rates were 6% and 0% respectively, which implicate the difference of MDS subtypes. In another trials, Siitonen etal [41] reported that according to IWG criteria,3 patients(16%) of 19 MDS responded to treatment following VPA,13-cis RA and 1,25(OH)2D3 combination. All the responses were hematological improvement. One patient responded to the treatment with an increase in platelet value from 67x109/l to 105x109/l. His peripheral blood and bone marrow blast cells decreased from 4% to 0% and from 19% to 7%, respectively. Furthermore, the disease remained stable in 11 patients but progressed in 5 during treatment. This is encouraging results.
Table 1: Results of Retinoic acid therapy in MDS.
A series of these studies are summaried in (Table 1). While some patients experienced improvement in peripheral blood counts, complete responses were reported in only a small proportion of these studies [42-43]. The sole exception was a patient who presented with 29% marrow blasts and 90% abnormal metaphases with 13-cis RA. He obtained a complete clinical and cytogenetic remission therapy [44-49]. This clinical response to 13-cis RA drug was due to in vivo growth inhibition of malignant monocytoid clone [50]. Continued follow-up of this study in this field will be of interest [51-54] (Table 1).
Retinoic Acids in Skin Disease
Vitamin A is necessary for normal epithelial cell differentiation and maturation [55-57]. Retinoids influence on skin keratocyte proliferation, epidermal differentiation and kerintinisation. Those retinoids including natural and chemically synthesized vitamin A derivatives are common used as systemic and topical treatment of various skin disorders. At present there have well developed three generations: the naturally occurring retinoids (all-trans retinol, Aretinoin, Isotretinoin, Alitretinoin) the monoaromatic retinoid and the polyaromatic retinoid derivatives [58].
Table 2: Results of 13-cis RA in severe acne treatment.
Isotretinoin is an orally active retinoic acid derivative for the treatment of acne (papulo- pustular,nodulo-cystic, conglobata) [59],since it shows an excellent efficacy against severe refractory nodulocystic acne. Peck’s [60] original observation in 1978-79 of the effectives of 13-cis RA in cystic acne has been well supported. In double-blind studies using small doses of 13-cis RA regimen, Farrell [61] in 15 patients, Jones [62] in 76 patients, Plewig [63] in 79 patients and Rapini [64] 150 patients reporting have confirmed this results. A summary study of limited review on 365 affected persons are presented in (Table 2). The drug action involves an inhibition of sebum excretion rate(SER) in sebaceous glands and production rate of free fatty acids[60,61,65-68] through trigerring NGAL (neutrophil gelatinase-associated lipocalin) expression [17] normalise follicular keratinisation [69] and the decrease in colonisation of propionibacterium acnes and associated inflammation in skin surface microflora [70].This response, mediated by toll-like-receptor 2(TLR2), is increased in acne patients due to high expression of TLR2 [71] (Table 2).
Figure 4: An advanced squamous cell carcinoma of skin before(left) and after(right) isotretinoin [57].
Encouraging results have also been used 13-cis RA in small numbers of patients with rosacea, Gram-negative folliculitis, Darier’s disease, ichthyosis and pityriasis rubra pilaris [72,73]. In the treatment of rosacea, isotretinoins led to a significant reduction of erythemia, papules and pustules in several studies [72,73]. During treatment of rosacea,13-cis RA act as a potent anti-inflammatory and sebum-suppressive agent. Long-lasting remission can be reported for first patient over 12 months [72]. The use of low dose isotretinoin (0.15-0.3mg/kg bw daily) showed high efficacy and was well tolerated. Isotretinoin is only partially effective in psoriasis, in contrast etretinate which is effective in psoriasis but ineffective in severe acne. Promising, some trials have reported with isotretinoin in patients with squamous and basal cell carcinomas [74,75] cutaneous T-cell lymphoma [56] recurrent malignant glioma [76] malignant eccrine poroma [77] and keratoacanthomas [78,79] and xeroderma pigmentosum with squamous cell carcinoma [79]. In literature, there were at least 10 CR patients with squamous cell carcinoma (SCC). Skroza etal [74] reported a CR patient with well-differentiated SCC following the daily dosage of 0.5mg/kg/day for 5 months. Dring 1-year follow up, he remained all in normal range. Using combination chemotherapy and isotretinoin for 4 months, Zaman [80] reported a complete clinical remission of tumors in a case of 15 year old female of xeroderma pigmentosum with SCC. Another collection of four SCC of skin obtained CR through isotretinoin at daily dose of 1mg/ kg/day twice a day for 4 months (Figure 4) [57]. The mechanism may involve the modification of epidermal growth factor receptor (EGFR) and certain protein kinase. At present, It has clearly known the results that amplified (50-fold EGF receptor in SCC relative to normal skin keratinocytes) or mutant EGFR is oncogenic in origin of some SCC [81]. This oncogenic receptor EGFRvIII has also been found in malignant glioma and invasive breast carcinoma [82-89]. Zhu [90] conduct a short CR using chemotherapy and topical 5% Fu of retinoic acid ointment in a 75-year old patient with SCC. She had a 8x5cm rodent ulcer in her left ear and facial area. A shrinkage of irregular and harden marginal valgus converted to flat and superficial red and scar noted after one month treatment. These findings suggest that retinoids may be effective and well-tolerated therapy for advanced epidermoid SCCs in some studies [91-95] (Figure 4) .
ATRA in patients with gastric cancer (GC)
Recently [96], two cohorts of group presented ATRA trials on patients with GC. Jin etal presented a better benefits of gastric dysplasia with omeprazole and sucralfate and the addition of ATRA (68% vs 37%) compared to patients treated with omeprazole and sucralfate alone. Hu etal also showed that ATRA significantly prolong overall survival following the combination of conventional chemotherapy. ATRA anticancer mechanisms of action against GC cells included cell cycle blocking and differentiation initiation(p21WAF1/CIP1 induction, decreased ERK/MAPK pathway), decreased expression of HER2 oncogenic receptor in patient’s gastric mucosa, apoptosis initiation and inhibiting CSC(cancer stem cell) properties such as tumorspheres formation and patient derived xenografts(PDX) growth in mice. In GC cells, CD44+ stem/progenitor cells and a high ALDH (aldehyde dehydrogenase, R-ALDH, ALDH1A1 and ALDH1A3) activity could be considered as putative targets to inhibit tumor growth, to overcome resistance to cancer therapy and to improve GC prognosis.
The-Structure-of-Retinoic-Acid-Receptors-Molecular-Basis-of-Retinoic-Acid-Action-and-the-RAR-Gene-Transcription RARs structure
The retinoic acid receptors (RAR) belong to the large family of ligand responsive gene regulatory proteins that includes receptors for steroid and thyroid hormones [97]. There are three retinoic acid receptors (RAR), RARα, RARβ and RARγ which are conserved throughout vetebrates encoded by their different RAR (chr 17q21, chr 3p24 and chr12q13) gene, respectively. The RARA contains 462 amino acids(aa) [98,99] RARB consists of 455aa [100] and RARG contains 454aa [101] respectively. The RAR is a type of nuclear receptor which act as a transcription factor that is activated by both all-trans RA and 9-cis RA. The RARs have different functions and may activate distinct target genes. The RARa is expressed in a wide variety of different hematopoietic cells [98,99] the RARβ in a variety of epithelial cells [100] and the RARr in differentiation of squamous epithelia and human skin tissue [101,102]
All RARs contain a variable N-terminal region(A/B), a highly conserved cysteine-rich central domain(C) responsible for the DNA binding activity, and a relatively well-conserved C-terminal half(E) functionally its role in ligand binding and nuclear translocation. These three main domain are separated by a hinge region(D) [12,97,102].The central DNA binding domain(88-153aa) exhibits an array of cysteine residues compatible with the formation of two so-called zinc finger(Miller,1985).Each of them a zinc atom tetrahedrically coordinated to four cysteine and each of the hypothetical zinc finger is encoded by a separate exon of the receptor gene (Figure 5) Zinc finger 1, 88-108aa, Zinc finger 2, 124- 148aa] [97-103].The N-terminal zinc finger of the DNA binding domain confers hormone responsiveness to HREs, determing target gene specificity and responsible for functional discrimination between HREs whereas the C-terminal finger contains the sugarphosphamide backbone of the flanking sequences [103,104] (Figure 5) .
Figure 5:Amino acid sequence of the DNA binding domain of the hRARa into two putative zinc–binding finger (Figure from George Zhu a feeling for scientific drawing based on Evans RM, Science, 1988, 240:899- 895; Beato M,Cell,1989, 56: 335-344; Giguere V,Nature,1987;330:624-29; Petkovich M, Nature, 1987,330: 444).
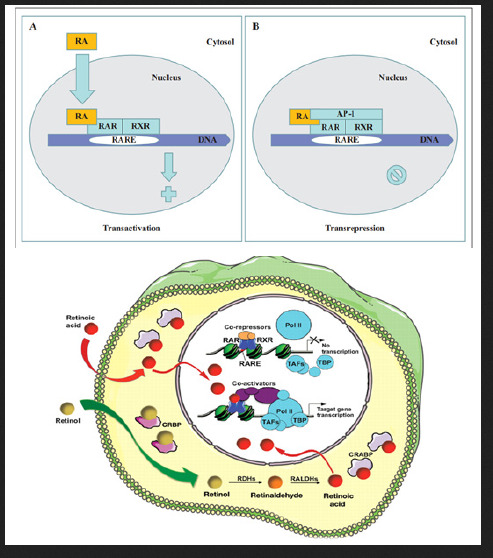
The molecular basis of retinoic acid action and the RAR gene transcription
Retinoic acid (RA) is a lipophilic signal molecule which is able to induce acute and direct activation of the expression of specific genes supports its molecular model of action that resembles that of steroid hormones [105]. The cellular retinoic acid-binding protein (CRABP) may be involved in this transfer [9,10]. In the nucleus, RA receptors (RAR) function as a heterodimer with retinoid X receptors (RXRs) [106-109]. RAR/RXR can bind to DNA motif at RA-response elements (RAREs, also HRE) in the regulatory sequences of target genes in the absence of ligand, thereby interacting with multiple protein complexes that include co-repressors N-CoR [110] SMRT [111] and histone deacetylases (HDACs), and maintaining gene repression. Here, RAREs consist of a direct repeat of a core hexameric sequence 5’ (A/G)G(G/T)TCA-3’ [112] or of the more relaxed 5’-(A/G)G(G/T) (G/T)(G/C)A-3’ motif, separated by 1,2,5 bp [113]. A corepressor represses expression of genes by binding to and activating a repressor transcription factor, the repressor in turn bind to target gene’s operator including RARE sequence, then blocking transcription of that gene (see corepressor-wikipedia). Transcriptional regulation thus drives from the binding of hormone-receptor complexes to RARE sites on target DNA [12,97,103]. In the presence of RA(all-trans RA,9- cis RA),binding of the RA ligand to RAR alter the conformation of the RAR, a conformational change in the DNA-bound receptor leads to the release of co-repressor complexes associated with the RAR/RXR dimer and the recruitment of co-acitivator complexes. These induce chromatin remodeling and facilitate assembly of the transcription pre-initiation complex including RNA polymerase II (Pol II) [114], TATA-binding protein (TBP) and TBP-associated factors (TAFs) [2,12,103,115,116] (Figure 6). Subsequently, transcription of target genes is initiated. This also represent liganddependent transcriptional activation which mediated by nuclear receptors. Like thyroid hormone receptor (THR) [117,118] retinoic acid act as ligand for RARs, converting RARa from transcriptional repressor to activators [2,12,119-122]. Numerous RAR target genes after RA induction have been identified including genes within retinoid pathway, such as RARB,Crbp1/2 (Rbp1/2),Crabp1/2 and CYP26a1.And also, several members of HOX gene family, including HOXa1,HOXb1,HOXb4 and HOXd4,and other genes Tshz1 and Cdx1 [123] the function of which has been demonstrated in vivo in the normal roles of retinoids in patterning vertebrate embryogenesis, early neurogenesis, cell growth and differentiation (Figure 6).
Figure 6:Retinoid receptor-dependent gene regulation [116] & (b): Gene regulation by retinoic acid signalling [2].
Molecular Model of the Gene Regulation of Retinoic Acid Action in APL
Acute promyelocytic leukemia (APL) is a clonal expansion of promyelocytic precursors . Retinoic acid(RA) (initial 13-cis RA, later ATRA and tamibarotene) plus chemotherapy is currently the standard of care [124-131]. APL has a very good prognosis, with long-term survival rates up to near 70%-90% [132]. Molecular analysis has uncovered the facts that approximately 98% of APL, RARa translocates and fuses with the PML gene on chromosome 15 [133-136]. The resulting RAR chimeric genes encode pml/RARa fusion protein, which is specifically expressed in the promyelocytic lineage [20]. In addition to oncogenic receptor derivative pml/RARa [108,137-139] the translocation involves oncogenic TBL1XR1- RARB [140] and NUP98/RARG [141] and oncogenic PML-RARG [142] which share high homolog (90%) of three RAR family that were also detected in APL rare cases.
Most studies have shown in APL that oncogenic pml/ RARa act as constitutive transcriptional repressor that blocks neutrophil differentiation at the promyelocyte stage. Without its ligand, retinoic acid (RA), PML-RARA functions as a constitutive transcriptional repressor of RARE-containing target genes, abnormally associating NcoR/HDACs complex and blocking hematopoietic differentiation. In the presence of pharmacological concentration of RA (about 350ng/ml), RA induce the corepressors NcoR/ HDACs dissociation from PML-RARA, thereby activates transcription and stimulate differentiation [11,12,108,139]. In vitro by using a dominant negative RAR construct transfected with interleukin 3(IL-3)-dependent multipotent hematopoietic cell line (FDCP mix A4) and normal mouse bone marrow cells, GM-CSF induced neutrophil differentiation was blocked at the promyelocyte stage. The blocked promyelocytes could be induced to terminally differentiate into neutrophils with supraphysiological concentration of ATRA [143]. Similarly, overexpression of normal RARa transduced cells displayed promyelocyte like morphology in semisolid culture,and immature RARa transduced cells differentiate into mature granulocytes under high dose of RA(10-6M) [144]. Moreover, mutation of the N-CoR binding site abolishes the ability of PML-RARa to block differentiation [145,146]. Therefore, ectopic expression of RAR fusion protein in hematopoietic precursor cells blocks their ability to undergo terminal differentiation via recruiting nuclear corepressor N-CoR/histone deactylase complex and histone methyltransferase SUV39H1 [147]. In vivo, transgenic mice expressing PML-RARA fusion can disrupt normal hematopoiesis, give sufficient time, develop acute leukemia with a differentiation block at the promyelocytic stage that closely mimics human APL (APL-like syndrome, even in its response to RA in many studies. These results are conclusive in vivo evidence that PML/ RARa is indeed oncogenic, and oncogenic pml/RARa is etiology of APL pathogenesis [148-150]. This also represent a steroid receptor in tumorigenesis (Figure 7).
Figure 7: pml/RARa fusion in differentiation block at promyelocytic stage in transgenic mice [149].
Moreover, in Rousselot’s group experiments, HL-60 cells transfected with 15-30ug of PML-RARa fusion in culture show no features of granulocytic differentiation after 7 days of incubation with 10-7,10-6 uM RA (5.5-9.5% of differentiated cells by the NBT test). At 5ug of PML-RARa plasmid concentration, the blockage of RA-dependent myeloid differentiation could be overcomes with high doses(10-6M) of RA (99% of differentiated cells by NBT test) (Figure 8) [151]. The results clearly indicate that PMLRARa mediated transcriptional repression, as well as PML-RARa oncoprotein blocks RA-mediate promyelocyte differentiation. (Figure 8).
Figure 8: Expression of pml-RARa in HL-60 cells blocks ATRA-induced promyelocytic differentiation a(in the presence of 10-7 M RA, top), and transcriptional repressive properties of pml-RARa in human myeloid cells as βRARE-luc assay(bottom) [151].
By using Xenopus oocyte system to uniquely the comparison of the transcriptional properties of RAR and PML-RAR is due to the lack of endogenous nuclear receptors and the opportunity to evaluate the role of chromatin in transcriptional regulation. The results shown in (Figure 9) demonstrated that, indeed, PML-RARA is a stronger transcriptional repressor that is able to impose its silencing effect on chromatin state even in the absence of RXR. Only pharmacological concentration of RA,pml/RARA become transcriptional activator function [139]. (Figure 9).
Figure 9: Shows pml/RARa as a constitutive transcriptional repressor in xenopus oocyte system, as measured by RARE3 CAT and GAL4 assay [139].
In vitro experiments, ATRA induce pml-RARA itself cleavage into a 85-97kd delta PML-RARA product (a truncated pml/RARA form) in RA sensitive NB4 [152-156] (Figure 10). Delta PML-RARa is not formed in ATRA differentiation resistant NB4 subclones [152,155] which indicate the loss of PML/RARa may be directly linked to ATRA-induced differentiation [152,155].This induction of of PML-RARa cleavage and degradation by RA(ATRA,9-cis RA,Am80) involve the proteasome-dependent [152-154] and caspase mediated pathway [155] or independent of proteasome and caspase cleavage[156] and possibly ubiquitin-activating enzyme EI-like(UBEIL) induction in NB4 cells. This is reason that proteasome inhibitor MG-132 and caspase inhibitor ZVAD do not block ATRA-induced pml/RARa cleavage and differentiation whereas this delta pml-RARA is blocked by RARA itself antagonist Ro-41-5253 [156].The proteasome-dependent pml/RARA degradation, by using proteasome inhibitor lactacystin test, allows APL cells to differentiation by relieving the differentiation block [153]. These data suggest a set of multiple molecular mechanisms for restoration by RA induced myeloid differentiation in APL cells. (Figure 10).
Figure 10: Shows delta pml/RARa cleavage products independent of proteasome and caspase in the presence of ATRA(a,b), and pml/RARa act as transcriptional repressor even in the presence of ATRA(0.01uM,1uM) in RARE-tu-luc assay while delta pml/ RARa is less potent activator of RARE-tk-leu activation than wild-type RARa(c) in NB4 cells [155].
Next we further examine the pml/RARa three region functions,in vitro deletion of the RARa DNA binding domain decreased the ability of pml/RARa to inhibit vitD3 and TGFinduced the myeloid precursor U937and TF-1 cell differentiation [145]. This is also supported by functional analysis of DNA binding domain mutation in vitro. The RARa zinc finger is a sequencespecific DNA binding through which RARa contacts the RA target genes. Moreover, deletion of PML coiled-coil region also blocked the differentiation capacity of TF-1 cells [145]. The coiled-coil region directs the formation of pml/RARa homodimers tightly interact with the N-CoR/HDACs complex, so that transcriptional derepression cannot occur at RARA target gene promoter even if the presence of ATRA [RA resistant, 12,157]. In vitro, using established subclones of NB4 resistant to both ATRA and 9-cis RA, they were significantly less able to stimulate transcription of a RARE driven CAT-reporter gene induction by ATRA and showed altered DNA binding activaty on a RARE [158]. In the resistant cases, mut PML stabilizes PML-RARa [159]. PML-RARA with ligand-binding domain (LBD) mutation, ligand RA binding with LBD is impaired. These results have clearly shown that PML protein dimerization and RARa DNA binding domain are indispensible for the myeloid precursors differentiation which was blocked by PML/RARA and eventually leukemic transformation.
In accordance,the pml/RARa/RXR target genes is found to block differentiation by consitutively silencing a set of RA-responsive genes in the control of hematopoietic precursor cells. Five major transcription factors, Ap-1 [160] C/EBPepsilon [161,162] Pu.1/ DAPK2 [163] PTEN [164] and p21WAF/CCKN1A [165] directly regulate genes important in myeloid differentiation. PML/RARA fusion is oncogenic transcriptional repressor of five genes. Inhibited expression or functions of these five transcription factors lead to a block in myeloid differentiation, which is a hallmark of APL.
In vitro cotransfection of pml/RARA with plasmid expressing AP-1 of c-Jun and c-fos proteins in MCF-7 cells, by using CAT assay, PML-RARa is a repressor of AP-1 transcriptional activity in the absence of RA while RA treatment converted the chimera into a strong activator [160]. Since high AP-1 activity is associated with differentiation of leukemic cells in several context [160] the stimulatory effects in the presence of RA could be relevance to its reversal by provoking differentiation. Another, in pml/RARacontaining cell lines, a close link exists between induction of differentiation and induction of C/EBP epsilon expression [161]. C/ EBPepsilon knockout mice had a block in myeloid differentiation [162]. In absence of retinoic acid (RA), induction of pml/RARa expression in U937PR9 cells stably transfected with zinc-inducible pml/RARa suppressed the expression of C/EBPepsilon. In contrast to its repression, in the presence of a pharmacologic concentration of RA, pml/RARa significantly increased the level of C/EBPepsilon expression in a time and dose-dependent manner [161]. The findings implicate that C/EBPepsilon is critical downstream target gene in RA-dependent granulocytic differentiation in the treatment of APL [163-165].
Phosphotase and Tensin homolog (PTEN) is a protein and lipid phosphatase, which plays a pivotal dual role in tumor suppression and self-renewal of hematopoietic stem cells as its promoting exhaustion of normal hematopoietic stem cells (HSCs) and generation of leukemia-initiating cells (LICs) [166,167]. PTEN expression is downregulated in APL, while ATRA treatment increases PTEN leves by inducing PU.1 transcriptional activity via pml/RARa degradation, allowing the binding of PU.1 in PTEN promoter, in turn promotes PTEN nuclear re-location and decreases expression of the PTEN target Aurora A kinases. Therefore, PTEN is one of the primary target gene of oncogenic pml/RARa in APL
Importantly, restoring DAPK2 expression in PU.1 knockdown APL cells partially rescued neutrophil differentiation [168]. In addition, DAPK2 interacts with other cyclin- dependent kinase inhibitors such as p15INK4b and p21WAF1/CIP, which is needed for the cell-cycle arrest in terminal differentiation of neutrophils. Moreover, DAPK2 can bind and activate the key autophagy gene beclin-1 [169]. DAPK phosphorylates beclin 1 on Thr 119 located at a crucial position within its BH3 domain and thus promotes the dissociation of beclin 1 from BCL-XL inhibitor and induction of autophagy [169]. Here, beclin 1 was initially identified as a BCL-2- binding protein, which is part of a class III PI3K (phosphatidylinositol -3-kinase) multiprotein complex that participate in autophagosome nucleation. Death- associated protein kinase (DAPK1) is a calcium/ calmodulin (CaM) serine/threonine kinase for mediator of cell death [170]. PU.1, an ETS transcription factor known to regulate myeloid differentiation. Silencing of PU.1 in the adult hematopoietic tissue produces dysfunctional stem cells and impaires granulopoiesis by inducing a maturation block. Overexpression of PU.1 overcomes the differentiation block in SCa 1+/Lin- HSC with transduction of PML/ RARa fusion, as measured by the Gr-1 and Mac-1 expression [171]. Thus, pml/RARa represses PAPK2/PU.1 - mediated transcription of myeloid genes in APL,linking a novel autophagy mechanism of pml/ RARA degradation [172].
Figure 11:Molecular model of the gene regulation of retinoic acid (RA) action (George Zhu, January 1991, revised in 2012, further revised in 2018 and in this paper). Schematic alignment of the receptor protein. The two highly conserved regions, identified as the putative DNA-binding (C) and hormone- binding (E), a hinge region (D) and the non-conserved variable NH4-terminus (A/B) as described above. CAT:CAAT box, CCAAT-enhancer binding proteins(or C/EBPs); GC:GC box; TATA:TATA box. Note: In APLcells, pml/RARa fusion point is located in the first 60 amino acids from the N-terminus(A/B) of RARa [12,182].
The elucidation of the molecular basis of retinoic acid and retinoid pharmacology in APL has been illustrated in several publications [157,173-176] the detail molecular model of gene regulation had also been proposed by Zhu in 1990s [12,177,178]. As an approach to APL treatment, one possible the action of retinoic acid, A consensus sequence (TCAGGTCA motif) has been postulated for thyroid hormone (TRE) and retinoic acid responsive element(RARE)-containing in the promoter region of target genes [112]. High dose of RA-RARE-PML/RARa complexes in intracellular localization appears to relieve repressors from DNA-bound receptor [11,12,117,145,158,179] including the dissociation of corepressor complexes N-CoR, SMRT and HDACs from PML- RARa or partially PML-RARa/RXR [11,12,146,157,179]. Also release PML/RARa -mediated transcription repression [175]. This transcriptional derepression occurs at RARa target gene promoter [12,157,180]. Consquentially, PML-RARa chimera converted receptor from a repressor to a RA-dependent activator of transcription [156,157,160]. Co-activator complexes containing histone acetyltransferase (e.g. p300/CBP) are recruited. The resulting pml-RARA oncoprotein proteolytic degradation occurs through the autophagy- lysosome pathway [172] and the ubiquitin SUMO-proteasome system (UPS) [152-155] as well as caspase 3 [155] or lysosomal protease (cathepsin D) enzyme or/and EI-like ubiquitin-activating enzyme (UBEIL) induction [181]. An effect is to relieve the blockade of pml/RARa-mediated RA dependent promyelocytic differentiation and retinoic acid (9-cid RA, ATRA, Am80) in APL therapy Zhu G, March 1990- January 1991, revised in 2012). Here, RA can overcome the transcriptional repressor activity of pml/RARa [11,12,156,179,182]. The oncogenic pml/ RARa uncover a pathogenic role in leukemogenesis of APL through blocking promyelocytic differentiation. This oncogenic receptor derivative pml/RARa chimera is locked in their “off” regular mode thereby constitutively repressing transcription of target genes or key enzymes (such as AP-1, PTEN, DAPK2, UP.1, p21WAF/CCKN1A) [160-165] that are critical for differentiation of hematopoietic cells. This is first described in eukaryotes (Figure 11).
Read More About this Article: https://biomedgrid.com/fulltext/volume3/vitamin-a-and-its-derivatives-retinoic-acid-and-retinoid-pharmacology.000656.php
For more about: Journals on Biomedical Science :Biomed Grid
#biomedgrid#medical and medicinal journal#Journals on Biomedical Imaging#Journals on Medical drug and theraputics#American medical journal#Journals on Biomedical Science
0 notes
Text
ABIM: Endocrinology
ABIM syllabus can be found here
Let me know if you find any errors
Sources: UWorld, MKSAP 16/17, Rizk Review Course, Louisville Lectures, Knowmedge (free version)
Adrenal Disorders
Primary aldosteronism and mineralocorticoid excess:
- Sx: HYPERNATREMIA, hypokalemia, metabolic alkalosis, HTN
- Dx: aldosterone:renin >20 (aldosterone >15 with low renin levels), salt challenge fails to decrease aldosterone levels --> get CT scan
- Tx: thiazide for BP control, spironolactone for hyperplasia, surgery for adenoma
Adrenal insufficiency:
- Addison’s = primary: tan, orthostatic HTN, hyponatremia, hyperkalemia, hypoglycemia, prolonged QTc
- Dx: morning cortisol levels --> cosyntropin stimulation test <18mcg/dL = adrenal insufficiency --> check morning ACTH levels (if decreased ACTH, MRI brain; if increased ACTH, CT adrenals)
- acute Tx: dexamethasone
- chronic Tx: hydrocortisone +/- fludrocortisone only if primary
- x2-10 dose of steroids during stress
Pheochromocytoma:
- associated with MENIIa and IIb (increased calcitonin/medullary thyroid cancer is also associated with both MENII’s; if IIa: hypercalcemia/parathyroid, if IIb: Marfan’s/neuromas)
- Dx: serum or 24 hour urine metanephrines --> MRI/CT ab --> if negative: T-MIBG
Tx: surgery:
- pre-op BP control with phenoxybenzamine/doxazosin/nicardipine
- intra-op HTN crisis: nitroprusside or phentolamine
Incidentaloma:
- if <4cm, f/u CT; if >6cm, surgery
- Dx with 1mg overnight dex suppression test (Cushings) + urine metanephrines (PCC) +/i if hypertensive: aldosterone:renin ratio (hyperaldosteronism if elevated)
Thyroid Disorders
Hyperthyroidism:
- Grave’s: anti-TSHR Ab
- associated with vitiligo
- Tx: Methimazole (AE: sore throat/agranulocytosis, hepatotoxicity) > PTU (for first trimester; AE: hepatotoxicity)
Hypothyroidism:
- Hashimoto’s: anti-TPO antibodies
- associated with primary thyroid lymphoma
- Tx if TSH>10 or planning pregnancy or symptomatic
*FYI: increase Synthroid dose in pregnancy and in CELIAC DISEASE
Thyroiditis:
1. subacute/DeQuervain’s: PAINFUL, Tx with NSAIDs only
2. Peripartum: painless, autoimmune
3. amiodarone-induced
*subclinical presentation (decreased TSH, normal T4): repeat TFT in 4-6mo
Thyroid nodules:
- if <1cm and doesn’t look cancerous, repeat US in 3-6 months
- if >1cm, FNA > Sx
Euthyroid sick syndrome: transient and mild, weird TSH/T3/T4 levels during illness without prior thyroid issues; usually T3 is decreased while TSH/T4 is normal
- Tx underlying illness only (no need for Synthroid)
Thyroid storm: fever, HF, psych changes/coma; Tx: PTU, propranolol/BB, steroids (vs. Myxedema coma from hypothyroidism: hypothermia/hypotension/bradycardia/bradypnea, desaturation, AMS, hyponatremia, hypoglycemia; Tx with Synthroid + hydrocortisone)
*if TSH/T3/T4 are all weirdly increased or decreased, cause is likely a pituitary tumor
*suspect toxic multinodular goiter when patient experiences hyperthyroid symptoms after receiving IV iodine contrast
Hypertension
Hyperaldosteronism:
- hypernatremia, hypokalemia, metabolic alkalosis (basically the opposite of RTA type 4)
- Tx with thiazide
Renal artery stenosis: Tx with ACEi
Cushing’s Disease:
- round and squishy
- Dx: elevated 24 hour urine cortisol, 1mg dexamethasone suppression test (positive if fails to bring cortisol <5), elevated late salivary cortisol
Lipid disorders
- start screening every 5 years in >35yoM, >45yoF; or >20 if +CAD risk
- goals: LDL<160, Cholesterol <190; pretty much decrease both goals by 30 for every additional CAD risk up to 3 times
- diet > exercise
- offer Orlistat if BMI>30
- offer Bariatric surgery if BMI >40 OR >35 with obesity-related condition
Ovarian disorders and female reproductive health
Polycystic ovary syndrome:
- amenorrhea and virilization
- Dx: bleeds with progesterone challenge, has elevated LH
- Tx hirsuitism with OCP --> if fails: spirnolactone; use clomiphene if wants babies
Amenorrhea:
1. primary ovarian insufficiency: elevated FSH = menopause --> if normal: check karyotype to r/o Turner’s (obtain cardiac imagining and kidney US for all patients with Turner’s)
*if amenorrheic because of super athleticism: screen for bulimia
2. hypothalamic cause: functional/tumor/lymphoma; has decreased FSH and NO withdrawal bleed after progesterone challenge
3. anatomic cause/Asherman syndrome: adhesions basically retain periods; also has no withdrawal bleeding --> Tx: surgery
Ovarian cancer:
- hyperandrogenism; normal DHEA levels but elevated total testosterone levels >200 in a woman = ovarian cancer until proven otherwise
- Dx: TVUS --> adrenal CT
Testes and male reproductive health
Male hypogonadism: total testosterone <200 +:
- increased LH/FSH = primary testicular failure (Klinefelter’s, Mumps orchitis, XRT, autoimmune)
- normal/decreased LH/FSH, elevated prolactin = secondary cause (prolactinoma --> MRI brain, opiates, steroids) --> obtain iron study to rule out hemochromatosis*
*Hemochromatosis presents as tan Diabetes with elevated transaminases, and hypogonadism; has OA symptoms and is associated with CPPD/Pseudogout
Male infertility:
- Cystic Fibrosis is associated with azospermia, bilateral absence of vas deferens
Gynecomastia: associated with anabolic steroids, marijuana, spironolactone
- if elevated estradiol --> check testicular ultrasound to r/o neoplasm --> chest/adrenal CT to r/o choriocarcinoma (elevated beta hCG, lung infiltrates, hemoptysis)
- if elevated LH, decreased testosterone --> check karyotype to r/o Klinefelters (associated with increased risk of breast cancer)
*testosterone therapy can worsen OSA, erythrocytosis, and increases risk of clots
Diabetes mellitus
Type I:
- associated with other autoimmune diseases (Celiac, vitiligo, thyroid)
- DKA
Type II:
- Dx with two of the following on separate days: (1) fasting >126, (2) A1c >6.5%, (3) random >200, or just one of this: (4) 2 hour gtt >200
*if a health-seeming patient >35yo with h/o CAD or 2 CAD risk factors wants to do a vigorous exercise program --> do an exercise stress test first
Diabetes mellitus and pregnancy: STOP ACE/ARB/statin (teratogenic!)
- obtain eye exam once/trimester
- BP control with methyldopa, BB (labetalol), CCB, Hydralazine
- goal: preprandial BG <90, 1 hour postprandial <120 using NPH and short-acting insulin (NOT long-acting insulin or orals)
- require annual DM screening after delivery
Diabetes goals:
- BP <140/90: use ACEi
- start statin regardless of LDL if cholesterol >135 and check yearly
- cholesterol goal <135 > LDL goal <100
- annual eye exam with Q3-5 year dilated eye exam
- urine albumin excretion <30; if >30, start ACEi/ARB
Indications for continuous BG monitoring:
(1) postprandial hyperglycemia
(2) Dawn phenomenon: morning hyperglycemia (vs. Somogyi = rebound hyperglycemia)
(3) overnight hypoglycemia
Diabetes complications:
- acute mononeuropathy: spontaneously resolves, no Tx
- gastroparesis: Tx: small meals, Reglan/Erythromycin (vs. Rifaximin for Scleroderma-related bacterial overgrowth that presents similarly as bloating)
- orthostatic hypotension: Tx with compression stockings +/- fludrocortisone
- peripheral neuropathy: BG control --> DULOXETINE > pregabalin
- HHS: plasma osm >320, BG >600-1000, normal pH/ketones; Tx with NS --> insulin --> when BG<200 and tolerating PO --> SQ insulin
- DKA: pH <7.3, bicarb <15, BG >250, elevated ketones elevated AG
vs. Diabetes insipidus: excessive thirst for cold water, can’t concentrate urine
- r/o DM, hypercalcemia
- Dx: water deprivation test --> if urine osm still <200 --> desmopressin challenge:
(1) can concentrate after challenge = positive test --> brain MRI; Tx intranasal or PO desmo/vasopressin
(2) still can’t concentrate after desmo: negative test --> kidney ultrasound; Tx sodium restriction and thiazide
*if Lithium-induced: Tx Amiloride
Disorders of calcium metabolism and bone
Hypercalcemia:
- hyperparathyroidism associated with MEN I and IIa (increased PTH or normal PTH with increased calcium and decreased phos), chondrocalcinosis, bone cyst --> Dx: Sestamibi scan --> Surgery
*Indications for parathyroidectomy:
(1) Age <50yo
(2) Ca >12 or 1 above baseline
(3) GFR<60
(4) 24 hour urine Ca >400
(5) symptoms of hypercalcemia
- drug-induced hypercalcemia: lithium, thiazide
- sarcoidosis: increased calcitriol = active Vit D = 1, 25 Vit D
- cancer / multiple myeloma: CRAB, difference in urine vs dipstick protein due to presence of undetected light chain, hypervitaminosis D
- Dx: check ionized calcium first --> r/o decreased TSH --> PTH/Ca/Phos/25Vit D levels --> decreased urine calcium ( = familial hypocalciuric hypercalcemia --> Dx: CASR mutation, urine Ca;Cr ratio <0.01)
Hypocalcemia: check ionized level, because may be due to hypoalbuminemia
- associated with DiGeorge
- symptoms include circumoral paresthesia, Chvostek cheek tap, Trousseau BP cuff
Hyperphosphatemia:
- CKD (increased PTH, decreased Vit D) --> Tx: Calcitriol/1,25VitD
- hypoparathyroid (decreased PTH)
- pseudohypoparathyroid (increased PTH, normal Vit D)
Hypophosphatemia:
- bone tenderness due to vitamin deficiency
Paget’s:
- hat size changes, bone pains, fractures, femur/tibia bowing, cranial nerve compressions; heart failure
- elevated alk phos --> bone scan
- Tx: bisphosphonate
Osteoporosis: T-score<-2.5 (ignore age-adjusted Z-score)
- get DEXA every 10 years (if normal) in woman >65 OR younger if FRAX >9.3% (they smoke, have h/o hip fx, steroid use, etc)
Vitamin D deficiency and osteomalacia:
- proximal muscle weakness/falls (especially in elderly), bone pain
- decreased calcium, phosphate; increased alk phos
- associated with Celiac disease, liver disease, kidney disease
- Dx: bone marrow biopsy (BMB)
- Tx: ergocalciferol/Vit D2
Renal osteodystrophy: ESRD pt w decreased Ca, Vit D; increased Phos, PTH; chondrocalcinosis at knees and pubic symphysis
Anterior pituitary disorders
Pituitary tumors: MRI (order first if mass effect)
- associated with MEN I, pregnancy, and check TSH!
(1) Prolactinoma: prolactin >500, galactorrhea/amenorrhea/erectile dysfunction; Tx: Cabergoline
(2) Acromegaly: Dx: IgF1 or oral glucose tolerance test that fails to decrease GH; Tx: surgery
(3) Cushing’s: HTN, DM, proximal muscle weakness; Dx: 24 hour urine cortisol, elevated late night salivary cortisol --> elevated morning ACTH = pituitary tumor --> Tx: surgery > XRT
- incidental pituitary tumors: f/u repeat MRI with prolactin levels
- Rx (TCA, CCB, Reglan, opiates) and pregnancy can cause elevated prolactin!
Hypopituitarism:
(1) apoplexy: sudden HA, vision change, AMS; Tx: steroids
(2) Sheehans (after pregnancy): amenorrhea, no lactation
(3) lymphocytic hypophysitis (occurs peripregnancy): sellar mass with anti-pituitary antibody; Tx: steroids OR if vision changes, surgery
Posterior pituitary and water metabolism
Hypernatremia: DI: polyuria, inability to concentrate urine; Dx: water deprivation --> desmopressin: (1) concentrates urine (urine osms goes up) = MRI brain and Tx w desmo/vasopressin; (2) still doesn’t concentrate (urine osms stay low) = kidney ultrasound and Tx with salt restriction, thiazide
Hyponatremia: SIADH: urine osm > serum osm, urine osm >500, euvolemic (vs. psychogenic polydipsia where decreased serum AND urine osm)
Endocrine tumors and endocrine manifestations of tumors
Pancreatic tumors associated with MENI (hypercalcemia/hyperparathyroid, prolactinoma/pituitary tumor):
- Insulinoma: Dx 72 hour fast (BG<45, insulin >5) --> CT abdomen --> still not detected?: check endoscopic ultrasound
- VIPOMA: watery diarrhea
- Gastrinoma/Zollinger-Ellison syndrome: severe dyspepsia; Dx: gastrin levels --> secretin stimulation test causing increased gastrin >200
- Glucagonoma: hyperglycemia, pustular rash, diarrhea, DVTs
- Carcinoid: flushing, N/V/D/AP; Dx: 24 hour urine 5-HIAA
Malignancy-associated hypercalcemia (squamous cell): decreased PTH, normal/decreased phosphate, increased PTHrpeptide
Ectopic ACTH (Cushing’s) due to tumor: associated with small cell, medullary thyroid cancer (elevated calcitonin), bronchial carcinoid (flushing, wheezing)
SIADH from tumor: associated with small cell; hyponatremia and euvolemia; urine osm >500
Hypoglycemia
- most commonly after gastrectomy/gastric bypass
- insulin use: decreased C-peptide
- sulfonylurea: increased C-peptide --> check for medication in urine
- insulinoma: seen in MEN I with hypercalcemia/hyperparathyroid, prolactinoma/pituitary adenoma; Dx with 72 hour fast: if BG <45 and insulin >5 --> CT abdomen
- exercise-induced delayed hypoglycemia: Tx: complex carbs
Polyglandular disorders
MENI (”3P’s”):
(1) Pituitary: prolactinoma, acromegaly, Cushings
(2) Pancreas: insulinoma hypoglycemia, VIPoma diarrhea, gastrinoma GERD, carcinoid flushing, glucagonoma hyperglycemia
(3) Parathyroid: hypercalcemia
MENIIa:
(1) Parathyroid: hypercalcemia
(2) PCC: hypertension
(3) Medullary thyroid cancer: elevated calcitonin
MENIIb:
(1) Marfan’s/neuromas
(2) PCC: hypertension
(3) Medullary thyroid cancer: elevated calcitonin
#me#endocrinology#abim#internal medicine#im#exam#test#diabetes#metabolism#medical#medicine#study#notes
9 notes
·
View notes
Text
11 Cod Liver Oil Benefits
11 Cod Liver Oil Benefits
1. Source of Anti-Inflammatory Omega-3 Fatty Acids
Cod liver fish oil is one of nature’s richest sources of omega-3 fatty acids, called docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Due to their natural anti-inflammatory properties, omega-3s are often used to treat a variety of symptoms naturally, from coronary heart disease risk factors to depression or arthritis pain.
There are many studied benefits of omega-3 foods or supplements, including protecting and improving heart health, battling mental disorders and decline, reducing inflammation, fighting autoimmune diseases, lowering cancer risk, supporting the growth of healthy bones and joints, improving sleep quality, benefiting child growth and development, fighting menstrual pain, lowering the risk of macular degeneration, and improving skin health as you age.
The problem is that the vast majority of Americans have an imbalance of omega-3 to omega-6 fats in their diet. Omega-6 fats aren’t necessarily bad for you, but if they are consumed in large amounts without omega-3s, they cause inflammation, which is at the root of most diseases.
A healthy ratio is ideally around 2:1 omega-6 to omega-3 fats, but many people on the Standard American Diet (SAD) consume five to 10 times more omega-6 fatty acids than omega-3s. Omega-3 deficiency is on the rise because of the overconsumption of processed foods, fast or fried foods that contain refined vegetable oils (like soybean oil, canola oil, cottonseed oil and corn oil) filled with omega-6s. Supplementing with omega-3-rich cod liver oil is one way to lower inflammation and tip your fatty acid ratio back in favor of better health.
2. Supplies Critical Vitamin D
Vitamin D acts more like a hormone in the body than a vitamin, since it affects neurotransmitter functioning, heart health and inflammatory responses. Synthesized by your own skin when you’re exposed to the sun’s UV light, the best ways to get enough vitamin D are to spend a little time outdoors without sunscreen and to eat vitamin D-rich foods.
Not only does vitamin D play a part in healthy bone metabolism, but it supports important functions of all of our cells. Because low vitamin D levels can interfere with our cells’ ability to multiply and replenish, vitamin D deficiency might increase the risk of becoming sick short-term, or, even worse, developing a chronic autoimmune disorder, cognitive or cardiovascular disease, diabetes, osteoporosis or cancer.
Many adults and children don’t get enough regular sun exposure year round due to living mostly indoor lifestyles, which is why vitamin D is a vital component of most multivitamins. One researcher reviewing the prevalence of vitamin D deficiency states in his 2008 review that “Vitamin D deficiency is now recognized as a pandemic.”(4) Low levels of vitamin D are associated with rickets in children, osteopenia, osteoporosis (and related fractures), cancer, autoimmune diseases, high blood pressure, diabetes, rheumatoid arthritis, mental illness, autism and tuberculosis. (5, 4)
High doses of vitamin D taken alone might have potential side effects, which is why it’s now recommended to take vitamin D in combination with synergistic vitamin A and omega-3 fatty acids, such as how it’s found naturally in vitamin D–rich foods including cod liver oil. (6)
3. Great Source of Vitamin A
Vitamin A is an important antioxidant that reduces oxidative stress (also called free radical damage) and, therefore, inflammation levels. It’s tied to the prevention of eye-related disorders, supports brain health, helps fight cancer and is important for hormone production. While most people eating a pretty well-balanced diet get enough vitamin A daily, deficiency is a risk for some people eating highly processed foods or not enough calories in general.
Recently, supplementing with vitamin A has come under debate, since high levels are thought to actually be harmful and potentially toxic, causing a condition known as hypervitaminosis A. However, this is generally caused by high-dose vitamin A supplements, not the amount found in a teaspoon of cod liver oil, which contains about 90 percent of your daily recommended allowance of vitamin A. (3)
When it comes to obtaining vitamin A from cod liver oil, the quality of the supplement seems to make a big impact. Many brands contain synthetic versions of vitamins A and D that aren’t well absorbed, in addition to dangerous ratios of these two nutrients. While consuming very high levels of vitamin A from supplements alone has been linked to some health concerns, obtaining vitamin A naturally from food sources can help improve the immune system. Eating plenty of vitamin A-rich foods is beneficial for bone growth, night vision, healthy cellular growth, testicular and ovarian function and much more. (7)
Vitamins A and D are fat-soluble nutrients often found together in animal foods. Both act like precursors to active hormones, so we produce certain enzymes that convert each of these to active forms the body can use to regulate our immune system. (8) In this case, vitamin A is converted to retinol. Obtaining vitamins A and D together is completely natural and allows these processes to happen in a way that protects us from toxicity.
4. Helps Prevent Heart Disease
Evidence shows that cod liver oil taken either in supplement or whole food form can help lower high triglycerides, a type of dangerous fat in the blood that raises the risk for heart disease. Cod liver oil also treats high blood pressure and helps to balance cholesterol levels. (9, 10)
A range of human and animal studies have proven high efficacy of omega-3 polyunsaturated fatty acids obtained from cod liver oil in preventing atherosclerosis (hardening and stiffening of the arteries) and its complications. Treatment either alone or in combination with statin drugs helps reverse risk factors in patients with hypertriglyceridemia and high triglyceride levels.
5. Lowers Risk for Cancer
High levels of vitamin D, obtained from both sun exposure and supplementing with cod liver oil, have been associated with lower risks of cancer. One study found that vitamin D from both the sun and cod liver oil could help prevent breast cancer in women due to having antiproliferative and proapoptotic effects on breast cancer cells, thereby reducing mammary tumors. (12)
While sun exposure still seems to be the most relevant protective factor for getting enough cancer-busting vitamin D, cod liver oil may also help offset common deficiencies.
6. Prevents or Treats Diabetes
As a great source of essential healthy fats, cod liver oil can help control insulin resistance, inflammation and manage glucose (sugar) levels in the blood. It’s even been shown to help lower symptoms of complications related to diabetes like kidney disease. Use of cod liver oil and vitamin D supplements during the first year of a baby’s life and use of cod liver during pregnancy can also help lower the risk for developing diabetes. (13)
One 2007 study published in the Journal of Pharmacy and Pharmacology found that giving diabetic rats cod liver oil supplements for 12 weeks acted as a natural diabetes treatment. The cod liver oil supplements completely prevented endothelial deficiency and helped correct several biochemical markers for metabolic syndrome (a combination of diabetes and cardiovascular disorder risk factors). (14)
Prior to supplementing with cod liver oil, the rats experienced elevated plasma glucose (sugar) levels and high triacylglycerol and high cholesterol concentrations in their blood. Cod liver oil helped manage the rats’ weight gain and entirely prevented plasma lipid abnormalities while also controlling insulin sensitivity and other factors.
7. Helps Treat Rickets and Rheumatoid Arthritis
At the beginning of the 20th century, scientists established that cod liver oil was a natural ���antirachitic,” which meant thousands of mothers began forcefully spoon-feeding the stuff to unwilling children to avoid painfulrickets. Cod liver oil use is associated with a reduction in pain, joint stiffness and swelling in patients with arthritis and other inflammatory conditions. (11)
Cod liver oil may even help reduce the amounts of non-steroidal anti-inflammatory drugs patients with rheumatoid arthritis need. (15)
8. Boosts Reproductive Health and Infant Development
Omega-3 fatty acids are important for reproductive health and ideal for a pregnancy diet. These fats found in cod liver oil and other aquatic sources are “precursors” to eicosanoids, compounds that affect cellular activity. Eicosanoids are involved in the inflammatory process and help to keep that in check, but they’re also associated with menstrual cycles, fertility and many hormone-related functions.
When you don’t have enough omega-3s in your diet, the functions fulfilled by eicosanoids can be disrupted, which is why increasing omega-3 intake may improve issues with the following hormone and reproduction-related conditions: (16, 17, 18, 19)
dysmenorrhea (menstrual cramps)
infertility
inconsistent ovulation
premature birth
low birth weights
infant brain development during pregnancy and breast-feeding
preeclampsia
postpartum depression
menopause
postmenopausal osteoporosis
breast cancer
low sperm count
poor sperm motility
Results from a 2003 study conducted at the University of Oslo in Norway showed that children who were born to mothers who had taken cod liver oil during pregnancy and lactation scored higher on intelligence tests at age four compared with children whose mothers had taken corn oil instead. (20)
9. Improves Brain Function
Regularly supplementing with fish oil as a natural remedy for depression has been associated with lower risks for depressive symptoms, due to both the higher intake of essential omega-3 fats and vitamin D. (21)
A 2007 study published in the Journal of Affective Disorders found that omega-3 fatty acids from cod liver oil improved the outcome of depression and anxiety in the general population. The Hordaland Health Study followed 21,835 adults living in Norway for two years and discovered that the prevalence of depressive symptoms in those who used cod liver oil daily was 2.5 percent, as compared to 3.8 percent in the rest of the population. They also found that the prevalence of high levels of depressive symptoms decreased with increasing duration (0–12 months) of cod liver oil use. (22)
There’s a strong connection between omega-3 intake or fish oil supplementation and prevention of cognitive decline, including Alzheimer’s disease. While no long-term human trials have successfully used fish oil to offset the development, there is tentative evidence that omega-3s it contains (as well as nutrients like vitamin B types, vitamins E, C and D) may serve to protect against cognitive decline and potentially decrease a person’s chance of developing this debilitating condition. (23, 24, 25)
10. Helps Maintain Bone Health
Vitamin D is important for building and maintaining strong bones. Studies show that women who live in cold, northerly latitudes and don’t get enough sunlight tend to produce less vitamin D, which increases the risk for bone turnover, bone loss, fractures and also obesity. (26)
Vitamin D supplementation, including from cod liver oil, has been associated with a significantly lower risk of bone fractures and might help naturally prevent osteoporosis from developing. (27)
11. Fights Ulcers
In laboratory settings, cod liver oil has shown benefits for improving gastric ulcer healing and reducing gastric antisecretory effects observed in rats. The oil also seems to produce gastric cytoprotective effects and causes a significant reduction in the development of stress and pain caused by gastric ulcers.
0 notes
Text
An Introduction To Vitamins
Vitamins are the organic compounds which are required in small quantities for normal growth, reproduction and maintenance of the human body. They are different from other organic food stuff because they do not go through the degradation processes to provide energy and do not enter the tissue structure. Several B vitamins act as a coenzyme in various metabolic reactions of the body. Vitamins are also different from hormones as they are not produced by the body and have to be taken through diet. They are classified on the basis of their biological and chemical activity.
There are 13 vitamins in total that a human body needs. They are as follows:
• Vitamin A - Retinol, Retinoic Acid, Retinal
• B vitamins:
◦ Vitamin B1 - Thiamine
◦ Vitamin B2 - Riboflavin
◦ Vitamin B3 - Niacin
◦ Vitamin B5 - Pantothenic acid
◦ Vitamin B6 - Pyridoxine
◦ Vitamin B7 - Biotin
◦ Vitamin B9 - Folate (Folic Acid)
◦ Vitamin B12 - Cobalamin
• Vitamin C - Ascorbic Acid
• Vitamin D - Calciferol, Calcitriol
• Vitamin E - Tocopherols
• Vitamin K - Quinone
They are classified based on the biological and chemical activity that they perform. They are classified as:
• Fat soluble (vitamins A, D, E and K)
• Water soluble (B vitamins, vitamin C and vitamin A in its beta-carotene form)
Fat soluble vitamins are hydrophobic and lipid soluble. They travel to the general body circulation through the lymphatics of small intestine absorbed in fat globules known as the chylomicrons. These are easily stored in the body.
Therefore, an increased intake of fat soluble vitamins predisposes a person to hypervitaminosis. Main storage sites are liver and fat tissue. Only a small quantity of these vitamins is required, but a deficiency can lead to certain diseases such as rickets.
Digestion of these vitamins is by the pancreatic enzymes. Absorption of fat-soluble vitamins requires adequate liver and pancreatic secretions. Too much of fat in the stool, i.e., steatorrhea can be caused by:
• Pancreatitis
• Pancreatic cancer
• Liver disease
• Crohn's disease
• Celiac disease
• Cystic fibrosis
• Gallstones
Common Causes of Steatorrhea
Basically, anything that reduces enzymes secretion from pancreas leads to steatorrhea.
Water-soluble vitamins cannot be stored in the body and a daily intake of these vitamins is necessary. They are hydrophilic and dissolve in the body. Any excess of these vitamins is simply excreted in urine. Therefore, toxicity is less common.
Vitamin A - Retinol, Retinoic Acid, Retinal
• Dietary source: butter, milk cheese, egg yolk, tomatoes, carrots, spinach, mangoes and corn
• Daily requirement: 400 µg for 2,000 calorie intake
• There is a higher requirement for growing children, pregnant women and in hepatic disease.
Functions
• Role in vision: rhodopsin has a role in night vision and retinal is a part of it.
• Role in epithelialization: it prevents keratinization of epithelial cells.
• Helps in bone and teeth formation
• Required for growth
• Plays a role in the metabolism of DNA and protein synthesis.
• Skin becomes dry and scaly in a deficiency of vitamin A.
Therapeutic uses
• Oral leukoplakia
• Acute myeloid leukemia
• Acne
Deficiency
• Nyctalopia (poor night vision)
• Dry and scaly skin
• Decreased endochondral bone formation and osteoblastic activity
Toxicity
• Nausea
• Headaches
• Irritability
• Teratogenicity
• Skin and hair problems
Vitamin E - Tocopherols
• Dietary source: wheat, sunflower, grape seed, canola, almond, margarine, cottonseed oil, spinach and corn oil
• Daily requirement: 10 mg for 2,000 calorie intake
• There is a higher requirement in pregnancy and lactation.
Functions
• It has antioxidant properties and contributes to the oxidation of free fatty acids.
• Involved in aerobic cellular respiration
• Combines with reactive oxygen species and works in conjugation with vitamin C
• More oxygen to RBCs
Therapeutic uses
• Nocturnal muscle cramps
• Atherosclerosis
• Fibrocystic breast disease
• Intermittent claudication
Deficiency
• Hemolytic anemia
• Muscular dystrophy
• Hepatic necrosis
• Immune impairment
Excessive Intake
Interferes with vitamin K and impacts clotting.
Vitamin K - Quinone
• Dietary source: fish, meat, broccoli, parsley, lettuce, kale, collards
• It is synthesized in the body by the normal intestinal flora. Newborns cannot produce vitamin K due to the sterile gut.
• Daily requirement: 80 µg for 2,000 calorie intake
Functions
• Promotes blood coagulation. Factor II, VII, IX and X require vitamin K.
• It is an important co-factor in oxidative phosphorylation.
• It enhances the capacity of calcium-binding proteins to deposit calcium in the concerned tissues.
• Deficiency: There is a rare chance of deficiency. Prolonged use of antibiotics and other drugs such as warfarin.
Vitamin D - Calciferol, Calcitriol
• Dietary source: fish liver oil, egg yolk, margarine, lard
• Daily requirement: 5 µg for 2,000 calorie intake. Sun exposure is very important for conversion into an active form.
• There is a higher requirement in infants, children, pregnant and lactating women.
Activation
7-dehydrocholesterol is converted into cholecalciferol (vitamin D3) in the presence of sunlight. It then combines with vitamin D2 (ergocalciferol) and gets converted to 25-hydroxyvitamin D3 (calcidiol) in the liver. It is then converted into 1,25-dihydroxyvitamin D3 (calcitriol) in the kidney. This is the active form.
Functions
• Absorption of calcium and phosphate from the intestines
• Enhanced mineralization of the bones
• Absorption of calcium and phosphate from the renal system
• Vitamin D lowers the pH in colon
• It has a role in increasing the citrate content of blood and bones
Rickets in Children
Deficiency
• Rickets in children
• Osteomalacia in adults
• Renal osteodystrophy
Toxicity
Hypervitaminosis D occurs if it is taken in large doses, 500 times that of the normal dose. Toxicity produces immediate and delayed effects.
• Immediate effects: anorexia, constipation, lassitude, thirst, polyuria, nausea, vomiting, diarrhea
• Delayed effects: metastatic calcification, urinary lithiasis
Vitamin B1 - Thiamine
• Dietary source: rice, peas, beans, whole white bread, bran, prunes, nuts, liver, meat, eggs and milk. It is also synthesized by the intestinal flora.
• Daily requirement: 1.4 mg. This is related to the carbohydrate content taken in diet, not the caloric value of food.
• Dietary requirements increase in case of hemorrhage, increased alcohol intake, prolonged illness and use of antibiotics, increased caloric burnout, fever, hyperthyroidism and pregnancy and lactation.
Functions
• It is a coenzyme in multiple metabolic reactions of the body, e.g., oxidative decarboxylation, trans-ketolation reaction in glucose metabolism, etc.
• Required for the activity of enzyme tryptophan pyrolyse (tryptophan metabolism)
Deficiency
Deficiency is rare due to wide distribution in foods. Beriberi is the result of thiamine deficiency. It has the following three types:
1. Dry Beriberi
◦ Symmetrical muscle wasting
◦ Peripheral neuropathy
◦ Confusion
◦ Difficulty in speech
◦ Involuntary eye movements
2. Wet Beriberi
◦ Signs and symptoms of dry Beriberi along with shortness of breath, rapid heart rate, edema in lower legs and cardiac failure.
3. Wernicke-Korsakoff ◦ Confusion
◦ Nystagmus
◦ Ataxia
◦ Confabulation
◦ Mammary body damage
◦ Aphasia
Vitamin B2 - Riboflavin
• Dietary source: yeasts, beans, peas, green vegetables, nuts, liver, kidney, milk, crab meat and eggs
• Daily requirement: 1.6 mg for 2,000 calorie intake
• There is a higher requirement in case of burns, acute illness, broad spectrum antibiotics use, pregnancy and lactation.
Functions
• Acts as a coenzyme in certain H-transfer reactions in metabolism
• Red blood cell production
Vitamin B3 - Niacin
• Dietary source: liver, meat, fish, kidney, legumes, coffee, tea and nuts
• It is also synthesized from tryptophan and in a limited amount by the gut flora.
• Daily requirement: 18 mg for 2,000 calorie intake
• There is a higher requirement in illness, infection, high corn or maize diet, pregnancy and lactation.
Functions
• Synthesis and formation of NAD+ and NADP which further act as co-enzymes in different metabolic reactions
• Nicotinamide formation
• Cholesterol production
• Nerve function
Deficiency
It can result from B3 or tryptophan. It results in a disease called Pellagra. Following are the clinical feature of Pellagra:
• Dermatitis
• Diarrhea
• Dementia
Therapeutic use
• To treat high cholesterol
Toxicity
• Skin irritation
• Liver damage
• Flushing of skin
Vitamin B5 - Pantothenic Acid
• Dietary source: kidney, yeast, egg yolk, liver, skimmed milk, chicken, royal jelly (richest source) and molasses
• Daily requirement: 6 mg for 2,000 calorie intake
• There is a higher requirement in case of burns, acute illness, severe injury, use of broad spectrum antibiotics, growing children, in convalescence, pregnancy and lactation.
Functions
• Formation of active acetate, acetyl Co-A
• Formation of succinyl Co-A which is involved in heme synthesis and degradation of ketone bodies
• Involved in oxidation of fatty acids
• Plays a role in adrenal cortical function
Deficiency
It is rarely seen due to wide distribution in food and its supply and synthesis by the gut flora but can lead to burning feet syndrome.
Vitamin B6 - Pyridoxine
• Dietary source: yeast, rice polishings, egg yolk, royal jelly and cereal grains
• Daily requirement: 2 mg for 2,000 calorie intake
• There is a higher requirement in the second half of pregnancy and in antituberculous treatment with isoniazid.
Functions
• Co-enzyme in transamination reaction and decarboxylation reactions
• Co-enzyme for deaminases and kynureninase
• Takes part in transsulfuration
• Co-enzyme for desulfhydrase
• Neurotransmitter production
• Protein metabolism (transaminases)
• Production of RBCs
Therapeutic uses
• Morning sickness of pregnancy
• Muscular dystrophies
• Hyperoxaluria
• Recurring oxalate stones of kidney
• Radiation sickness
Deficiency
The deficiency of vitamin B6 does not cause any disease but following clinical manifestations are seen:
• Decreased immunity
• Oxaluria
• Peripheral neuropathy
• Epileptiform convulsions in infants
• Anemia
Most common cause of deficiency is isoniazid treatment.
Vitamin B7 - Biotin
• Dietary source: liver, milk, kidney, milk products, molasses, legumes, vegetables and royal jelly. Bacterial flora of intestines can also synthesize this vitamin.
• Daily requirement: 30 µg for 2,000 calorie intake
• There is a higher requirement in pregnancy and lactation and long-term antibiotic therapy.
Functions
• It is a co-enzyme for carboxylases enzyme
• Involved in CO2 fixation reactions
• Fatty acid metabolism
• Cellular growth
• RBC production
Deficiency
There is no specific deficiency disease, but two conditions are related to biotin deficiency. These are:
• Genetic deficiency of holocarboxylase synthase
• Leiner's disease
Vitamin B9 - Folate (Folic Acid)
• Dietary source: liver, kidney, yeast, green leafy vegetables, cauliflower, spinach, wheat, meat and fish
• Daily requirement: 400 µg for 2,000 calorie intake
• There is a higher requirement in pregnancy and lactation, cancers and hemolytic anemia.
Functions
• Role in one-carbon metabolism reactions. One carbon moiety can be utilized to form several compounds.
• An important role in hematopoiesis
• Myelination
• DNA nucleotide production
Deficiency
• Macrocytic anemia
• Weakness
• Growth retardation
• Granulocytopenia
• Thrombocytopenia
• Megaloblastic anemia (decreased RBCs and hematocrit, increased homocysteine levels, increased MCV)
• Trimethoprim and methotrexate are the drugs that inhibit folate production.
Excessive intake
• Vitamin B12 deficiency
• Renal damage
Vitamin B12 - Cobalamin
• Dietary source: liver, fish, egg, meat and kidney. It is not present in plants.
• Daily requirement: 6 µg for 2,000 calorie intake
• There is a higher requirement in pregnancy, lactation and pernicious anemia.
Absorption
Stomach acid and digestive enzymes are necessary to remove vitamin B12 from proteins. It then binds to intrinsic factor released by parietal cells of the stomach. Intrinsic factor chaperones B12 to the terminal ileum where it is absorbed.
Functions
• Important for the conversion of homocysteine to methionine
• Nerve cell function
• RBCs production
• Fatty acid oxidation
Deficiency
It is due to the following factors:
• Pernicious anemia (autoimmune disease affecting parietal cells)
• Metformin use
• Crohn's disease
• Bowel resection
• Use of antacid and proton pump inhibitors
Deficiency of B12 leads to megaloblastic anaemia because folate cannot be recycled.
Nervous system issues arise because of methyl malanoic acid which causes demyelination. Peripheral numbness, spasticity and loss of proprioception are seen in the case of deficiency.
Vitamin C - Ascorbic Acid
• Dietary source: citrus fruits, papaya, pineapple, banana, strawberry, cabbage, cauliflower, green peas, tomatoes and potatoes. Amla has the highest concentration of vitamin C.
• Daily requirement: 75 mg for 2,000 calorie intake
• There is a higher requirement during infections.
Functions
Deficiency of vitamin C can produce Scurvy. One of the main clinical manifestation is swollen gums.
Image: "Deficiency of vitamin C can produce Scurvy. One of the main clinical manifestations is swollen gums."
• Involved in cellular oxidation-reduction reactions
• Important for collagen synthesis
• Required for the activity of osteoblasts and fibroblasts
• Cofactor in hydroxylation of tryptophan
• Tyrosine metabolism
• Helps in the maturation of RBCs along with folic acid
• Formation of tissue ferritin
• Iron absorption
• Important in electron transport system of the body.
• Actives arginase and papain enzymes and inhibits beta amylase and urease activity
• Co-enzyme for dopamine hydroxylase
• Carnitine formation in liver
• Fatty acids alpha-oxidation
• Plays important role during stress
Therapeutic uses
• Treatment of scurvy
• Methaemoglobinaemia
• Wound healing
• Treatment of infectious diseases
Deficiency
Low intake of vitamin C leads to a disease known as scurvy. It has the following clinical manifestations:
• Delayed wound healing
• Poor dentine formation
• Fragile capillaries
• Swollen and bleeding gums
• Poorly laid osteoid of bone
• Hypochromic microcytic anemia
• Painful swelling of joints and bones
• Loose teeth
• Bruising
Health and Medical Information online published by Dr Vivienne Balonwu.
Read more »
0 notes
Text
Fat Soluble Vitamins
Vitamins A, D, E, and K are the fat-soluble vitamins. Unlike water-soluble vitamins, these vitamins dissolve in fat and are stored in body tissues. Because they are stored, over time they can accumulate to dangerous levels and can lead to a condition called hypervitaminosis, meaning excess amounts of a vitamin in the body, if more than the recommended amount is taken.
“Too much vitamin A, D, or K can lead to increased levels that are unhealthy and can cause health consequences,” says Frechman. She adds that too much vitamin A can lead to birth defects, and too high levels of vitamin E may increase the risk of hemorrhaging. Excess vitamin K can lessen or reverse the effect of blood thinner medicines and prevent normal blood clotting.
•Vitamin A
Vitamin A has multiple functions: it is important for growth and development, for the maintenance of the immune system and good vision.
-Food containing Vitamin A
Beef liver, carrots, sweet potatoes, eggs, kale, spinach, broccoli, dairy
•Vitamin D
Your body must have vitamin D to absorb calcium and promote bone growth. Too little vitamin D results in soft bones in children (rickets) and fragile, misshapen bones in adults (osteomalacia). You also need vitamin D for other important body functions. Only vitamin produced in the body via the absorption of sun-rays. Also protects you from sunburn.
-Foods containing Vitamin D
Cheese, eggs, beef liver, fish
•Vitamin E
It is a fat soluble antioxidant, which can be obtained only as a food supplement. The most widely known health benefits of vitamin E are protection against toxins such as air pollution, premenstrual syndrome, eye disorders such as cataracts, neurological diseases such as Alzheimer’s disease, and diabetes.
-Foods containing Vitamin E Almonds, seeds, Swiss chards, plant oils, turnips, kale, spinach, seeds •Vitamin K Vitamin K is a fat-soluble vitamin that is most well known for the important role it plays in blood clotting. However,vitamin K is also absolutely essential to building strong bones, preventing heart disease, and crucial part of other bodily processes. -Foods containing Vitamin K Leafy greens, broccoli, prunes
0 notes