#samd software as a medical device
Text

Explore Nu10's innovative SAM-D, a cutting-edge samd software as a medical device designed to revolutionize healthcare. SAM-D offers advanced solutions for diagnostic accuracy, patient care, and medical data management, ensuring the future of healthcare is secure, efficient, and patient-centric. Elevate your medical practice with Nu10's SAM-D technology.
0 notes
Text
#Brazil's ANVISA#Medical Device#Software as Medical Devices (SaMDs)#ANVISA#LATAM#RDC#IFU#RDC 751#labeling#Regulatory Affairs#Medical Device Regulations
0 notes
Text
How Patient Care Is Being Improved by Technology Everyday
With technology becoming more integral to patient care in recent years, innovation in the medtech space is progressing at an unprecedented level. From streamlining long-held manual processes to creating entirely new diagnostic devices, new tech is cropping up all over the industry—resulting in a real-world impact on patients’ quality of life.
These technologies are helping companies to enable faster and more efficient engagement with patients, improve traditional devices, incorporate more software solutions into patient care, and even launch wholly new products. Further, these modern tools help medtech companies keep up with the rapid pace of innovation, by launching new products faster and quickly extending to new markets.
As hospitals face consolidation and CIOs are asked to increase staff productivity, tech companies that can support automation with solutions that are easy to onboard are getting heightened interest.
Streamlining quality processes for the better
In medtech especially, quality is the name of the game. The speed at which companies can address post-market or field issues is vital to success. After all, if a product meant to improve patient care isn’t performing the way it’s intended to, there could be tangible life-saving implications.
The secret to speedy post-market action is automating and integrating what was once a manual and disparate case-to-complaint and reportability process, by using a single enterprise platform.
For instance, imagine a company that wants to decrease their issue-to-resolution times. Their cases can be automatically tied to complaints and propagate common data, enabling faster investigations, reportability determination and electronic submissions.
The Covid-19 pandemic has forced life sciences companies to take a fresh look at how they do business. But as they reinvent their operations, companies often overlook a critical function: Their learning and development programs.
Also, their enterprise technology enables them to capture nonconformances with batch details which can quickly trigger ship holds, dispositions, and field actions.
And many of these major innovations trickle downstream to corrective and preventive actions (CAPA), Engineering Change Orders (ECOs), and business processes.
Having the ability to close the quality loop throughout the entire enterprise value chain can not only revolutionize a necessary process, it can deliver better patient experiences and outcomes.
Improving traditional therapies
While headlines about new strains of Covid-19 and Monkeypox continue to circulate, the most common chronic diseases continue to grow at alarming rates.
Technology is helping to both manage difficult symptoms of these diseases, and find proactive ways to prevent them before the onset.
Advances in technology are giving patients their independence back. Think of how much easier a diabetes patient’s day-to-day life would be by conducting therapies in their own home instead of adjusting their scheduling around trips to the hospital for recurring appointments. Not to mention the lowered cost.
Imagine a world where therapies for type 2 diabetes (T2D) have the potential to prevent or even reverse its course. In the U.S. alone approximately 30 million people are diagnosed with T2D and half of them have difficulty controlling it.
Leveraging software as a medical device (SaMD)
Smart technology is virtually inescapable today. We use software devices in many aspects of our lives—in our homes, in our cars, in our pockets. And our medical needs are no exception.
Software as a Medical Device (SaMD) is one of the fastest growing areas of medtech innovation, enabling tangible life-saving results from:
Remote patient monitoring Faster, more accurate disease diagnosis and treatment decisions, such as stroke and pulmonary issues
Streamlined workflows & coordination for care teams
Digital Therapeutics and Digital Companions
…and the list is increasing exponentially. SaMD has changed how patients manage their health and interact with healthcare professionals.
Ushering in the future of care
The pace of medtech innovation is accelerating so much it almost feels as if we’ve stepped into the future. There are groundbreaking companies focused on eradicating certain cancers that are offering comprehensive genomic profiling (CGP) which provides results from a simple blood draw in days. This allows oncologists to obtain information quicker and advise the right treatment to each of their cancer patients.
This is just one example of how patients at all stages of cancer can live longer and healthier through the power of blood tests and the data they unlock.
The secret weapon
The secret weapon is the ability to connect all business processes on a single platform solution. Keeping pace with top innovators requires a modern system to manage integrated change processes and cross-functional collaboration across the entire product and software lifecycle.
The critical and complex products that patients rely upon demand responsibility to ensure the best availability, quality, outcomes and uptime.
Technical Doctor's insight:
Contact Details :
[email protected] or 877-910-0004
www.technicaldr.com
4 notes
·
View notes
Text
The Evolution of Regulatory Affairs in the Digital Health Era
The Evolution of Regulatory Affairs in the Digital Health Era
In the rapidly evolving pharmaceutical industry, regulatory affairs have always been crucial to ensuring compliance, safety, and efficacy. However, with the advent of digital health technologies, the landscape of regulatory affairs is undergoing a significant transformation. This blog explores the evolution of regulatory practices in the digital health era, with a focus on the pharmaceutical sector, and the implications for professionals seeking to advance their careers through regulatory affairs courses.
The Role of Regulatory Affairs in Pharma
Regulatory affairs in pharma are the backbone of the industry, ensuring that products meet all legal and regulatory requirements before they reach the market. These professionals navigate a complex web of guidelines, from drug development to post-market surveillance, ensuring that patient safety is paramount. Traditionally, this role involved a significant amount of documentation, manual processes, and close interaction with regulatory bodies like the FDA, EMA, and other global entities.
The Impact of Digital Health on Regulatory Affairs
The digital health era, characterized by the rise of health apps, telemedicine, wearable devices, and artificial intelligence (AI), has introduced new challenges and opportunities for regulatory affairs. These technologies are rapidly changing how healthcare is delivered and consumed, necessitating a shift in regulatory strategies.
Emergence of New Regulations: The integration of digital technologies in healthcare has led to the creation of new regulations and guidelines. For instance, the FDA has issued guidance on software as a medical device (SaMD), addressing the unique challenges posed by AI and machine learning algorithms. Regulatory affairs professionals must now stay updated with these evolving guidelines to ensure compliance.
Data Privacy and Security: Digital health solutions often involve the collection and processing of vast amounts of patient data. Regulatory affairs professionals must ensure that these technologies comply with data privacy regulations, such as GDPR in Europe, while also safeguarding against cybersecurity threats.
Streamlined Regulatory Processes: The adoption of digital tools has also streamlined many regulatory processes. Electronic submissions, digital signatures, and automated workflows have reduced the time and resources required for regulatory compliance. This shift allows professionals to focus more on strategic decision-making and less on administrative tasks.
The Growing Need for Specialized Training
As the digital health era continues to evolve, the demand for regulatory affairs professionals with specialized knowledge in digital health and emerging technologies is on the rise. Enrolling in a Regulatory Affairs Course that covers these new areas can be a significant advantage for those looking to advance their careers in this field. These courses provide in-depth knowledge of the latest regulations, digital health technologies, and best practices for navigating this complex landscape.
Key Insights for the Future of Regulatory Affairs
Continuous Learning: The pace of change in digital health is unprecedented. Regulatory affairs professionals must commit to continuous learning and staying informed about new regulations, technologies, and industry trends.
Collaboration Across Disciplines: The intersection of digital health and regulatory affairs requires collaboration between tech experts, healthcare professionals, and regulatory bodies. This multidisciplinary approach is crucial for developing effective and compliant digital health solutions.
Adapting to Global Regulations: With the globalization of the pharmaceutical industry, understanding and adapting to the regulatory requirements of different regions is more important than ever. Regulatory affairs professionals must be equipped to navigate these global challenges.
Conclusion
The evolution of regulatory affairs in the digital health era is a dynamic and ongoing process. As the pharmaceutical industry continues to innovate, regulatory practices must adapt to ensure the safe and effective delivery of new technologies to patients. By embracing the changes brought about by digital health and investing in specialized training, regulatory affairs professionals can play a pivotal role in shaping the future of healthcare.
Read This Also - Robotic Process Automation (RPA) A New Approach for Process Automation
0 notes
Text
Gen-AI Regulations in Life Sciences: Are You Ready for the Future?

How prepared are you for the changes in generative AI regulations affecting life sciences? With rapid advancements in AI, especially in the generative sector, the life sciences industry is undergoing transformative changes. These innovations bring new opportunities and challenges, particularly regarding regulatory compliance. Understanding these regulations is crucial for stakeholders in the life sciences sector to ensure safety, effectiveness, and ethical standards.
Understanding FDA's Role
In the U.S., the FDA has taken significant steps to regulate AI in life sciences. Their guidelines focus on ensuring that AI technologies used in medical devices and healthcare applications are both safe and effective.
AI/ML-Based Software as a Medical Device (SaMD) Action Plan: The FDA’s action plan for AI and ML-based software includes a regulatory framework that supports the continuous improvement of AI algorithms. This plan includes guidance on pre-market submissions, modifications, and real-world performance monitoring to ensure the technology meets the necessary standards.
Good Machine Learning Practice (GMLP): The FDA emphasizes Good Machine Learning Practice (GMLP) to maintain the quality and reliability of AI technologies. GMLP principles such as transparency, reproducibility, and robustness are crucial for the successful deployment of AI systems in healthcare. Companies developing AI tools must adhere to these guidelines to ensure their products are compliant with FDA standards.
What’s Happening in Europe?
The European Union (EU) provides a complementary approach with its comprehensive regulatory framework focusing on the classification and management of AI systems.
The AI Act: The AI Act classifies AI systems used in life sciences, particularly those impacting patient health and safety, as high-risk. These systems must comply with strict requirements, including rigorous testing, transparency, and post-market surveillance. This ensures that high-risk AI systems undergo thorough evaluations to prevent potential harm to patients.
General Data Protection Regulation (GDPR): GDPR plays a vital role in regulating AI in the EU by ensuring that personal data used in AI systems is handled with utmost care, emphasizing data privacy and security. Compliance with GDPR is mandatory for AI systems processing personal data, making it a critical aspect of regulatory compliance in life sciences. Companies must implement robust data protection measures to comply with GDPR requirements.
WHO’s Global Guidelines
The World Health Organization (WHO) provides global guidelines for the ethical and safe development of AI technologies in healthcare.
Ethical Guidelines: The WHO’s ethical guidelines emphasize principles like transparency, accountability, and inclusiveness. These guidelines aim to ensure that AI technologies in healthcare are accessible and do not increase existing inequalities. This means AI tools should be designed to serve all populations fairly, without introducing biases that could lead to discrimination.
Safety and Effectiveness: The WHO stresses rigorous testing and validation of AI systems to ensure safety and effectiveness, including pre-market evaluations and continuous monitoring for any adverse events. This involves ongoing assessments to confirm that AI systems continue to perform safely and effectively throughout their use.
Tackling Key Challenges
Despite these robust regulatory frameworks, several challenges remain in implementing AI in life sciences.
Bias and Fairness: Ensuring that AI systems are free from bias is a significant challenge. Bias in AI can lead to unfair treatment and worsen health disparities. Regulatory bodies emphasize transparency and fairness in AI algorithms to address this issue. Companies need to implement measures to detect and mitigate biases in their AI systems.
Data Privacy and Security: With AI's increasing role in healthcare, protecting patient data has become critical. Compliance with data privacy regulations such as GDPR ensures that patient information is handled securely and ethically. Companies must invest in advanced security measures to protect sensitive data from breaches and unauthorized access.
Continuous Learning and Adaptation: AI technologies continuously evolve, and regulatory frameworks must adapt to these changes. Ensuring that AI systems remain safe and effective throughout their lifecycle, even as they learn and improve over time, poses unique challenges. This includes updating regulatory guidelines to accommodate new advancements in AI technology.
What Life Sciences Companies Can Do
Life sciences companies must take proactive steps to ensure compliance with generative AI regulations.
Establishing Compliance Programs: Developing robust compliance programs that align with regulatory guidelines is essential. This includes regular audits, risk assessments, and implementing Good Machine Learning Practice (GMLP) principles. Companies should create comprehensive compliance strategies to address all regulatory requirements effectively.
Collaboration with Regulatory Bodies: Engaging with regulatory bodies such as the FDA, EMA, and WHO can provide valuable insights and guidance, helping companies stay ahead of regulatory changes. Establishing open communication channels with regulators can facilitate better understanding and compliance with new guidelines.
Investing in Ethical AI: Investing in ethical AI practices is crucial. This includes ensuring transparency, accountability, and fairness in AI algorithms and addressing potential biases. Companies should prioritize ethical considerations in their AI development processes to build trust with users and regulators.
Continuous Monitoring and Improvement: Implementing mechanisms for continuous monitoring and improvement of AI systems is essential. This includes post-market surveillance, real-world performance monitoring, and updating AI models based on new data and feedback. Regular updates and improvements to AI systems can help maintain their effectiveness and compliance with evolving regulations.
Conclusion
Generative AI is transforming life sciences, offering immense potential for advancements in healthcare. However, staying compliant with evolving regulations is crucial. Companies need to adopt proactive measures and invest in ethical AI practices to leverage the full potential of these technologies while ensuring safety, effectiveness, and compliance.
At iTech GRC, utilizing IBM OpenPages, we specialize in providing compliance software for life sciences, life sciences risk management, GRC tools for life sciences, and life sciences audit software. As an IBM OpenPages Premier Partner, we help you ensure regulatory compliance in life sciences, making sure your AI initiatives meet all necessary standards. Partner with us to stay ahead in this rapidly evolving field and make the most of generative AI in life sciences.
0 notes
Text
Software that heals? #SaMD is here!
SaMD stands for Software as a Medical Device. Think mobile apps for chronic disease management, or AI-powered analysis tools for medical imaging.
Here's how SaMD can benefit your healthcare biz:
Improved patient outcomes: SaMDs can empower patients & give providers better data for personalized care.
Reduced costs: Remote monitoring & early intervention can prevent costly complications.
Enhanced patient engagement: SaMDs can keep patients involved in their health journey.
Thinking of incorporating SaMDs? Do your research on regulations & security. But the future of healthcare is lookin' bright!
0 notes
Text
Navigating EU Medical Device Regulations: A Comprehensive Guide to EU MDR and IVDR Compliance
Introduction The European Union's Medical Device Regulations (EU MDR) and In Vitro Diagnostic Regulations (IVDR) represent a significant overhaul of the regulatory framework governing medical devices and in vitro diagnostics within the EU. Compliance with these regulations is essential for manufacturers, distributors, and other stakeholders in the healthcare industry. In this comprehensive guide, we delve into the latest EU MDR and IVDR requirements, provide insightful charts, graphs, and tables, and offer problem-solving strategies to help businesses navigate through these complexities effectively.
Understanding EU MDR and IVDR The EU MDR and IVDR aim to enhance the safety and performance of medical devices and in vitro diagnostics while ensuring a transparent and harmonized regulatory framework across the European Union. Key provisions of these regulations include stricter requirements for clinical evidence, post-market surveillance, and traceability of devices. Below is a chart summarizing the key changes introduced by EU MDR and IVDR:
[Insert EU MDR and IVDR Chart Here]
Trends in EU Medical Device Regulations In addition to the regulatory changes brought about by EU MDR and IVDR, several trends are shaping the landscape of medical device regulations in Europe:
Focus on Patient Safety: EU MDR and IVDR place a strong emphasis on patient safety by requiring manufacturers to demonstrate the safety and performance of their devices through comprehensive clinical data and post-market surveillance.
Increased Scrutiny of Notified Bodies: Notified Bodies play a crucial role in the conformity assessment process for medical devices. With the implementation of EU MDR and IVDR, Notified Bodies are subject to stricter requirements and increased oversight to ensure consistent and rigorous evaluation of devices.
Digital Health Technologies: The rise of digital health technologies, such as software as a medical device (SaMD) and mobile health applications, presents new regulatory challenges and opportunities under EU MDR and IVDR.
EU MDR and IVDR Compliance Challenges While EU MDR and IVDR aim to strengthen the regulatory framework for medical devices and in vitro diagnostics, compliance with these regulations presents several challenges for stakeholders:
Transition Period: The transition period for EU MDR and IVDR implementation has been extended due to the COVID-19 pandemic, posing challenges for businesses in meeting the compliance deadlines.
Data Requirements: EU MDR and IVDR impose stringent requirements for clinical data and evidence, which may be particularly challenging for small and medium-sized enterprises with limited resources.
Supply Chain Management: Ensuring compliance throughout the supply chain, including contract manufacturers and suppliers, requires robust systems for traceability and quality management.
Problem-Solving Strategies for EU MDR and IVDR Compliance To address the challenges associated with EU MDR and IVDR compliance, stakeholders can implement the following problem-solving strategies:
Early Planning and Preparation: Start planning for compliance early and allocate sufficient resources to ensure a smooth transition to EU MDR and IVDR requirements.
Engage with Regulatory Experts: Seek guidance from regulatory experts and consultants who specialize in EU MDR and IVDR compliance to navigate complex regulatory requirements effectively.
Invest in Technology Solutions: Leverage technology solutions, such as electronic quality management systems and regulatory intelligence platforms, to streamline compliance processes and enhance efficiency.
Conclusion Compliance with EU MDR and IVDR is essential for ensuring the safety and efficacy of medical devices and in vitro diagnostics marketed within the European Union. By understanding the latest regulatory requirements, staying informed about emerging trends, and implementing effective problem-solving strategies, businesses can navigate the complexities of EU MDR and IVDR compliance successfully.
Here are top ESG Consulting Companies:
VECTRA International
KPMG
Deloitte
0 notes
Text
SaMD: Transforming Healthcare to Enhance Patient Outcomes

In today's rapidly evolving landscape of healthcare, technology plays an increasingly pivotal role in improving patient outcomes, enhancing diagnostics, and transforming treatment methodologies. One such technological innovation that has garnered significant attention is Software as a Medical Device (SaMD).
Software as a Medical Device refers to software intended to be used for medical purposes without being part of a hardware medical device. It is designed to perform medical functions, whether for diagnosis, prevention, monitoring, treatment, or alleviation of disease. It encompasses a broad spectrum of applications, ranging from mobile health apps to sophisticated diagnostic algorithms and telemedicine platforms. By leveraging the power of software, it has the potential to bridge geographical barriers, streamline healthcare delivery, and empower both patients and healthcare providers with actionable insights derived from data.
This blog explores the Software as a medical device solution, focusing on its applications in remote patient monitoring (RPM), wireless Holter monitoring, cognitive anxiety management, scoliosis screening, and gamified therapy for ADHD.
Market on the Rise: The Growing Impact of SaMD
The Software as a medical device market is expected to grow at a CAGR of 21.9% between 2020 and 2027. It has experienced rapid growth in recent years. It helps medical professionals predict, monitor, and diagnose diseases, allowing them to take preventive measures at the appropriate time. Because it does not require any hardware, it can use fast feedback loops for improvement. In addition, the advancement of technologies such as AI/ML, IoT, Telehealth, Cybersecurity, AR, and VR has accelerated the growth of software as a medical device.
SaMD's Transformative Impact on Various Healthcare Aspects :
The Software as a medical device market is experiencing phenomenal growth, fueled by its transformative impact on healthcare delivery. Here is how software as a medical device is transforming specific areas:
Remote patient monitoring :
Remote Patient Monitoring (RPM) transforms healthcare by allowing you to monitor vitals and physiological parameters remotely and in real-time. Software as a Medical Device is the backbone of this revolution, providing a secure and efficient platform to manage your patients' remote monitoring needs.
Real-time Tracking and Analytics: Software as a medical device platforms seamlessly collect vital signs and physiological data from wearables and biosensors. Advanced analytics engines analyze this data to identify trends, patterns, and potential health concerns. Customizable dashboards provide both you and the patient with clear visualizations of health data, facilitating informed decision-making.
Alerts and Notifications: It allows you to set personalized alerts for vital signs that exceed pre-defined thresholds. This enables prompt intervention and prevents potential complications. You also receive notifications of device malfunctions or errors, ensuring data integrity and system uptime.
Secure Communication: HIPAA-compliant Software as a medical device platforms prioritize patient data security. Multi-factor authentication and encryption ensure that only authorized personnel can access sensitive health information. Secure messaging features within the platform enable you to communicate with patients regarding treatment plans, medication adjustments, and any necessary follow-up actions.
Interoperability: The platforms designed for RPM seamlessly integrate with existing Electronic Health Records (EHR) systems. This eliminates the need for manual data entry, reduces errors, and ensures a complete view of the patient's medical history. They also facilitate data exchange with various wearable devices and biosensors, providing flexibility in choosing the most suitable monitoring tools for each patient's needs.
For more information click the below link :
https://nu10.co/samd-transforming-healthcare-to-enhance-patient-outcomes/
#techsolutions#healthcare#nu10#remote patient monitoring#wireless holter monitoring#machine learning#artificial intelligence#gaming therapy#adhd problems#startup#scoliosis#Scoliosis Screening
1 note
·
View note
Text
Cybersecurity for Medical Devices – FDA and EU MDR Perspective - OMC Medical Limited

FDA –Food and Drug Administration
The revolution in the digital sector has resulted in the Internet of Things (IoT), Software as a Medical Device (SaMD), Internet of Medical Things (IoMT) and other connected devices permeating the healthcare environment, both in hospital and home, has ended up with the possibility of cyberattacks and intrusions against the connected medical devices and the networks to which such a device is connected.
Most Medical devices are connected to the Internet, hospital networks, and other medical devices to provide health care and increase the ability of healthcare providers to treat patients.
These features also increase potential risks for Cybersecurity. Medical devices, like other computer systems, are vulnerable to security breaches, potentially impacting the safety and effectiveness of the device.
Since 2005, the FDA has tried to accomplish and enhance medical device cybersecurity, and the latest FDA effort is to create draft guidance that expects security throughout the total product life cycle (TPLC).
Another effort is the Protecting and Transforming Cyber Health Care Act of 2022 (PATCH Act of 2022),which, if passed, would revise the existing Federal Food, Drug, and Cosmetic Act.
The FDA guidance establishes six broad expectations on the Secure Product Development Framework (SPDF), which covers all aspects of a product’s life cycle, for the development, release, support, and decommission and satisfy Quality System Regulations (QSR) under 21 CFR Part 820:
Cybersecurity is a fundamental part of device safety and the QSR
Security by design
Transparency
Security risk management
Security architecture
Testing/objective evidence
The FDA draft guidance, under QSR, also declares that verification and validation activities by the medical device manufacturers shall include sufficient testing performed on the Cybersecurity of the system, which validates their inputs and outputs.
Further, the FDA summarizes that cybersecurity controls require testing beyond standard software verification and validation to demonstrate that the device has a good assurance of safety and effectiveness.
The following cybersecurity testing and corresponding objective evidence would be considered as the minimum support for a premarket submission:
Security requirements
Evidence of their boundary analysis creates a rationale for their boundary assumptions.
Threat mitigation
Evidence that all the design input security requirements were implemented successfully
Evidence of testing their threat models that demonstrates effective risk control measures provided in the system and use case
Evidence of the adequacy of risk control.
Vulnerability testing – Evidence on the testing of malformed
Abuse case and unexpected inputs
Vulnerability chaining
Closed box testing of known vulnerability scanning
Software composition analysis of binary executable files
Static and dynamic code analysis
Penetration testing– Identify and characterize security-related issues that discover security vulnerabilities in the product.
Regular interval cybersecurity testing – It is performed at regular intervals to identify the potential vulnerabilities before exploitation
THE FDA’S ROLE IN MEDICAL DEVICE CYBERSECURITY
Dispelling Myths and Understanding
Download the Fact Sheet (PDF – 175kb)
04/07/2022 Draft Guidance: Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions
This draft guidance replaces the 2018 draft version, which emphasizes the importance of understanding that all medical devices are designed securely, enabling new cybersecurity risks to be mitigated throughout the Total Product Life Cycle, and it elaborates the outline of the FDA’s recommendations more clearly for premarket submission to address cybersecurity concerns.
03/08/2022 Cybersecurity Alert: Vulnerabilities identified in medical device software components: PTC Axeda agent and Axeda Desktop Server
The PTC Axeda agent and Axeda Desktop Server are cloud-based technologies that allow people to securely view and operate the same desktop through the Internet. The Axeda agent and its desktop server are owned by the computer software company PTC.
The FDA alerts all medical device users and manufacturers about a cybersecurity vulnerability identified for the Axeda agent and Axeda Desktop Server.
The agent and desktop server of Axeda are used in many medical devices across several medical device manufacturers, and all the versions of the Axeda agent and Axeda Desktop Server are affected.
On the 8th of March, 2022, the Cybersecurity and Infrastructure Security Agency (CISA) published an advisory, ICSA-22-067-01, on these vulnerabilities.
Any successful exploitation of this vulnerability could allow an unauthorized attacker to take complete control of the host operating system, resulting in full system access, remote code execution, reading or changing the configuration, system file access, accessing log information, and other denial condition.
These vulnerabilities may result in changes to the functions of the medical device and impact the availability of the remote support functionality.
As a result, PTC recommends that affected manufacturers:
To upgrade Axeda agent Version 6.9.2 build 1049 or 6.9.3 build 1051 while running older versions of the Axeda agent.
Also, to configure the Axeda agent and Axeda Desktop Server to listen only on the local host interface 127.0.0.1.
Then, Provide a unique password in the AxedaDesktop.ini file for each and every unit.
Remove the installation file.
Make sure to delete the ERemoteServer file from the host device.
Never use ERemoteServer in production.
When running the Windows operating system, first configure Localhost communications (127.0.0.1) between ERemoteServer and Axeda Builder.
When running in Windows or Linux, only allow connections to ERemoteServer from trusted hosts and block all others.
Configure the Axeda agent for the authentication information required to log in to the Axeda Deployment Utility.
So, Cybersecurity is one of the crucial aspects of today’s fast pacing digital world. The threats caused by Cybersecurity, especially on medical devices, are hard to deny. It is important to learn how to defend themselves from them and create a safe environment for the usage of medical devices.
EU MDR and IVDR

The NIS Directive also provides for legal measures to increase the overall level of Cybersecurity in the EU.
GDPR (General Data Protection Regulation) helps the manufacturers of medical devices in regulating, protecting and processing personal data by the individual, company or organization that relates to the EU.
The EU Cybersecurity Act certifies Cybersecurity for ICT products, services, and processes.
According to the Cybersecurity Act, manufacturers are required to demonstrate state of art in the design, development, and improvement of their medical devices throughout their life cycle.

The MDCG has proposed some key philosophies of the staged security concept strategy (“defense in depth strategy”) as follows:
Security management
Specification of security requirements
Security by design
Secure implementation
Management of security-related issues
Security update management
Security risk management
The list of possible IT security requirements for the operating environment according to MDCG:
Compliance with national and EU regulations (e.g., GDPR).
Ensuring appropriate security controls are in place
Ensuring the physical security of the medical device through security measures
Ensure control and security of network traffic through proper measures
Life Cycle Aspects
Security measures specific to their workstations connected to the medical device.
Security vulnerabilities related to the device hardware/software and third-party hardware/software used with the medical device.
During the life of the devices, the manufacturer should implement the process to collect post-market information about the security of the device.

Based on the EU Cybersecurity Act, the manufacturer must provide the following information to the user of the medical device:
Specifications of the operating system
IT security risk assessment information.
Provisions for ensuring the integrity of software updates and security patches
Product installation
Security configuration options
Initial configuration guidelines
Step-by-step instructions for deploying security updates
Description of the backup and restore functions for data and configuration settings
Procedures for using all the medical devices in failsafe mode
The manufacturers are required to establish a post-market surveillance (PMS) system and actively keep these PMSs (Post Market Surveillance) up to date. Medical device cybersecurity requirements should be part of this PMS system.
Depending on the class of medical device, a PMS report or PSUR report will be generated, which concludes the analysis of all data from the market.
Originally Published at: https://omcmedical.com/cybersecurity-for-medical-devices-fda-and-eu-mdr/
0 notes
Text
Crafting Healthcare Excellence with SaMD

In the rapidly advancing digital healthcare realm, one of the most exciting developments is the rise of Software as a Medical Device (SaMD). This innovation is revolutionizing patient care for millions worldwide, offering a more convenient and cost-effective approach to healthcare.
SaMD, as defined by the International Medical Device Regulators Forum (IMDRF), refers to software intended for medical purposes that operates on mobile phones, tablets, and computers. They aid in diagnosis, screening, monitoring, and treating various physiological conditions, covering a wide range of patient needs, from diabetes management to cloud-based solutions offering crucial insights to clinicians.
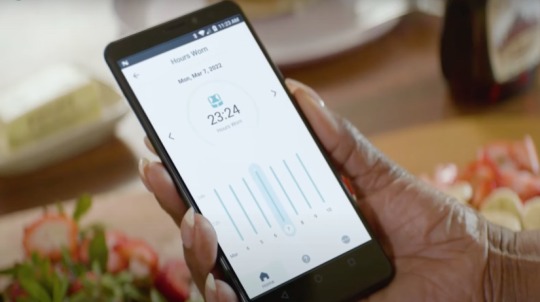
With time, there has been a significant shift in medical device functionality. SaMD applications have benefited from advancements in software engineering, enabling the integration of third-party hardware and software. This integration optimizes patient care, making it more efficient and cost-effective. Additionally, SaMD applications adhere to rigorous regulatory standards, ensuring safety and efficacy.
SaMD applications often take the form of patient engagement applications or patient companion apps that serve as the patient’s primary link and point of interaction with the medical device system. Patient companion apps have become integral in managing chronic health conditions and supporting remote patients.
For instance, diabetic patients can now effectively manage their glucose levels through integrated systems that include wearable insulin pumps, Continuous Glucose Monitors and Cloud Analytics, all accessible through a patient companion application.
As the world embraces digital healthcare, SaMD patient companion apps are increasingly becoming indispensable for companies striving to achieve their clinical and operational goals and ensure a future where healthcare is patient-centric and accessible.
Discover how GlobalLogic, a Hitachi group company, utilizes its digital engineering expertise to help Medtech companies businesses in creating Software-as-a-Medical Device (SaMD) apps
https://social-innovation.hitachi/en-us/think-ahead/healthcare/transforming-healthcare-with-software-as-a-medical-device-patient-companion-app/
#patient engagement app#patient care#remote patient monitoring#advanced healthcare analytics#healthcare innovation#cardiac care#cardiac recovery#patient app#patient experience
0 notes
Text
SaMD (Software as a Medical Device): nu10
At nu10, we are leaders in developing SaMD (Software as a Medical Device) solutions that revolutionize the healthcare industry. Our team of experts is committed to creating cutting-edge software applications that meet the highest regulatory standards and provide accurate, reliable, and secure medical insights. With a focus on innovation and patient-centricity, our SaMD solutions leverage advanced technologies such as artificial intelligence and machine learning to improve diagnosis, treatment, and patient outcomes. Join us at nu10 to transform healthcare with SaMD solutions that prioritize safety, efficiency, and effectiveness, making a positive impact on the lives of patients and healthcare providers.
0 notes
Text
Samsung Medications New Tracking on Health App!

Samsung Medications Tracking Feature
Samsung Electronics announced a new Medications tracking feature for the Samsung Health app to help users manage their health. Users can easily track their prescription and over-the-counter samsung medications and get important information and tips with the new feature. It can help regular medication users and general health supplement users.
“Samsung Health connects devices, services, and people to help people better understand and manage their health,” said Hon Pak, Vice President and Head of Digital Health Team, MX Business at Samsung Electronics. “They believe the new Samsung Medications tracking feature will make medication management easier, improve adherence, and improve overall health.”
Samsung Health’s Medications feature provides general descriptions and side effects for Samsung medications entered into the app. Drug-to-drug interactions and adverse reactions from food and alcohol are also listed. Samsung Health will warn users taking Simvastatin that grapefruit juice can cause serious side effects. Users can even log the shape and colour of their pills to easily distinguish them. To avoid confusion, add dosage, consumption time, and other details.
Users can set reminders to take and refill their medications. Samsung Health sends reminders ranging from “gentle” to “strong” depending on how urgent or important a prescription is, and these alerts are customised to the user. For important Samsung medications, users can set a “strong” reminder that displays a full-screen smartphone alert with a long tone. Vitamin supplements have a discreet pop-up reminder. Galaxy Watch users will receive wrist reminders to stay on top of their medication schedules even when away from their phones.
The Samsung Health app offers advanced sleep management, mindfulness, and irregular heart rhythm detection. Samsung Health’s Medications tracking feature creates a holistic wellness experience that helps users manage their medications and live healthier.
Samsung Health app updates later this week will add Medications tracking to the U.S
Samsung Health Medications helps users organise and schedule their Samsung medications. Information is evidence-based and licensed from Elsevier, a global leader in information and analytics.
Android 8.0 and Samsung Health app 6.26 required. Devices may have different feature availability.
Sleep features are for fitness and wellness only. Measurements are for personal use only. Seek medical advice.
IHRN is only available in certain markets. Available on Wear OS 4.0+ devices. Notifications are not intended to be sent for every episode of irregular rhythm suggestive of AFib, and silence does not indicate no disease process. Users with other arrhythmia’s should not use it. The Samsung Health Monitor app supports them. Market and device availability may vary. For market restrictions in obtaining approval/registration as a Software as a Medical Device (SaMD), it only works on watches and smartphones purchased in markets with service (although service may be limited when users travel to non-service markets). Only people 22 and older can use this app.
Read more on Govindhtech.com
0 notes
Text

A Brief Insight Into the Ever-Evolving Nature of SaMD Compliances and Their Significance in Europe
🏥💻 Dive into the complexities of Software as a Medical Device (SaMD) and European data laws. From patient privacy to regulatory nuances, discover key insights for success. 🚀🔒
Read the full blog here: https://wi4.org/news/insights/how-to-bring-samd-medtech-to-european-markets-a-patient-data-and-eu-compliance-perspective/
SaMD #DataPrivacy #InvestmentInsights #HealthTech #Wi4 #vineetagrawal
1 note
·
View note
Link
Regulatory Affairs Internship OPPORTUNITY FOR LIFESCIENCES STUDENTS at OMC Medical Limited About the Company (OMC Medical Limited) OMC Medical Limited specializes in providing regulatory affairs services to global medical device companies. With over a decade of experience, we excel in various regulatory domains, including UK MHRA Registration, European Authorised Representative services, Brexit Customs Clearance, and more. Our team is committed to ensuring compliance with medical device regulations worldwide, offering services such as global product registrations, document translation, and EU-MDR labeling for software as a medical device (SaMD). Company Vacancies List Position Title: Regulatory Affairs Intern (Full-Time) Company Name: OMC Medical Limited Salary: Commensurate with experience Detailed Job Description: Full-time remote Regulatory Affairs Internship role assisting in medical device and combination product registrations, submissions, and licensing activities globally. Duties include preparing regulatory applications, supporting regulatory tracking activities, interpreting regulations, and engaging in risk assessments. Training and mentorship provided in healthcare regulatory affairs. Role: Regulatory Affairs Intern Industry Type: Medical Device Regulatory Affairs Department: Regulatory Affairs Employment Type: Full-Time, Remote Role Category: Internship Educational Background: Bachelor's or graduate degree in regulatory affairs, bioscience, or related field preferred. Previous internship experience and understanding of medical device regulations are advantageous. Key Skills: Regulatory affairs, attention to detail, analytical skills, written and verbal communication, organizational skills, proficiency in Microsoft Office Products, familiarity with FDA, EU MDR, or other regulatory bodies preferred. How to Apply: Apply via LinkedIn Apply Via LinkedIn [caption id="attachment_58210" align="aligncenter" width="1200"] OMC Medical Limited's Lifesciences Internship Opportunity[/caption]
0 notes
Text
Demystifying Medical Device Software Validation: Ensuring Safety and Compliance
Briefly introduce the importance of medical device software validation.
Explain that the blog post will provide an overview of the key concepts, processes, and regulations related to medical device software validation.
I. Understanding Medical Device Software
Define what medical device software is and its significance in healthcare.
Discuss the different types of medical device software (e.g., embedded, standalone, mobile apps).
Emphasize the increasing reliance on software in modern medical devices.
II. Why Software Validation Matters
Explain the critical role of software validation in ensuring patient safety.
Discuss the potential risks and consequences of inadequate software validation.
Highlight real-world examples of medical device software failures and their implications.
III. Regulatory Framework
Introduce the regulatory bodies and standards governing medical device software validation (e.g., FDA, ISO 13485, IEC 62304).
Explain the classification of medical devices and how it affects validation requirements.
Provide an overview of the FDA's Software as a Medical Device (SaMD) guidance.
IV. The Software Validation Process
Outline the key steps in the software validation process:
Requirements analysis and specification
Risk assessment and management
Design and development
Testing and verification
Validation and documentation
Emphasize the importance of traceability and documentation.
V. Common Challenges and Solutions
Discuss common challenges in medical device software validation (e.g., changing requirements, evolving technology).
Provide practical solutions and best practices for overcoming these challenges.
VI. Case Studies
Present real-world case studies of successful medical device software validation projects.
Highlight the benefits of proper validation, including improved patient outcomes and reduced liability.
VII. Future Trends in Medical Device Software Validation
Explore emerging trends and technologies in medical device software validation (e.g., artificial intelligence, machine learning).
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in design and development of highly sophisticated implantable devices at industry leading companies, with direct expertise in software V&V. This team, with the support of IZiel’s regulatory and clinical experts, are decidedly equipped to handle complex software validation activities for medical device manufacturers. Integrating risk assessments into the validation lifecycle and documenting the basis for what was done also provides a level of assurance to management and regulatory authorities that the system was properly defined, designed, built, tested, operated, and maintained.
0 notes