#titration curve
Text
I miss art tbh. Like I’m doing art but it’s for money or it’s for things I don’t care about (inktober. I must finish though). I got high last night, listened to dreamsong on repeat and painted some Klimt studies, effervescent. I need more of that. Instead I have lab D;
#I just had a midterm and they slapped a whole unit onto us. a very stressful lab.#and 3 hours to preform and calculate titration curves. bitch???#I wish I got accommodations this semester. math is not my friend
26 notes
·
View notes
Text
For the titration curve in figure 11.17, the initial pH will be:
pH = -log[H3O+]
= -log(0.200)
= 0.70

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#logarithmic curve#logarithm#titration#acidic#alkaline#sodium hydroxide#hydronium
2 notes
·
View notes
Note
quastion. if you were a high schooler looking to take the least stressful lab science would you taking physics or chemistry
i think this is a simpler question of which do you enjoy more? for me i would choose physics over chemistry any day but that’s because i hate chemistry and so all chem labs i took were boring and stressful, and i love physics so the labs were fun and interesting
however if both subjects are equally uninteresting to you, then i do recommend physics! maybe i'm biased, but i think even personal interest aside, my high school chem labs were definitely more involved and complicated than my high school physics labs. physics experiments in lower level classes such as high school (and even introductory college physics to some extent) are pretty simple to set up and conduct, the main understanding comes from doing the math afterwards to get any sort of result out of it, whereas chem was more like, actually mixing stuff and then observing
not only were physics labs easier, simpler, and more engaging, but each lab also was unique and distinct from each other. i really can only recall the same basic setup of like, mixing stuff together as the idea behind every single chem lab i did. the exact way you mixed them together differed of course but that was the main idea
meanwhile some examples of physics labs i recall from high school include:
projectile motion: most likely you will roll a marble down a ramp off the edge of the lab table, measure the horizontal and vertical distances it traveled, maybe time it also or use a photogate to measure the velocity, or something, and then use the kinematic equations to find any missing variables, and then through all that you will probably be to told to find the value of g, what is known as the acceleration due to gravity, aka the rate at which things fall.
circular motion: you may be using a FLYING PIG to demonstrate circular motion!!! figuring out the tension in the string, the idea of centripetal force, centripetal acceleration, rates of revolution, etc.
harmonic motion: push some slinkies around, demonstrate hooke's law and spring force, calculation of frequency and oscillation, maybe observing resonant frequencies and resonant modes
standing waves: using some sort of low tech version of a standing wave generator to observe, well, standing waves. the high school version of this lab i believe was very surface level and was mostly just drawing how different standing waves looked, counting the nodes and antinodes, and predicting it for different frequencies. i think the teacher even got us a giant rope and we had to recreate the lower frequency standing waves together as a class by just oscillating it ourselves
all around, in my experience at least, high school physics labs are so much more involved and engaging than chem ever was. and while the math involved in the physics class was more daunting than chem, it was such a fun and interactive class. and again i may be biased but i think, if both chem and physics are uninteresting to you but you need to choose one anyway, i think having a basic background in physics is a lot more useful and goes a lot further than a basic background in chem does. i truly believe that knowing stuff about the kinematic equations, circular motion, free body diagrams, harmonic motion, etc etc will enrich your life further and change the way you see the world around you. high school physics will not make you an expert but it can certainly make observing patterns in life and how the natural world operates a lot more fun and exciting
#sorry i am INCAPABLE of ever giving a brief response when it comes to physics stuff lol#ask#Anonymous#literally the only chem lab i remember is titration and i cant even tell you what titration is anymore#all i know is that it was long and frustrating and the word makes me shudder years later#like some acid and base type shit i really could not tell you#but by far my most vivid memory of any science class i took in my 4 years of high school .was the fucking flying pig in physics#i will say i did get far better grades in chem. but despite that i also felt like i understood way fucking less as i was going through it#which made it its own brand of stressful#my grades in physics were my worst in high school but even despite that i still felt like i was retaining and learning so much more#and despite the complexity of the subjects increasing throughout the school year my grades actually increased as well#its truly just like a rough learning curve at first adjusting to the class compared to previous science classes#and if your physics class is like how mine was and you all get bad grades then a good teacher will offer opportunities#for you to earn points back and that also means that concepts get reinforced in your head#so despite getting a 60 on an exam he will make us basically redo the exam and relearn the concepts#and earn an 80 on it once we're done with exam corrections#so you will get a better grade in the end AND actually LEARN from doing badly on the exam#so what im saying here is: it also depends on the teacher. so if you get a bad teacher who just gives you a bad grade and moves on#then like. the class will not be enjoyable. and will be stressful. but if you have a good teacher then it should be fine#and you WILL get bad grades. you just will. but dont sweat it because literally everyone will always get bad grades#and a good teacher will give you the opportunity to make up for those bad grades. bc its unfair to punish you for it.#since everyone always gets bad grades. lol
2 notes
·
View notes
Text
I swear to god chem pract made me nearly kill myself I couldn't figure out q2 and I was freaking out and then I came up with the answer at 8pm and almost died. (forgot to add the 0.7 and was wondering why I had 9 grams of copper) and then I spilled the stupid hcl on my hand and now I have some weird burn thing. also forgot to add boiling stones and water bath for the propanoic acid
#lia vents#prelims made too confident :/#everyone say ty to me for bringing down the bell curve#also did anyone use the filter paper and funnel#titration my beloved I shd fave treasured you more ���💔💔💔#don't reblog. I dw to know how well y'all did.
0 notes
Text
𝐋𝐚𝐭𝐞 𝐍𝐢𝐠𝐡𝐭 — Kuroo Tetsurou

+ established friendship, college au, lab partners, no warnings | 1k words

Kuroo was only certain about a few things in his life.
The first thing was he absolutely hated black coffee. No matter how many times he’d tried it, or pretended to like it to impress you, they never amounted to anything.
The second thing was you never failed to make his heart beat just a little bit faster. Even as he watched you run through the titration wrong for the second time.
“I don’t think that’s how you’re supposed to do it.”
“It’s fine,” You started, obviously focused on the experiment in front of you. “It’s only the second trial, we’ve got a couple more.”
“We’re supposed to get the endpoints within a certain range, or did you forget?” He leaned against the counter once more as he stared at you fidgeting with the stopper. “We’re not even within the thousandths yet-”
“It’s fine.” You said once more, finally looking up at him to stop him from rambling on about whatever series of numbers that were about to come out of his mouth, and again Kuroo felt his heart skip just slightly.
“Sure.” He mumbled, looking away from you quickly. The last thing he wanted to do was annoy you, it was obvious from the grade you held as lab partners that both of you knew what you were doing.
After a long almost awkward silence, you sighed loudly, stepping away from the bench with your hands up.
“You do it.”
“You sure?”
You only nodded as he happily switched places with you, starting to work on the titration immediately.
“Don’t look too happy.” You rolled your eyes before laughing slightly. “I didn’t have time this morning to read the lab manual, don’t let it get to your head. I just want to pass.”
“Yeah, sure.” His lips curved into an annoying grin as he glanced at you between the steps of the lab. “I won’t celebrate too much when my numbers are better than yours.”
“Oh, whatever. Do you want me to do it again? I can.” You started to move towards the setup again, laughing when his eyes widened as he shook his head quickly. “Okay, then shut it.”
You should’ve known the silence wouldn’t last too long, after just two minutes he looked at you again with yet another smirk.
“So, why were you late this morning?”
“Don’t you have another trial to do?” You rolled your eyes again, glancing at the lab manual for backup.
“I have to wait for five minutes on this one still.” He responded smugly, pointing to step eight on the paper where his words were written down on it. “So... you going to tell me?”
“Is it any of your business?”
You looked away from him grimacing slightly at the reminder of last night. Most of the latter part was a blur but you remember texting him. What it was that the two of you talked about, you had no idea. Every time you looked at your messages you felt nauseous again.
“Hmm, yeah I’d say it is.” Kuroo said after checking on the time left, “Your texts were pretty interesting. What was it that you said? That I-”
“Shut up.” You all but sneered in his direction, hoping he wouldn’t talk about it anymore, or at least until the hangover plaguing your head would go away.
He laughed at your reaction, and your heart jumped at the sound, it hadn’t been the first time in this lab but it still nearly stopped you in your tracks.
Ever since last night, you hadn’t been able to look him in the eye but you couldn’t tell why. You had a pretty good idea of what the reason was, but the sinking of your stomach every time you thought about it stopped you.
“Oh, I see. You don’t remember talking to me.” Kuroo said after what seemed like forever of him staring at you. “Is that it?”
“Yeah..” You looked away from him once more, pretending to go over the data you’d written down.
“C’mon, you don’t remember anything?”
You shook your head in response, trying to ignore the pulsing headache growing behind your eyes. It was your own fault for going out with your roommates on a Wednesday.
“Hmm, okay.”
He quieted down after that, finishing the rest of the lab quickly which you were very grateful for.
It was a little odd though, seeing how quiet he was compared to how talkative he normally was. You couldn’t manage to bring last night up again, head still swirling a little as you tried to think about it.
As the both of you cleaned up the lab equipment you couldn’t fight the way your heart started to flutter fast when you were close to him. Comparing data nearly killed you, your shoulder brushing his just barely completely distracted you.
Though, despite the way you dreaded talking about last night with him, you felt a bit of disappointment growing inside of you as you finally packed up your stuff for the day. This being your only class today, you weren’t sure if you’d see him after it.
“I’ll see you tonight, right?” Kuroo asked stopping at the end of the hall, he always stopped there for a moment when you walked out of the class together. He went right when you had to continue straight.
“What?” You stared at him, mouth gaping slightly as you wracked your brain for plans.
“You asked me out last night.” He grinned, obviously enjoying your confusion. “Said to meet you at 7 downtown.”
“You’re kidding.”
“Nope.” He laughed this time, nudging your shoulder with his, “See you tonight. 7 p.m. downtown, don’t be late.”
All you could do was stare at him as he headed down the right hall towards the exit.
“Ass..” You whispered under your breath despite the smile lacing your lips matching your racing heart now.
“Oh, and check our messages!” Kuroo called out, turning around to wave at you before disappearing into the other half of the building.

Note From Em: I am yet again drinkin wine sooo, disregard mistakes :) <3

#haikyuu fluff#kuroo fluff#haikyuu imagine#kuroo x reader#kuroo tetsurou x reader#haikyuu x reader#haikyuu drabble#haikyuu x y/n#kuroo#kurroo tetsurou#kuroo x y/n#kuroo x you#kuroo tetsurou x you#haikyu!!#haikyuu#haikyu!! fluff#haikyu!! x reader#haikyu!! x y/n#haikyu!! imagine#Kuroo tetsurou oneshot#haikyuu oneshot#kuroo oneshot#kuroo x gn reader
191 notes
·
View notes
Note
hi vanya 😭😭 idk if you guys have it on the west coast, but we had the solar eclipse today on east coast and it happened during school so we went outside to the amphitheater (idk if this is normal thing or a rich, pretentious priv school thing) and tell me why when i take off the little light blocking glasses (everything looks black except the sun) i see this guy staring at me?? i was fully convinced we were all just looking up at the sun but ok!! im also rly sad bc i'm failing chem rn and my gpa is tanking 😭 i just dont understand acid and bases!! what are even titration curves i have no idea
please keep us updated on the lion dancer guy!! im so invested omg
-key
HALLOOO
we did have the solar eclipse today too! except my school is full of nerds so no one cared abt it.... they had class to attend... this is me included, i was busy doing work to care. i think all schools have ampitheaters too, my district just calls it the center of the arts or the performing arts center
i think he wants you girl........ its giving "the fireworks are beautiful but you are more beautiful"
also dont even worry i hate chem too, i cannot stand that class
IM HAVING MY FRIEND PUT IN A GOOD WORD FOR ME, like im gonna make her glaze me every single time he's around so that he thinks about me more
wish me luck i miss lion dancer already 😞
4 notes
·
View notes
Text
when he says damn girl i love your curves but you're not sure if he means heating-cooling or titration
2 notes
·
View notes
Text
do any chemists here want to help me figure out how to determine the concentration of hcl in an mixture of h3po4 and hcl given the experimental Ka values of a naoh and h3po4 titration i have titration curves and everything
#things that might not make sense to anyone but me but i’m suffering in chem lab about this#they’re literally making us do labs on material that are 2 weeks ahead content wise#misc
9 notes
·
View notes
Text
Way too fucking pleased with how good my acid-base titration curves look for this lab
15 notes
·
View notes
Text
I'm gonna vent for a hot minute about work frustrations. I just need to shout into the void.
Feel more than free to ignore this.
🙃🙃🙃
So.
I got a new job. I am leaving my current job on May 12. I have, for the last 6 years, been the primary analyst for about a dozen titrations, most of which were only run a couple times a year, but 5 of them are high volume tests.
I have been telling my manager, for Years at this point, that it is important we get back ups trained in my big methods. For times when I go on vacation, take a work trip, get sick, or find a new job. My manager continued to drag her feet and hem and haw about who should be trained on what. A few times I got someone trained, and then they left a month later. And more than once the available people were just bumblefucking incompetent and couldn't grasp the goddamned basic Follow the Recpie steps of the methods.
It is now officially Panic Train crunch time. I have 5 full working days left here before I leave forever.
I have spent every goddamned day this week, and a good portion of last week trying to train one (1) person on a single basic ass test method.
She is:
1. Already technically trained on the method, this is a refresher for something she hasn't done in 7 years
2. Someone who has been in our work group longer than I have and has, along with everyone else, had to use the same goddamned electronic notebooking program we all have been using for the last 2 years.
You will NEVER GUESS what she doesn't know how to fucking use! The gmp notebooking program that she has been using for all of her other work!
I will give her all of the allowances for refreshing herself on a method she hasn't done in almost a decade. I will give her all of the allowances for the method-specific quirks of the notebooking software.
We have, over the last 4 months, made an effort to regularly shadow-train-supervised run on this method. She should get this by now. We've done intensive training, all hands on for her, over the last week. And I am STILL having to coach her on software basics and it it driving me absolutely Bananas.
And I'm getting testy about it because we are officially out of leisure time and I need to cram in 6 methods' worth of training over 2 weeks and I don't have time to be nice about things anymore. I've repeated myself 500 times we don't have fucking time for me to be nice.
And the person I'm training is stressed out and she doesn't even WANT to do this, but she has to. Alongside all of her other work so she can train my replacement in a few weeks. So she's being extra reticent about it all.
Plus I have to train other people on other methods. And there is literally no fucking time for people to be bumblefucking their way through goddamned lab work basics that I Shouldn't need to be teaching them at this point but here we are!
I am also, on top of everything else, trying to wrote up comprehensive step-by-step guides for How to work the Titrator Softwares, to leave something of my 7+years of experience behind for others. I am doing this At The Request of the people I am training. I spent 4 hours putting together one guide already. With pictures. And sent it to her days before we were supposed to start training. So she would have time to read it and study (again AT HER REQUEST) AND SHE DIDN'T EVEN LOOK AT IT ONCE.
I am losing my goddamned mind. I am stressed out. I am not sleeping well. I am busy beyond all reason, and there is just too much bullshit for me to handle with a smile on my face.
And I know it's not fair at all for me to expect someone to immediately pick up on everything I have spent 40hrs/week for the last 7+ years doing flawlessly. But we are so far beyond "this is the learning curve" to "this is straight up reticent incompetence" and I can't fucking fix that. Like this is sink or swim crunch time and I CANNOT STILL be holding people's hands as they stand on the top step of the pool.
The only thing keeping my hubris in check right now is the fact that I know for sure I will be the person bumblefucking my way through a new lab and a new set of methods on a few weeks, and my trainers will likely be shouting into the void about me too.
#i am so. so. SO happy i allowed myself a week off inbetween jobs#because I need a fucking break. and a fucking drink#vent#i'll probably delete this later
4 notes
·
View notes
Text
titration lab
when i first learned about titrations
i hated them
equivalence points and pKa’s and molarity calculations
i carved S shaped curves into my mind and still couldn’t understand
what exactly made them work
i am not a patient person
i dreaded sitting there for hours watching the
drip, drip, drip,
of the strong base or acid
one drop at a time, to get an accurate result
one drop the difference between pale success and bold failure
it didn’t take me long to figure out that
one drop is nothing.
we would be here forever if we did what we were supposed to and
took our time
so i turned the stopcock and let it run too long
five mLs, ten mLs
until the solution was solid pink
and my lab report had 200% error.
when you snapped at me for the first time, it was a drop in clear solution.
i am not a patient person. i never swirled my flasks thoroughly enough. i let tens of milliliters of titrant flow out of the burette before i even thought about slowing down.
one time, my lab partner and i forgot to add the indicator
and it took us 40 mLs of sodium hydroxide before we realized something was wrong
one drop of phenolphthalein
and the whole solution bloomed bright pink
sudden and all at once and way too strong
you held a pillow to my face until i begged you to let me breathe.
one drop is nothing.
the solution stays crystal clear
until it rides the S shaped curve up
and approaches the equivalence point
but by then, it is often too late
and i haven’t swirled enough, or been vigilant enough
and instead of disappearing into clear liquid
the phenolphthalein pink stays
bright as cherry lipstick
and the mark blooming on my cheek
the day you hit me was the day i finally figured out titration curves.
weak acids buffer the pH change
when you add a strong base
and they share the same ions
but eventually, add enough base
and the delicate balance is ruined with one drop
hot pink flaring in an erlenmeyer
how much does it take?
five? ten? twenty?
how many drips before i’ve had enough?
#poetry#stream of consciousness#chemistry#my poem#poets on tumblr#original poetry#writing#spilled ink
4 notes
·
View notes
Text

Labtron Kjeldahl Analyzer is for titration and distillation, with a measuring range of 0.1~200
mgN and a recovery rate of ≥ 99.5%. ambient temperatures from 10°C~35°C, a distillation time of 3~8 minutes
per sample, and storage for up to 5000 records. Real-time titration curve display.
0 notes
Text
(Recall from chapter 11 that the smaller the pKa the more acidic is the group. At lower pH, carboxylic acids are found in the RCOOH form and amines are found in the RNH3+ form. At higher pH, the opposite is true; carboxylic acids are present as the salt RCOO- and amines are present as uncharged RNH2. Figure 24.3 on p. 1061 shows how this looks at different pH.)
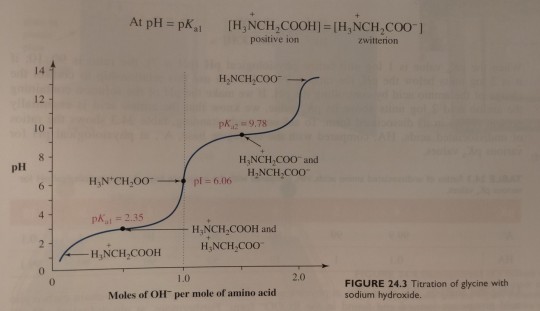
Next, the solution is titrated with 1.00 M NaOH; the volume of base added and the pH of the resulting solution are recorded and then plotted as shown in figure 24.3. (...) By examining the titration curve (figure 24.3), you can see that the isoelectric point for glycine falls halfway between the pKa values for the carboxyl groups and the ammonium ion:
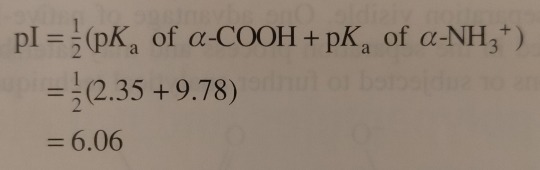
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#acidic#carboxylic acid#ph#salt#amine#titration#glycine#sodium hydroxide#carboxyl#ammonium#ions
1 note
·
View note
Text
i hope weak acid + strong base titration curves specifically die forever.. stop mixing weak acids and strong bases altogether. no one wants that!
0 notes
Text

Potentiometric Titrator
Potentiometric titrator is a touch screen instrument that displays the titration methods and curves in detail. The device can save up to 100 sets of GLP compliant data, also has 100 user-defined methods and 10 user-defined shortcuts for easy operation. It comprises four titration modes Dynamic endpoint titration (DET), Monotone equivalent point titration (MET), Preset endpoint titration (SET), and Manual titration (MAT). Auto calculation and the formula are pre-installed which provides time-efficient work.
Potentiometric titrator provides results which are based on analysis methods that are highly important in the field of electrochemistry and are commonly used for the determination of different organic and inorganic ions in various areas such as process control and environmental, industrial, agricultural analysis and medicinal drug analysis.
#potentiometrictitrator, #titrator, #titrationmethods
0 notes
Text
Therefore, at this point in the titration curve, the reaction:
OH-(aq) + H3O+(aq) → 2H2O(l)
has gone to completion, and the solution contains only Na+ and Cl- ions in water.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#chemical reactions#sodium#chlorine#ions#anion#cation#water
0 notes