#Nitrocellulose Transfer Membranes
Text
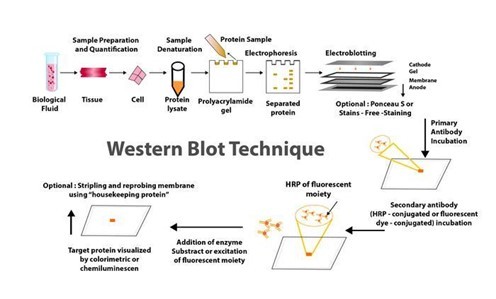
The blog "The Concept, Processes, and Applications of Western Blotting" published on PraxiLabs outlines the Western blot (or protein immunoblot) technique, highlighting its indispensability in modern biomedical research and various laboratories. This analytical method, rooted in molecular biology and immunogenetics, employs antibodies to specifically detect antigens, allowing for the identification of a particular protein within a sample alongside information on protein size and abundance.
Originating from the collaborative work of Harry Towbin, Julian Gordon, and Theo Staehelin in 1979, the development of protein blotting techniques marked a significant advancement in the field. Their pioneering work facilitated the electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets, thereby setting a foundational technique for numerous scientific investigations.
Western blotting operates on the principle of separating proteins within a sample based on molecular weight through gel electrophoresis. These proteins are then transferred (blotted) onto a more durable surface, such as a nitrocellulose or PVDF membrane. The detection of the protein of interest is facilitated by the use of specific antibodies.
Let’s explore the Western Blot method step-by-step.
Sample preparation: Involves preparing and loading the sample onto a gel for electrophoresis.
Electrophoretic separation: Proteins are separated by size using gel electrophoresis.
Protein transfer: The separated proteins are transferred from the gel to a membrane.
Blocking: Nonspecific binding sites on the membrane are blocked.
Antibody probing: The membrane is incubated with primary and secondary antibodies to detect the protein of interest.
Detection: Various methods, including chemiluminescence and fluorescence, are used to visualize the protein-antibody complexes.
Imaging and analysis: The final steps involve capturing images of the membrane and analyzing the detected signals to estimate protein size and quantity.
The article highlights the benefits and applications of Western Blotting.
One of the key advantages of Western Blotting lies in its sensitivity and specificity, making it highly effective in analyzing complex protein structures. This is crucial not only in research for obtaining molecular insights but also in clinical settings for making accurate diagnoses.
Sensitivity: demonstrates the ability to identify minuscule protein quantities within samples, as slight as 0.1 nanograms, which is an invaluable tool for early diagnostics in detecting minimal immune reactions triggered by viruses or bacteria in patient samples.
Specificity: originates from two principal factors attributing to the western blot technique. Firstly, the application of gel electrophoresis allows the classification of proteins based on their size, charge, and structure. This initial sorting is instrumental in the identification process, as the resulting patterns on the gel can provide preliminary insights regarding the protein or peptide of interest.
What about the applications of Western Blotting?!
The Western blot technique has a wide range of uses in scientific and clinical approaches, such as:
Biological sciences: It enables researchers to ascertain the presence, size, and amount of protein expression related to various tissues or cell types. This technique is pivotal for understanding protein expression levels, monitoring protein purification processes, and studying the effects of diseases or treatments on protein behavior. Its application extends to comparing protein expressions across different tissues or observing how proteins respond to specific treatments or diseases.
Medical diagnosis: Western blotting has found significant applications in the medical diagnostic field, especially as a confirmatory test for various diseases. For instance, it is widely used in the diagnosis of HIV, where it confirms the presence of HIV antibodies in blood samples following a preliminary ELISA test. In addition, Western blotting assists in diagnosing other conditions, such as Lyme disease, by detecting antibodies against specific pathogens, offering a layer of confirmation that enhances the accuracy of initial screening tests.
If you are interested in exploring more details about the Western Blotting Technique, check our PraxiLabs blog about “How to Analyze Western Blot Data”
0 notes
Text
METHODS FOR PURIFYING AND ANALYSING PROTEINS VIA WESTERN BLOTTING
For many years, western blotting was a primary method in molecular biology and techniques such as proteomics (the large-scale study of proteomes: a set of proteins produced in an organism, system, or biological context). Given that western blot is a multistep process that frequently necessitates specialised interpretation, potential errors and variations at any step can jeopardise the reliability and reproducibility of its results.
Given this situation, many advanced and sophisticated approaches to improving the western blot protocol have been developed over the last two decades. These enhancements are more automated and sensitive, with high potential for reducing potential issues with the traditional western blot method. Western blotting's improved sensitivity and innovative equipment advancements have the potential to broaden the field of clinical applications for this fundamental method.
Helvetica Health Care explains the western blot technique as a method of purifying and analysing proteins, as well as new developments in western blotting methodology, in this article.
WHAT EXACTLY IS THE WESTERN BLOT TECHNIQUE?
Traditionally, the western blotting method involves seven steps, as below.
Sample preparation from cells or tissue lysates
Separation of proteins by gel electrophoresis
Protein transfer in a nitrocellulose or polyvinylidene fluoride (PVDF) membrane,
Blocking of non-specific proteins on membrane
Primary Antibody incubation
Secondary Antibody incubation
Protein detection & analysis
WHAT ARE THE NEW DEVELOPMENTS IN THE WESTERN BLOT DOMAIN AND WHAT CHARACTERISTICS DO THEY HAVE?
Below are some innovations that have enhanced the western blot protocol and addressed the potential problems in its application in protein purification and analysis.
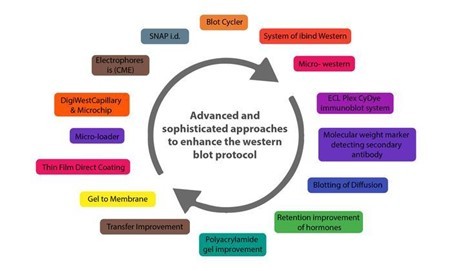
CAPILLARY AND MICROCHIP ELECTROPHORESIS (MCE)
MCE has higher sensitivity and resolution than traditional techniques, allowing for the detection of multiple target proteins from a single cell lysate sample. The method not only allows for parallel multiplexed tests of a set of proteins using a small sample amount, but it also eliminates blocking stages and has faster analysis times (8 min for electrophoretic resolution). MCE, while still in development, has the potential to be significantly improved with additional work.
AUTOMATED MICROFLUIDIC PROTEIN IMMUNOBLOTTING
Automated protein immunoblotting is a programmable and controllable technology that combines PAGE (polyacrylamide gel electrophoresis) and blotting in a single device. It saves time, avoids multiple test stages, and reduces the amount of equipment and reagents required. Because it can detect free prostate-specific antigens in a sample of human seminal fluid in less than 5 minutes, this technology is both cost-effective and reagent-efficient. The method is constantly being improved to improve sensitivity and enable protein quantification.
SINGLE CELL-RESOLUTION WESTERN BLOTTING
This sensitive method has also been used to investigate protein variations in stem cell signalling and differentiation. It can detect specific cell-to-cell changes in protein expression. MiloTM (ProteinSimple), the world's first Single-Cell Western platform, can measure protein expression (up to 4 proteins per cell simultaneously) in 1000 single cells in about 4 hours.
DIGIWEST
DigiWest, a simplified version of the standard procedure, improves western blotting throughput by requiring less lysate, target detection, and antibody. However, this method requires biotinylation of the target proteins, requires specialised reagents and equipment, incurs initial startup costs, has issues with translating the digital DigiWest results to western blot mimics, and has specifics about some procedures, such as the complex and difficult elution of proteins from PVDF membranes.
SIMPLE WESTERN
Simple Western is a method developed by ProteinSimple (San Jose, California) that is based on CE-SDS (Capillary electrophoresis sodium dodecyl sulphate), in which separated proteins are linked to the capillary wall by a patented photo- (ultraviolet) induced chemical crosslink. The method can use up to 90 different antibodies at the same time, is relatively quick, and requires small amounts of sample. It can also perform standard curve quantification, similar to high-pressure liquid chromatography (HPLC), and improve reproducibility. However, it necessitates the use of specialised reagents.
MICRO-LOADER
The Micro-loader technology has improved the sensitivity of the western blot assay and PAGE. To load samples, it employs a sample micro-loader device with a funnel-like design. This technique is highly effective for measuring protein expression and phosphorylation in samples that are few, difficult to find, and limited in quantity because it only requires a small number of loading samples for signal detection.
THIN-FILM DIRECT COATING WITH SUCTION-WESTERN BLOTTING (TDCS)
Western blotting has significant limitations due to high antibody consumption and lengthy operating times. TDCS, a capillary-tube-based technique, aims to reduce antibody and time consumption in western blotting. This quick and sensitive detection method allows for the quantitative study of multiple antigen-antibody interactions, as well as multiple protein interactions.
BLOTTING OF DIFFUSION
SDS-PAGE Single-prefabricated gels on plastic support are a quick and easy way to make multiple blots using diffusion blotting. Diffusion blotting allows for the comparison of several proteins on blots from the same gel. Protein transmission rates by diffusion blotting are 10% in 3 minutes, 20% in 20 minutes, and 40% to 60% in 3 hours. When compared to electroblotting, the technique may increase the amount of proteins efficiently delivered to the membrane surface. As a result, when quantitative protein exchange is not required, diffusion blotting is the preferred method.
MICRO WESTERN
Soon, micro western will be a proper western blot technique for confirming the diagnosis of purified HIV p24 and gp120 proteins in blood plasma. The Micro-Western may be vital for detecting infectious diseases and cancer, despite not being widely available.
SYSTEM OF IBIND WESTERN
Thermo Fisher Scientific's iBind Western procedure makes use of a low-cost semi-automated western blotting structure that uses sequential flow technology to distribute chemicals and antibodies to different compartments. Cleaner western blots will result from using the iBind Western Systems and highly specialised primary and secondary antibodies.
BLOT CYCLER
Every step of membrane processing is automated with the BlotCycler (Precision Biosystems, USA), and up to 12 membranes can be processed at once. The BlotCyclerTM is an Automated Western Blot Development system that improves repeatability and sensitivity by completing all blot cleaning, trapping, and incubation steps. It is also simple to programme all of the actions for specific operations.
SYSTEM OF SNAP I.D.
In a low-cost system of SNAP i.d. 2.0, solvents are uniformly distributed via the tissue using a technique based on a vacuum and a flow distribution system (Merck, USA). Its main advantage is that washing, antibody incubation, and blocking all take about 30 minutes. The SNAP i.d.® 2.0 gadget actively forces reagents through the membrane, unlike conventional Western blotting, which depends on diffusion to transfer reagents.
Washes, antibody recollection, and antibody-antigen binding have been improved. The SNAP i.d.® 2.0 delivers new, cutting-edge western blotting characteristics with an immunodetection capability in 30 minutes without using additional reagents (e.g., antigen, antibody, or detection reagents).
RETENTION IMPROVEMENT OF HORMONES OF THE PEPTIDE ON THE MEMBRANE OF BLOTTING
Western blotting is significantly improved by treating the PVDF membrane used in this method with glutaraldehyde (0.2%) for fifteen minutes in a saline solution containing tween-80. In western blotting, the addition of glutaraldehyde to the membrane prevented or reduced the quantity of insulin loss.
Apart from these, two more techniques that have improved the conventional method are
Analysis of western blot utilising molecular weight marker detecting secondary antibody
Total and target protein co-detection by immunoblotting of fluorescent ECL and labelling of Cydye
Many innovations in western blotting techniques to transfer proteins from gel to membrane have also been observed, such as vacuum blotting, centrifuge blotting, multiple tissue blotting, and so on. The Trans-Blot Turbo, the iBlot® dry blotting system, and the PierceTM Power Blot Cassette are all improvements in protein transfer methods from gels to membranes. The transfer time is reduced from an hour or overnight to at most 10 minutes when these new techniques are used.
HHC offers the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV in serum or plasma and is available in 10 or 30-strip kit formats. We also provide a bench top, a western blot processor, and the AUTOBLOT 3000. It controls incubation times, dispensing volumes and washing programs. Ten user-defined protocols can be programmed easily. Contact us now to know how we can help you get accurate and high-quality output with our products when performing the western blotting procedure.
0 notes
Text
Lzip pgc1a

Membrane Blocking and Antibody Incubations Electrotransfer to nitrocellulose membrane ( #12369).Ĭ.NOTE: Loading of prestained molecular weight markers ( #59329, 10 µl/lane) to verify electrotransfer and biotinylated protein ladder ( #7727, 10 µl/lane) to determine molecular weights are recommended. Load 20 µl onto SDS-PAGE gel (10 cm x 10 cm). Heat a 20 µl sample to 95–100☌ for 5 min cool on ice.Sonicate for 10–15 sec to complete cell lysis and shear DNA (to reduce sample viscosity).Immediately scrape the cells off the plate and transfer the extract to a microcentrifuge tube. Lyse cells by adding 1X SDS sample buffer (100 µl per well of 6-well plate or 500 µl for a 10 cm diameter plate).Aspirate media from cultures wash cells with 1X PBS aspirate.Treat cells by adding fresh media containing regulator for desired time.Protein Blotting A general protocol for sample preparation. Detection Reagent: SignalFire™ ECL Reagent ( #6883).ī.Secondary Antibody Conjugated to HRP: Anti-rabbit IgG, HRP-linked Antibody ( #7074).Pore size 0.2 µm is generally recommended. Blotting Membrane and Paper: ( #12369) This protocol has been optimized for nitrocellulose membranes.Blue Prestained Protein Marker, Broad Range (11-250 kDa): ( #59329).Biotinylated Protein Ladder Detection Pack: ( #7727).Primary Antibody Dilution Buffer: 1X TBST with 5% BSA for 20 ml, add 1.0 g BSA to 20 ml 1X TBST and mix well.Blocking Buffer: 1X TBST with 5% w/v nonfat dry milk for 150 ml, add 7.5 g nonfat dry milk to 150 ml 1X TBST and mix well.10X Tris Buffered Saline with Tween ® 20 (TBST): ( #9997) To prepare 1 L 1X TBST: add 100 ml 10X TBST to 900 ml dH 2O, mix.10X Tris-Glycine Transfer Buffer: ( #12539) To prepare 1 L 1X Transfer Buffer: add 100 ml 10X Transfer Buffer to 200 ml methanol + 700 ml dH 2O, mix.10X Tris-Glycine SDS Running Buffer: ( #4050) To prepare 1 L 1X running buffer: add 100 ml 10X running buffer to 900 ml dH 2O, mix.1X SDS Sample Buffer: Blue Loading Pack ( #7722) or Red Loading Pack ( #7723) Prepare fresh 3X reducing loading buffer by adding 1/10 volume 30X DTT to 1 volume of 3X SDS loading buffer.10X Tris Buffered Saline (TBS): ( #12498) To prepare 1 L 1X TBS: add 100 ml 10X to 900 ml dH 2O, mix.20X Phosphate Buffered Saline (PBS): ( #9808) To prepare 1 L 1X PBS: add 50 ml 20X PBS to 950 ml dH 2O, mix.NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water. Solutions and Reagentsįrom sample preparation to detection, the reagents you need for your Western Blot are now in one convenient kit: #12957 Western Blotting Application Solutions Kit NOTE: Please refer to primary antibody product webpage for recommended antibody dilution. For western blots, incubate membrane with diluted primary antibody in 5% w/v BSA, 1X TBS, 0.1% Tween ® 20 at 4☌ with gentle shaking, overnight.

0 notes
Text
Transfer Membrane Market | Increasing R&D Spending By Pharmaceutical and Biotechnology Companies

According to the new market research report " Transfer Membrane Market by Type (PVDF, Nitrocellulose, Nylon), Transfer Method (Tank, Semi-dry, Dry), Application (Western, Northern, Southern Blot, Protein Sequencing), End user (Academia, Diagnolab, Pharmaceutical Companies) - Global Forecast to 2023" the transfer membrane market is projected to reach USD 187.9 million by 2023 from USD 174.8 million in 2018, at a CAGR of 1.5% during the forecast period.
Factors such as increasing public and private funding for life science research, the significantly high prevalence of target diseases across the globe, and increasing R&D spending by pharmaceutical and biotechnology companies are expected to drive the growth of market.
Browse 64 market data Tables and 35 Figures spread through 124 Pages
Download Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=36975865
The report analyzes the global market by type, transfer method, application, end user, and region.
On the basis of type, the market is divided into nylon membranes, nitrocellulose membranes, and PVDF membranes. In 2018, PVDF Membranes are expected to command for the major share of the market. This can be attributed to the advantages of PVDF membranes over its counterparts, such as better protein retention, strength, chemical compatibility, and wide applications in western blotting.
Based on end user, the market is segmented into academic and research institutes, pharmaceutical and biotechnology companies, diagnostic laboratories, and other end users. Among these, the academic and research institutes segment is expected to account for the largest share of the transfer membrane market in 2018, owing it to the rising financial support from private as well as government bodies for life science research in various nations.
Get Sample Copy of This Report
@https://www.marketsandmarkets.com/requestsampleNew.asp?id=36975865
Regional Growth, Development and Demand Analysis:
North America is expected to account for the largest share of the global transfer membrane market in 2018, followed by Europe. The large share in the North American region is mainly attributed to the presence of leading transfer membrane manufacturers in the region, availability of government and private financial support for life science research, and high target disease prevalence in the region.
Some of The Major Players In Transfer Membrane Market :
Merck KGaA (US), Thermo Fisher Scientific (US), Bio-Rad Laboratories (US), GE Healthcare (US), PerkinElmer (US), Pall Coporation (US), Advansta (US), GVS (Italy), Santa Cruz Biotechnology (US), Abcam (UK), ATTO Corporation (Japan), Carl Roth (Germany), Macherey-Nagel (Germany), Azure Biosystems (US), and Axiva Sichem Biotech (India)
#Transfer Membrane Market#PVDF Transfer Membranes#Nitrocellulose Transfer Membranes#Nylon Transfer Membranes
1 note
·
View note
Photo

Nitrocellulose Transfer Membrane Market Size By Technology By Operating Principle By End-Use, Industry Outlook Report, Regional Analysis, Application Potential, Price Trends, Competitive Market Share & Forecast, 2019 – 2024 Nitrocellulose Transfer Membrane Market Report The report offers an overview of the Nitrocellulose Transfer Membrane with the help of application segments and geographical regions (United States, Europe, China, Japan, Southeast Asia, India, Central & South America, ROW) that govern the market currently.
0 notes
Text
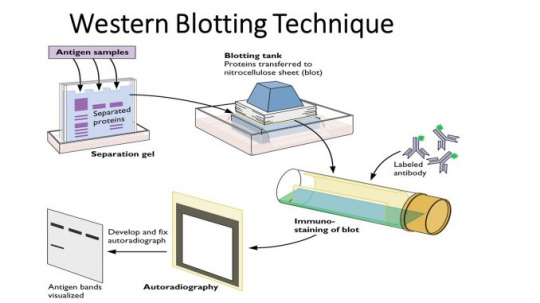
Western Blot
A western blot can detect specific protein molecules from a mixture of proteins. They can also be used to evaluate the size of a protein of interest, and to measure the amount of protein expression.
Gel electrophoresis
The proteins of the sample are separated by isoelectric point (pI), molecular weight, electric charge, or a combination of these factors.
Samples are often boiled first to denature the proteins - prevents proteases (enzymes that break down proteins) from degrading samples.
Most commonly, this uses polyacrylamide gels and buffers loaded with sodium dodecyl sulfate (SDS). SDS-PAGE (SDS polyacrylamide gel electrophoresis).
Proteins are covered in the negatively charged SDS - becoming anionic - and migrate towards the positively charged (higher voltage) anode through the acrylamide mesh of the gel.
Smaller proteins migrate faster through this mesh, and the proteins are thus separated according to size.
The different rates of advancement (different electrophoretic mobilities) separate into bands within each lane.
The concentration of acrylamide determines the resolution of the gel.
One lane is a marker/ladder - a mixture of proteins of known molecular weights, to act as a control and compare results to so as to estimate the sample’s molecular weight.
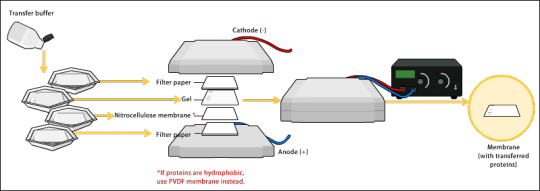
Transfer
To make the proteins accessible to antibody detection, they are moved onto a membrane made of nitrocellulose or polyvinylidene difluoride (PVDF).
Electroblotting uses an electric current to pull the negatively charged proteins from within the gel onto the membrane while maintaining the organization they had within the gel.
Blocking
Blocking of non-specific binding prevents interactions between the membrane and the antibody used for detection of the target protein.
Membrane is placed in a dilute solution of protein – typically 3–5% bovine serum albumin (BSA) or non-fat dry milk in tris-buffered saline (TBS) or I-Block, with a minute percentage (0.1%) of detergent
The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached.
When the antibody is added, it cannot bind to the membrane, and therefore the only available binding site is the specific target protein.
This reduces background - clearer results, and eliminates false positives.
Primary antibody
A solution of primary antibody (diluted in either PBS or TBST wash buffer) is incubated with the membrane under gentle agitation.
This binds to the target protein.
The membrane is washed several times in wash buffer to remove unbound primary antibody,
Secondary antibody
Membrane is exposed to another antibody
Which recognises and binds to the species-specific portion of the primary antibody (eg anti-mouse secondary antibody will bind to almost any mouse-sourced primary antibody).
Secondary antibody is commonly linked to biotin or a reporter enzyme
Several secondary antibodies will bind to one primary antibody and enhance the signal.
Western blot binding
As with most immuno tests, an enzyme can be attached to the secondary antibody that will produce a coloured reaction product - visible on the membrane so that the test can be read.
Another method uses a near-infrared (NIR) fluorophore-linked antibody - light produced from the excitation of a fluorescent dye is static, making fluorescent detection more precise and accurate
A radioactive label could also be coupled to the secondary antibody.
#medicine#biomed#biomedicine#notes#medblr#studyblr#sciblr#premed#nursing#biology#human biology#med#biomedical science#science#immuno#clinical#immunology#assay#western blot#2#3#electrophoresis
144 notes
·
View notes
Text
protocol: western blotting
sample prep and running protein gel
1. Take concentrations from BCA protein assay and diluted to 100-fold (calculated). Total 40ug volume from measured ug/ul concentration.
2. Calculate lysis buffer amount needed to reach 24 ul/sample x 2.2 for enough to duplicate and 0.2 extra for loading.
3. Pipet lysed samples to mix before adding volumes from (1) to volumes from (2) and mixing.
4. Add 10% 2-mercaptoethanol to blue loading dye 7.5.19: 20 -> 200 uL) and vortex to mix. Add 2.2x 8ul/sample to each tube from (3) and pipet to mix.
5. Vortex gently before incubating @95°C for 5 minutes. Vortex 3s and put on ice. Centrifuge at max rpm for 2 minutes at 4°C. Vortex gently on 1/2 speed.
6. mix 75 uL lysis buffer and 25uL loading buffer for blank practice/addition to marker. No incubation necessary.
7. set up WB gel for loading. Fill to line and inside gel space with running buffer (diluted). (Dilution: 100mL 10x: 1000mL ddH2O). Remember to sub in a plastic plate if you only have 1 gel to run!
8. using butterfly tips, load max 40uL into each well. For gel facing away, R-> load. For gel close to you, L-> R load.
9. load marker "protein dual core" from -20°C fridge into wells flanking and dissecting gel (2.5uL, 10uL, 10uL). Weigh down marker lanes with 20-25uL soln from (6).
10. Add H2O and LB/2-mercaptoethanol to extra lanes to prevent marker running sideways. If needed, add more RB while running w/cap on for 2hours (120 min) at 80V
gel-to-membrane transfer process
1. make transfer buffer - 2 gel = 1L. 800 mL H2O, 200mL methanol, 5.8g Tris, 2.9g glycine. Stir w/ teflon and place in ice.
2. >>transfer order <<i. pour TB in large, flat tray. soak 5 sponges in soln + 4 papers, nitrocellulose membrane.ii. membrane with gel attached will go face down in TB. Start with "u" then flip to "n"iii. [bottom to top] 3 sponge. 2 papers. gel. membrane. 2 papers. 2 sponge. membrane goes to ANODE. <</transfer order>>
3. pry gel out from between glasses to place on membrane, via green rectangle cut below last marker line and above @ gel lanes to remove excess gel. Remove gel lanes at top and excess at bottom.
4. remove air bubbles w/ TB pour onto gel. Lay paper gently on top and then flip and add membrane.
5. "make" the sandwich within the white plastic holder. drip each layer with TB using a sponge, until everything is secure and TB overflows. Run transfer 30V for 3-5 hours. Flip on palm, lay down.
antibody application and binding
1. rinse with wash buffer and place in plastic compartment with 10-15 mL blocking buffer (2.5g BSA + 50mL wash buffer, shake 20+ minutes before use) per cut. Shake 30min-1hr to reduce noise.
2. wrap in plastic and close off edges to prevent membrane drying. Cut into sections for antibody binding and snip corners to distinguish between each membrane portion.
3. wash with WB. Add primary antibodies from cold room into each well of a plastic WB container. Place membrane sections into correct antibody lanes. Slowly shake overnight in cold room.
4. wash new container with WB and transfer membranes. Place primary antibodies back in original tubes in cold room. Wash in 8mL WB per well, 10 minutes, 3 times, on shaker at room temperature
5. add 20000x dilution of secondary antibody from protein imaging kit in -20° fridge to prepared 6mL BSA per membrane piece (i.e., 1.25uL rabbit antibody for 25mL BSA) and gently shake on rocker at RT for 1 hr
6. perform another 3 • 10 minutes wash with WB, 6-8mL per well
membrane image capture and b-actin stripping
1. reserve imaging machines in CORE. Bring basket, p1000 pipet, antibody substrate on ice, and membranes with antibody/BSA binding to 9th floor CORE imager
2. clean imaging screen with ddH2O and immerse each membrane in 250uL antibody substrate on separate dish for 1-2 minutes before placing on screen and imaging. Save files onto USB drive and place membranes back in WB
3. make stripping buffer (recipe in comments). Wash membranes with WB, drain, and place 7.5mL stripping buffer to membranes that contain b-actin/other control bands to remove experimental protein-specific antibody binding
4. block with BSA for 0.5 hr and wash with WB before adding b-actin antibodies from cold room and incubating 1.5+ hours at RT or overnight at 4°C.
5. repeat steps (3 from previous section) - (1 from this section) with b-actin or other control band, to obtain control WB images. Assess control results to ensure consistent protein concentrations and procedures.
6. clean all equipment used with ddH2O and place antibodies, BSA, substrates and kits in cold room or fridge.
3 notes
·
View notes
Text
The rough surface is the receiving protein surface
Transfer film: transfer cryo rack sandwich structure sequence: black plate, mesh pad, a layer of filter paper, gel (habitually placing Marker on the right side), NC film (upper left corner of the protein side of the film) Mark well), two layers of filter paper, mesh mat, white board. Note: The filter paper used is also divided into rough surface and fine surface. The delicate surface faces the gel and NC membrane, and the rough surface faces the mesh pad. When sandwich clips are placed in the electrodes, the black plate corresponds to the black side (negative electrode) of the electrode and the white plate corresponds to the red side (positive electrode) of the electrode. Emphasis: The order of gel and NC membrane (nitrocellulose membrane) must not be reversed. The NC membrane used is divided into rough surface and smooth surface.
The rough surface is the receiving protein surface, and this surface is in direct contact with the gel. When covering the slot cover, also pay attention to black corresponding to black and red corresponding to red; when the wire electrode is connected to the power supply, it is also black to black and red to red. Reversing any of the above steps will cause protein transfer to membrane failure. Remember. Parameters used for film transfer: steady current 45mA, overnight (999min).
4. Blocking solution used for blocking: 5% Nonfat milk, prepared with TBST, RT (room temperature), 30min ~ 1h (determine the time according to the actual situation, such as the number of times the blocking solution has been used and the background of the ECL color). In order to prevent the milk powder from deteriorating, add sodium azide, NaN3 (a spectral preservative), to a final concentration of 0.02% (the concentration of sodium azide stock solution in this laboratory is 2%).
Wash: TBST to wash away the remaining milk powder. 5. Antibodies for primary antibody incubation: mouse, Anti-Flag. Primary antibody dilution: 4% BSA by Super Block, 1: 1000. Also added 0.02% sodium azide NaN3. Incubation: RT, about 3h. Wash: TBST, 3 x 5min6, secondary antibody incubation: HRP, anti-mouse. Secondary antibody dilution: 5% Nonfat milk by TBST, 1: 2000. (Secondary antibodies are usually updated two or three times) (emphasis: NaN3 cannot be added to secondary antibodies, because it will inhibit HRP activity) Incubation: RT , 1h. Wash: TBST, 6 x 5min. 7, color rendering: ECL. Liquid A / B, each with a volume equal ; l, was mixed with the current one.
1 note
·
View note
Text
DNA - FINGERPRINTING
DNA fingerprinting is also know as DNA profiling is a process of determining an individuals DNA characteristics. This is a forensic technique in criminal investigations, comparing criminal suspect's profiles to DNA to identify the like hood of their involvement in the crime. Also can be used for paternity tests. 99 % of human DNA sequences are the same in every person. Remaining sequences are different . It makes every individual unique.

This is used to distinguish and identify the individuals of a same species by using their DNA samples. These biological samples may be blood, hair, saliva, semen, body tissue cells. The DNA samples are isolated first. Then digested by restriction endonucleases. After this electrophoretic separation of fragments are done. These fragments are transferred into nylon or nitrocellulose membrane. This is followed by probing, hybridization and autoradiography. From this banding pattern is analyzed.
youtube
FOR FURTHER INFORMATION VISIT : -
https://readitupwithswathy.blogspot.com/2022/04/dna-fingerprinting.html
#dna#dna fingerprinting#dna profiling#forensic science#crime#suspect#dna sequence#restriction endonucleases#nylon#nitrocellulose membrane#probe#hybridization#autoradiography#banding pattern#genetics#genome
1 note
·
View note
Text
ANALYSIS OF WESTERN BLOT PROCEDURES
Proteins play many vital roles in our bodies. Essentially proteins or polypeptides are complex compounds of amino acids that are needed by our bodies to regulate, function and construct organs and tissues in the body.
Proteins are integral to organisms, helping almost every cell process and are also critical to metabolism. For these reasons, detecting the presence or absence of protein, their size or molecular weight is vital. Protein analysis is crucial in
investigating a disease,
detecting the presence of allergenic protein in food samples and
evaluating whether genetic manipulation experiments were successful or not
There are different types of proteins in terms of their size and the arrangement of amino acids, having diverse molecular structures, nutritional characteristics, and chemical properties. Given the diverse nature of this vital biological element, there are three traditional techniques that researchers and scientists use for protein analysis:
protein separation,
western blotting, and
protein identification.
In this blog, our team at HHC will help you explore the western blot technique to understand the procedure and its uses for protein analysis
WHAT IS WESTERN BLOTTING?
Developed by Harry Towbin and his colleagues in 1979, the western blot procedure is a typical cell and molecular biology procedure widely used in the analysis of proteins. Often known as protein immunoblotting, the method allows researchers to determine the presence, size and quantity of particular proteins in a given sample. Three elements comprise the western blotting method:
1) separation by size,
2) transfer to a solid support or membrane, and
3) marking target protein using a proper primary and secondary antibody to visualise.
Western blotting is very useful for identifying individual proteins from a complicated mixture of proteins isolated from cells that may have similar characteristics or sizes.
THE WESTERN BLOT PRINCIPLE
The Western blotting technique is based on the use of polypropylene gel electrophoresis and antibodies. In this technique, researchers can separate and identify proteins based on their molecular weight and type from a gel-like sample using electrophoresis, which acts as a molecular sieve. Next, the separated proteins are transferred to a membrane that produces a band for each protein. The membrane is then incubated with labelled antibodies specific to the protein of interest to create a coloured band. These antibodies can be identified, and the size and abundance of the bound proteins can be analysed in relation to established standards or controls.
HOW IS THE WESTERN BLOT PROCEDURE PERFORMED?
The western blotting technique is performed in 7 steps. They are
1) Sample preparation
2) Gel electrophoresis
3) Proteins transfer
4) Blocking
5) Primary Antibody incubation
6) Secondary Antibody incubation
7) Protein detection & analysis
1) Sample preparation
The first step in the western blotting technique involves the extraction of proteins from different samples such as cells or tissues. The extracted samples need to be broken down by a homogeniser or sonication. Phosphatase inhibitors and protease are used to avoid the digestion of the sample at cold temperatures. Once the protein is extracted, the quantity of proteins needs to be determined.
2) Gel electrophoresis
This step involves the separation of the proteins based on their charge and molecular weight. This is done using polypropylene gel electrophoresis like sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) or native PAGE. Usually, separating gels come in 5%, 8%, 10%, 12% or 15%. The size of the prey protein is taken into consideration when choosing the right percentage of separating gel. The relation is inverse. Higher percentage gels are suitable for proteins that weigh less.
3) Proteins transfer or blotting
Once the proteins are separated using gel electrophoresis, they are transferred to a solid support membrane such as nitrocellulose (NC) or polyvinylidene difluoride (PVDF) membrane to facilitate antibody probing. Protein transfers can be performed using various techniques: diffusion transfer, capillary transfer, and vacuum blotting. But the most used is the electroblotting technique, wherein an electric current is applied to the protein gel sandwiched between the membrane and the filter papers. An electric field is aimed perpendicular to the gel’s surface that extracts the protein from the gel and transfers them onto the membrane in a tight manner.
Protein gel transfer through electroblotting can be performed in different ways, such as wet, semi-dry and dry transfer. Wet or semi-dry transfer strategies usually provide reliable results as the buffering is more flexible. However, these methods are time-consuming. On the other hand, the dry transfer method is efficient and quick but offers less buffering flexibility.
4) Blocking
The next and crucial step in the western blot procedure is blocking. Because blotting membranes have a high affinity for proteins, blocking any residue binding sites after the transfer is critical to avoid further non-specific binding of the assay detection antibodies. This is accomplished by exposing the membrane to a liquid that contains protein, such as milk or serum. BSA and non-fat dry milk are the most used typical blockers.
When submerging the membrane in the diluted protein solution, the proteins in the solution bind to all the areas of the membrane that are not bound by the prey protein. The process reduces the “noise” in the western blot’s result to provide more precise findings.
After blocking, antibody probing is performed. There are two strategies for this:
direct detection using a single or primary antibody and
indirect detection using a secondary antibody
For the western blot technique, the indirect method is used most often.
5) Primary Antibody incubation
At this stage, the primary antibody is used to probe the membrane and binds to the prey protein. Depending on the antigen to be determined, the primary antibody is selected.
In the direct detection method, the membrane is then incubated with the secondary antibody to bind to the primary antibody and is detectable.
6) Secondary Antibody incubation
When using indirect assay detection, it is vital to wash the membrane using an antibody-buffer solution to reduce background and remove excess or unbound antibodies before the secondary antibody is used to incubate the membrane. After the wash, the specific enzyme-conjugated secondary antibody incubates the membrane. When the second antibody incubation is performed, the labelled secondary antibody binds to the primary antibody that has interacted with the prey protein and is detectable. The selection of the suitable secondary antibody is based on the variety or the species of the primary antibody.
7) Protein detection & analysis
At this final stage, protein detection is achieved when the conjugated enzyme interacts with a substrate introduced to the membrane, causing a chemical change in the form of a coloured substance allowing the researcher to mark the location and the densitometry of the prey protein. Horseradish peroxidase (HRP) and alkaline phosphatase (AP) are the most used enzymes. Due to its stability, suitability for most conjugations, and affordability, HRP is frequently preferred.
Protein detection and the subsequent visualisation can be performed using chemiluminescent, colourimetric, radioactive and fluorescent detection techniques, the most common being chemiluminescent detection.
When using the chemiluminescent detection method, it is essential to note that the duration of the signal or the chemical change lasts only until the substrate reacts with the conjugated enzyme (usually between 1 and 24 hours), making it a very sensitive technique. Creating a permanent record of the signal is possible by using an X-ray film or using digital imaging.
WHAT ARE THE USES OF THE WESTERN BLOTTING TECHNIQUE?
In brief, the western blotting procedure determines the presence or absence, size, and abundance of target proteins in a sample which is beneficial for various scientific reasons across many fields of study. In this regard, the technique proves beneficial to evaluate the following:
Protein DNA interactions
Protein-protein interactions
Post-translational modifications (PTMs)
Protein isoform detection
Antibody characterisation
Epitope mapping
Subcellular protein localisation
Among all the applications of the Western blotting method, the most common are as below.
Diagnosis of infectious diseases
Although time-consuming when compared to other alternatives like the PCR technique, Western Blot proves highly useful in testing and confirming cases of HIV (human immunodeficiency virus), BSE (bovine spongiform encephalopathy), Lyme disease and aspergillosis.
Biomarking non-infectious diseases
As Western Blot is an antibody-based method, the technique often supports and produces reliable results for detecting non-infectious diseases using HTS (High throughput screening). For example, when determining cancers, incongruous isoforms of proteins can become potential markers of the pathology of the disease. Autoantibodies may also indicate an autoimmune disease.
Identifying the differences in protein levels between groups or over time
Western Blot is very efficient in determining changes in protein levels or even different cycle phases for samples studied in a temporal experiment. Additionally, contrasting samples from various disease or treatment groups can draw attention to protein-level changes that could point to a disease’s underlying aetiology or impact a treatment’s effectiveness.
Determine if a protein is expressed
When performing experimental genomic manipulations, Western Blot can help compare the changes made at the genomic level with those that occur at protein levels. (for example, is a novel protein expressed in the expected tissue or location? Or is its expression truncated or lost compared to a wild-type strain?).
Tagged protein detection
Tags can be engineered and assigned to proteins during genomic manipulations facilitating the identification of proteins attached to them from samples of a system under study or of a species. This method enables researchers to localise the protein and understand its course. The process can prove particularly useful for checking recombinant proteins produced through experiments.
Compared to methods like PCR (polymerase chain reaction), western blotting is not usually considered a quantitative procedure. However, the direct identification of proteins it delivers is efficient and has proven significantly advantageous for confirmatory testing for disease diagnosis.
The key to achieving reliable results from a successful blot procedure is the selection of the correct antibody and identifying the optimal concentration. The use of the right equipment is equally essential to get optimised results.
HHC provides in the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV (Simian Immunodeficiency Virus, the lentivirus most closely related to Human HIV) in serum or plasma. It is available in 10 or 30 strip kit formats. Additionally, we offer a bench top, western blot processor: the AUTOBLOT 3000, which controls incubation times, dispensing volumes and washing programs. The AUTOBLOT 3000 can programme ten user-defined protocols.
If you want to add the most updated lab equipment to enhance the protein detection services of your lab, don’t hesitate to get in touch with Helvetica Health Care Website now!
Originally Posted On HHC
0 notes
Text
Global Transfer Membrane Market worth USD 187.9 million by 2023 : Technological advancements in the field of Protein Sequencing
Global Transfer Membrane Market worth USD 187.9 million by 2023 : Technological advancements in the field of Protein Sequencing
Factors such as increasing public and private funding for life science research, the significantly high prevalence of target diseases across the globe, and increasing R&D spending by pharmaceutical and biotechnology companies are expected to drive the growth of market.
According to a new market research report “Transfer Membrane Market by Type (PVDF, Nitrocellulose, Nylon), Transfer Method…

View On WordPress
0 notes
Text
Global Transfer Membrane Market worth USD 187.9 million by 2023 : Technological advancements in the field of Protein Sequencing
Factors such as increasing public and private funding for life science research, the significantly high prevalence of target diseases across the globe, and increasing R&D spending by pharmaceutical and biotechnology companies are expected to drive the growth of market.
According to a new market research report “Transfer Membrane Market by Type (PVDF, Nitrocellulose, Nylon), Transfer Method (Tank, Semi-dry, Dry), Application (Western, Northern, Southern Blot, Protein Sequencing), End user (Academia, Diagnolab, Pharmaceutical Companies) – Global Forecast to 2023“, published by MarketsandMarkets™, the global market is projected to reach USD 187.9 million by 2023 from USD 174.8 million in 2018, at a CAGR of 1.5% during the forecast period.
Browse in-depth TOC on “Transfer Membrane Market“
64 data
35 Figures
124 Pages
Download PDF Brochure: @ https://www.marketsandmarkets.com/pdfdownload.asp?id=36975865
Factors such as increasing public and private funding for life science research, the significantly high prevalence of target diseases across the globe, and increasing R&D spending by pharmaceutical and biotechnology companies are driving the growth of this market.
By Type, the PVDF transfer membrane segment is expected to hold the largest share of the market in 2018
On the basis of types, the Transfer Membrane Market is classified into nylon transfer membrane, nitrocellulose transfer membrane, and PVDF transfer membranes. In 2018, PVDF transfer membranes are expected to command the major share of the Transfer Membrane Market.. The PVDF transfer membrane segment is expected to account for the largest share of the Transfer Membrane Market in 2018. Advantages of PVDF transfer membrane over nitrocellulose membranes such as higher durability and hydrophobicity, increasing R&D by pharmaceutical and biotechnology companies, and growing proteomic research are the major factors driving the growth of this segment.
By Transfer method, the dry electro transfer segment is expected to be the fastest growing segment in the forecast period
On the basis of transfer method, the Transfer Membrane Market is segmented into tank electrotransfer, semi-dry electrotransfer, dry electrotransfer, and other transfer methods (diffusion blotting and vacuum blotting). The Dry electrotransfer segment is expected to grow at the fastest CAGR during the forecast period. Growth in this segment is attributed to the advantages of dry-electrotransfer over conventional methods, including rapid, high-quality transfer and ease of use due to the non-requirement of additional buffers in the case of dry-electroblotting.
Request Sample Pages : https://www.marketsandmarkets.com/requestsampleNew.asp?id=36975865
North America is expected to dominate the market in 2018
Geographically, the Transfer Membrane Market is segmented into North America, Europe, Asia Pacific, and the Rest of the World. North America is expected to account for the largest share of the market in 2018, followed by Europe. The large share of North America can primarily be attributed to presence of leading transfer membrane manufacturers in the region, availability of government and private financial support for life science research, and a high disease prevalence in the region.
The key players in the Transfer Membrane Market include Merck KGaA (US), Thermo Fisher Scientific (US), Bio-Rad Laboratories (US), GE Healthcare (US), PerkinElmer (US), Pall Coporation (US), Advansta (US), GVS (Italy), Santa Cruz Biotechnology (US), Abcam (UK), ATTO Corporation (Japan), Carl Roth (Germany), Macherey-Nagel (Germany), Azure Biosystems (US), and Axiva Sichem Biotech (India), among others.
Browse Adjacent Markets: Biotechnology Market Research Reports & Consulting
Browse Related Reports:
Western Blotting Market by Product (Instruments, Consumables), Application (Biomedical & Biochemical Research, Disease Diagnostics), End User (Academic & Research Institutes, Pharmaceutical & Biotechnology Companies) – Global Forecasts to 2021
https://www.marketsandmarkets.com/Market-Reports/western-blotting-market-235810711.html
Membranes Market by Material (Polymeric, Ceramic), Technology (RO, UF, MF, NF), Application (Water & Wastewater Treatment, Industrial Processing), Region (North America, APAC, Europe, MEA, South America) – Global Forecast
to 2024
https://www.marketsandmarkets.com/Market-Reports/membranes-market-1176.html
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the “Growth Engagement Model – GEM”. The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write “Attack, avoid and defend” strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, “Knowledge Store” connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Salgarkar
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
0 notes
Link
0 notes