#Stem Cells
Text
UCLA scientists have identified a protein that plays a critical role in regulating human blood stem cell self-renewal by helping them sense and interpret signals from their environment.
The study, published in Nature, brings researchers one step closer to developing methods to expand blood stem cells in a lab dish, which could make life-saving transplants of these cells more available and increase the safety of blood stem cell-based treatments, such as gene therapies.
Blood stem cells, also known as hematopoietic stem cells, have the ability to make copies of themselves via a process called self-renewal, and can differentiate to produce all the blood and immune cells found in the body. For decades, transplants of these cells have been used as life-saving treatments for blood cancers such as leukemia and various other blood and immune disorders.
Continue Reading.
197 notes
·
View notes
Text
mitochondria miku

#hatsune miku#mikuhatsune#miku fanart#miku#mitochondria#stem cells#oc#my oc art#my art#digital art#concept art#artists on tumblr#character concept#concpet art
47 notes
·
View notes
Text
damn viruses really are out here gaslighting gatekeeping and girlbossing they’re way to taking over cells. stop it man that’s not cool :(
51 notes
·
View notes
Text
Bone Cells

I will try to draw the other types of cells without having an artblock
11 notes
·
View notes
Text
Signals for Embryos
A stem cell-derived mouse embryo model reveals the molecular signals that orchestrate early embryo tissue patterning [getting the cells and layers in the right place]
Read the published research article here
Video from work by Sina Schumacher and colleagues
Department of Systemic Cell Biology, Max Planck Institute of Molecular Physiology, Dortmund, Germany
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature Communications, June 2024
You can also follow BPoD on Instagram, Twitter and Facebook
11 notes
·
View notes
Text
Scientists create synthetic human embryos

from the exclusive Guardian story:
Using stem cells, scientists have created synthetic human embryos in a groundbreaking advance that sidesteps the need for eggs or sperm.
Scientists say these model embryos, which resemble those in the earliest stages of human development, could provide a crucial window on the impact of genetic disorders and the biological causes of recurrent miscarriage.
The work also raises serious ethical and legal issues, as the lab-grown entities fall outside current legislation in most countries.
Prof Magdalena Żernicka-Goetz, of the U of Cambridge and the California Institute of Technology, described the work in a plenary address at the International Society for Stem Cell Research’s annual meeting in Boston.
“We can create human embryo-like models by the reprogramming of embryonic stem cells,” she told the meeting.
There is no near-term prospect of the synthetic embryos being used clinically. It would be illegal to implant them into a patient’s womb, and it is not yet clear whether these structures have the potential to continue maturing beyond the earliest stages of development.
Previously, Żernicka-Goetz’s team and a rival group at the Weizmann Institute in Israel showed that stem cells from mice could be encouraged to self-assemble into early embryonic structures with an intestinal tract, the beginnings of a brain, and a beating heart. Since then, a race has been under way to translate this work into human models, and several teams have been able to replicate the very earliest stages of development.
In April, researchers in China created synthetic embryos from monkey cells and implanted them into the wombs of adult monkeys, a few of which showed initial signs of pregnancy but none of which developed beyond a few days. Scientists say it is not clear whether the barrier to more advanced development is merely technical or has a more fundamental biological cause.
“It’s going to be hard to tell whether there’s an intrinsic problem with them or whether it’s just technical,” said Robin Lovell-Badge, the head of stem cell biology and developmental genetics at the Francis Crick Institute. This unknown potential made the need for stronger legislation pressing, he said.
“Our human model is the first three-lineage human embryo model that specifies amnion and germ cells, precursor cells of egg and sperm,” Żernicka-Goetz told the Guardian before the talk. “It’s beautiful and created entirely from embryonic stem cells.”
so we're about to completely leap over the first steps of making test-tube babies - gathering eggs and sperm and having them get things rolling - and going straight to manufacturing embryos, huh
65 notes
·
View notes
Text
3D Bioprinting Takes Eye Tissue Engineering to New Heights at NIH

Scientists at the National Eye Institute (NEI), part of the National Institutes of Health, have made a significant advancement in the field of 3D bioprinting by successfully creating functional eye tissue using this innovative technique. The potential for this development to revolutionize the treatment of eye conditions and diseases is vast, and further research will undoubtedly continue to build upon these groundbreaking findings.
The research team used 3D bioprinting and patient stem cells to create eye tissue that will help researchers better understand blinding diseases. The team printed a combination of cells that make up the outer blood-retina barrier, a type of eye tissue that supports the retina’s photoreceptors. This technique could potentially provide an unlimited supply of patient-derived tissue for studying degenerative retinal diseases like age-related macular degeneration (AMD).
Continue Reading
#3d printing#biotechnology#amd#health#technology#stem cells#biomedical engineering#scicomm#medcomm#science news
115 notes
·
View notes
Text



Studying for histology and also trying to read more and more books in my free time.I am so afraid for the upcoming exams.I am praying that I do well.
#studyblr#medicine#histology#stem cells#fantasy#med studyblr#medstudent#medschool#studyspo#study motivation#medical school#gilmore girls#taylor swift#study aesthetic#study playlist#study desk#study space#bookworm#unilife#books and reading#booklr#book lover#sarah j maas#house of sky and breath#im so tired#keep moving forward#inspiration#exam stress#good grades
12 notes
·
View notes
Text

Brb I gotta sell my period blood to science
8 notes
·
View notes
Text
A new study using stem cell-based models has shed new light on how the human embryo begins to develop, which could one day benefit the development of fertility treatment.
The study led by the University of Exeter Living Systems Institute has revealed how early embryo cells decide between contributing to the fetus or to the supporting yolk sac. The paper is titled "Naive pluripotent stem cell-based models capture FGF-dependent human hypoblast lineage specification" and is published in Cell Stem Cell.
Understanding this decision is important because the yolk sac is essential for later development in the womb. Producing the right number of yolk sac forming cells may be critical for infertility treatment using in vitro fertilized (IVF) embryos.
Continue Reading.
48 notes
·
View notes
Text
A team of researchers in the United States and United Kingdom say they have created the world’s first synthetic human embryo-like structures from stem cells, bypassing the need for eggs and sperm.
These embryo-like structures are at the very earliest stages of human development: They don’t have a beating heart or a brain, for example. But scientists say they could one day help advance the understanding of genetic diseases or the causes of miscarriages.
The research raises critical legal and ethical questions, and many countries, including the US, don’t have laws governing the creation or treatment of synthetic embryos.
The pace of discoveries in this field and the growing sophistication of these models have alarmed bioethics experts as they push ever closer to the edge of life.
“Unlike human embryos arising from in vitro fertilization (IVF), where there is an established legal framework, there are currently no clear regulations governing stem cell derived models of human embryos. There is an urgent need for regulations to provide a framework for the creation and use of stem cell derived models of human embryos,” James Briscoe, associate research director at the Francis Crick Institute, said in a statement.
Dr. Magdalena Zernicka-Goetz described the work in a presentation Wednesday to the International Society for Stem Cell Research’s annual meeting in Boston. Zernicka-Goetz, a professor of biology and biological engineering at CalTech and the University of Cambridge, said the research has been accepted at a well-regarded scientific journal but has not been published. The research was first reported by The Guardian.
Zernicka-Goetz and her team, along with a rival team in Israel, had previously described creating model embryo-like structures from mouse stem cells. Those “embryoids” showed the beginnings of a brain, heart and intestinal tract after about eight days of development.
The embryo-like structures that Zernicka-Goetz says her lab has created were grown from single human embryonic stem cells that were coaxed to develop into three distinct tissue layers, she said. They include cells that would typically go on to develop a yolk sac, a placenta and the embryo itself.
She told CNN that the embryo-like structures her lab has created are also the first to have germ cells that would go on to develop into egg and sperm.
“I just wish to stress that they are not human embryos,” Zernicka-Goetz said. “They are embryo models, but they are very exciting because they are very looking similar to human embryos and very important path towards discovery of why so many pregnancies fail, as the majority of the pregnancies fail around the time of the development at which we build these embryo-like structures.”
She said that to her knowledge, it was the first time a human model embryo had been created with three tissue layers. But she stressed that while it mimics some of the features of a natural embryo, it doesn’t have all of them.
Researchers hope these model embryos will shed light on the “black box” of human development, the period following 14 days after fertilization, which is the agreed limit for scientists to grow and study embryos in a lab.
Right now, the synthetic model human embryos are confined to test tubes. It would be illegal to implant one in a womb, and animal research with stem cells from mice and monkeys has shown that even when scientists have attempted to implant them, they don’t survive – probably because researchers haven’t figured out how to fully replicate the conditions of pregnancy.
Zernicka-Goetz said that the aim of her research wasn’t to create life but to prevent its loss, understanding why embryos sometime fail to develop after fertilization and implantation.
“We know remarkably little about this step in human development, but it is a time where many pregnancies are lost, especially in an IVF setting,” Roger Sturmey, senior research fellow in maternal and fetal health at the University of Manchester in the UK, said in a statement.
“Currently, we can say that these ‘synthetic embryos’ share a number of features with blastocysts, but it is important to recognise that the way that synthetic embryos are formed is different to what happens when a normal embryo forms a blastocyst,” he said. “There is much work to be done to determine the similarities and differences between synthetic embryos and embryos that form from the union of an egg and a sperm.”
10 notes
·
View notes
Text
#itsbethdaniels#X39#reactivateYourStemCells#stem cell therapy#stem cells#phototherapy#light therapy#get patched#get paid#sharing is caring
7 notes
·
View notes
Text
A vegan cannibal who grows plant based human meat and organs
#tumblr#cannibal#vegan#good#science side of the internet#scientist cannibal#biologist#stem cells#research for food#food science#food porn#science#cannibalism#vegetables#vegetable
3 notes
·
View notes
Text
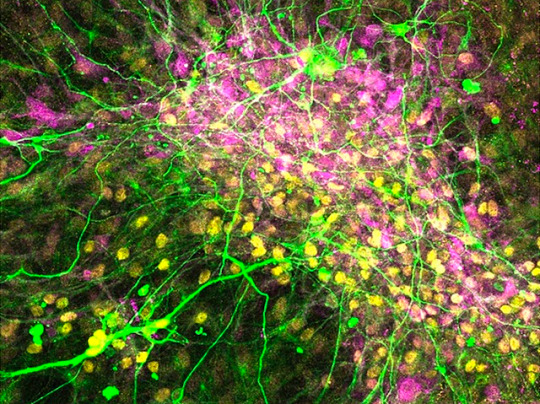
Nerve Meets Muscle
Aligned muscle fibres surrounded by innervating motor neurons forming functional neuromuscular junctions grown from human pluripotent stem cells creates a model for studying neuromuscular disease and drug targeting
Read the published research article here
Image from work by Alessia Urzi and colleagues
Stem Cell Modeling of Development & Disease Group, Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature Communications, December 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#biology#neuroscience#nerves#muscles#neuromuscular junction#immunofluorescence#stem cells#neuromuscular disease#neurobiology
23 notes
·
View notes
Text
I have been a regular Red Cross donor. Especially plasma and platelets the last 5 years.
Today I received the letter I have been waiting for. I’m officially an international registered stem cell donor!
If there is ever a match, I’ll gladly try to help save that person’s life!
5 notes
·
View notes
Text
youtube
6 notes
·
View notes