#beta-lactam resistance
Text
A Cross-Sectional Study of Cephalosporin Prescriptions for the Treatment of Respiratory and Urinary Tract Infections in Two Sudanese Hospitals
Abstract
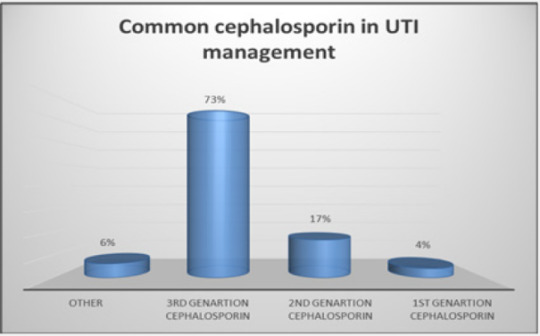
Cephalosporins representing a wide variety of β-lactam antibiotics. Cephalosporins have some desirable features, including a convenience of administration, a reasonably broad spectrum of efficacy and a low incidence of toxicity. A descriptive cross-sectional study on the usage of cephalosporin for the treatment of respiratory tract infections (RTI) and urinary tract infections (UTI) was conducted at Ibnsinaa and Alshaab Hospitals in Khartoum state. The data were acquired via questionnaires sent to doctors and community pharmacists, as well as 48 patient files with UTI and RTI diagnoses. SPSS was used to examine the data. The study’s findings indicated that 90% of physicians and pharmacists do not follow cephalosporin prescription and dispensing recommendations. 73% of cephalosporins (3rd generation) are used to treat UTI, whereas 54% of cephalosporins (2nd generation) are used to treat RTI. At conclusion, the findings of this research reveal that the use of cephalosporin in these hospitals is often inconsistent with accepted therapeutic principles. To prevent the emergence of cephalosporin-resistant pathogens, healthcare providers should be cautious when prescribing antibiotics and remain current on recommended antibiotic practices and dosages.
Keywords: Antibiotics; Cephalosporin; UTI; RTI; Infections; Sudan
Introduction
Infectious diseases were a major cause of morbidity and death before to the turn of the twentieth century. Even in the industrialized world, the average life expectancy at birth for men and women was 46 and 48 years, respectively. Plaque, diphtheria, smallpox, pneumonia, cholera, typhoid fever, syphilis, tuberculosis, typhus, and other contagious illnesses were common [1]. Alexander Flemming’s discovery of the first antibiotic (penicillin) in 1928 revolutionized medicine and saved millions of lives [2]. Following the end of Second World War, the golden era of antibiotic discovery began. From the 1950s until the 1970s, dozens new antibiotics were discovered each year, and they revolutionized medicine. Without antibiotics, routine treatments such as open-heart surgery, chemotherapy for cancer patients with compromised immune systems, and organ transplantation would be impossible [3-5]. However, bacteria quickly evolved resistance to antibiotics, and the frequency of infections caused by multidrug-resistant bacteria is growing globally. Since the turn of the twenty-first century, the threat of untreatable diseases has loomed [6,7].
Cephalosporins were not discovered by chance. World War II needs pushed the quest for antibiotics generated by microorganisms [8]. Cephalosporins are antibiotics with a beta-lactam ring that are derived from the Acremonium fungus, commonly known as cephalosporium, this important antibiotic is widely used against bacteria in a variety of serious diseases, including respiratory tract infection (RTI), skin infection, and urinary tract infection (UTI) [9]. Cephalosporins currently come in five generations. With the development of fifth generation cephalosporins, infection management has become even more difficult. However, their use must be strictly limited because if bacteria develop resistance to the fifth generation cephalosporins, infection management will become very difficult [10] Over the last few decades, the rise and spread of beta-lactam resistance in nosocomial Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa, has become a major global concern. Particularly concerning is the rising resistance to third- and fourth generation cephalosporins [11].
Antibiotics are widely utilized in Sudan, and the majority of hospitals in the country rely heavily on cephalosporin antibiotics, especially in surgical departments, as the preferred option for prophylaxis [12]. Accordingly, the current study aimed to evaluate use of cephalosporin in the treatment of respiratory and urinary tract infections in two Sudanese hospitals (Ibnsinaa and Alshaab Hospitals).
Methodology
Study design
This study used a descriptive cross-sectional survey to confirm and/or refute assumptions about the attitudes of health professionals in two hospitals in Khartoum that treat patients with UTI and RTI with cephalosporins, as well as to evaluate the results in order to comprehend and resolve the study’s issue.
Study area
The study took place in two hospitals in Khartoum, Sudan’s capital: Ibnsinaa and Alshaab Hospitals in the state of Khartoum.
Study duration
Two months, between May and July 2018, the surveys were performed utilizing a questionnaire to gather data.
Data collection
The sample size was chosen to be 96 prior to completing the survey. The questionnaire was anonymous. It elicited data on cephalosporins administered for UTI and RTI under treatment recommendations, the Protocol for Dispensing Cephalosporin, the Mode of Prescription, the Common Cephalosporin Used to Manage UTI and RTI, and Counseling Patients About Drugs.
Ethical approval statement
The research used a cross-sectional design. The study protocol was authorized by the ethical committee at Alneelain University’s Faculty of Pharmacy in Khartoum, Sudan, in accordance with the Helsinki Declaration for the conduct of human experimentation. Each participant completed an informed permission form after receiving a thorough verbal summary of the process.
Statistical analysis
The statistical analyses were performed, classified, and analyzed using SPSS. The descriptive data and results were presented using tables and figures. To compare and correlate variables, the chi-square test was utilized.
Results and Discussion
Cross-sectional studies often enable researchers to gather a large amount of data fast. Self-report questionnaires are often used to acquire data affordably. However, causal correlations might be difficult to deduce from cross-sectional data [13].
According to our current study, numerous significant facts were discovered throughout the present cross-sectional investigation. As seen in (Table 1), the protocol for treating RTI and UTI infections at the respective institutions which should be followed by healthcare providers. Clinical guidelines are gaining popularity as a tool for clinicians to use to influence their practice. No guideline, however, can be sufficiently detailed to apply to all clinical circumstances [14].
Additionally, 90 % of healthcare personnel (physicians and pharmacists) at these two hospitals do not adhere to cephalosporin prescription and dispensing guidelines (Table 2). These intriguing results highlight a global concern, especially in developing countries where antibiotic stewardship is poor. Regretfully, the irrational use of antibiotics in Sudan is well-documented [15,16]. According to previously published data, even developing countries with a better health situation than Sudan, a significant amount of antibiotics is provided without a prescription, and a large percentage of antibiotics supplied are unsuitable for the illnesses being treated [17]. The WHO acknowledged irrational antibiotic usage as a significant role in the development of antimicrobial resistance in its two publications, ‘Global Strategy for Antimicrobial Resistance Containment’ and ‘The Pursuit of Responsible Medicines’ and therefore, health authorities in developing countries should tackle this concern [18].
In our study, as shown in (Figure 1-3), 90 % of healthcare providers at these hospitals did not follow specific manner in prescription of cephalosporins for UTI and RTI patients. 4% of participants prescribed first generation cephalosporins, 17% prescribed second generation, 73% prescribed third generation, and 6% prescribed other antibiotics, as shown in Figure 2 & 3. As a result, the third-generation cephalosporin is the most often used antibiotic to treat urinary tract infections. Additionally, our survey found that 6% of respondents prescribed the first generation of cephalosporins to control RTI infections, 54% used the second generation, 31% used the third generation, and 8% used others, as shown in Figure 2 & 3. As a result, we discovered that second generation cephalosporins are effective in treating RTI infections in our investigation.
Numerous clinics worldwide give cephalosporins to patients in excess of what is necessary and with an excess of extravagance that borders on abuse, necessitating medical monitoring and control to prevent the establishment of anti-cephalosporin infections [19,20]. Fortunately, several institutions have recognized the negative repercussions and created control procedures aimed at possibly limiting antibiotic usage and abuse [21]. These control strategies must be implemented as soon as possible in developing countries such as Sudan, since some countries have reported infections and the rise of cephalosporin-resistant pathogens. For instance, Acinetobacter baumannii strains was detected highly resistant to cephalosporins and β-lactamases in Syria [22], In the United Kingdom, Enterobacter cloacae reported resistant to third generation cephalosporins [23], and Klebsiella infection which was found resistant to late-generation cephalosporins in a nosocomial outbreak in the United States [24]. Finally, Effective antibiotic resistance prevention strategies are available and should be adopted aggressively in critical care units. These strategies fall into three categories: nonpharmacologic infection control, antibiotic management and increasing existing efforts to avoid antibacterial resistance, particularly given the expected future scarcity of novel antibacterial medication classes [25].
To Know More About Novel Approaches in Drug Designing & Development
Please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#antibiotic discovery#beta-lactam resistance#cephalosporin-resistant pathogens#chemotherapy#juniper publishers#open acess journals#drug development
1 note
·
View note
Text
Augmentin: a step up from those other pedestrian penicillins, augmentin contains both a beta-lactam and a beta-lactamase inhibitor as an added defense against pesky bacteria. It's one of the most commonly prescribed medications in the world, and it can be used to treat a wide variety of infections (in both humans and animals!) with good coverage against both gram positives and gram negatives. While it's pretty low-risk overall, it is one of the most frequent causes of idiosyncratic drug-induced hepatic injury (though the incidence comes out to about 43 in every 100,000 prescriptions of augmentin) (1).
Penicillin: the OG. The first antibiotic. Who could stand against her? Famously isolated from mold following Dr. Alexander Fleming's observations that it could keep bacteria at bay, penicillin was instrumental in ushering in the Age of Antibiotics. It saved lives throughout WWII and continues to save lives today with coverage against gram positives, gram negatives, and anaerobes--it even treats syphilis! Though its use has waned in the face of increased bacterial resistance, penicillin is still a strong contender in this tournament.
Vote for the best antibiotic
(1) deLemos AS, Ghabril M, Rockey DC, et al. Amoxicillin-Clavulanate-Induced Liver Injury. Dig Dis Sci. 2016;61(8):2406-2416. doi:10.1007/s10620-016-4121-6
#antibiotic tournament#antibiotics#medicine#medblr#med student#med school#pharmblr#pharmacy#polls#poll tournament#antibiotic tournament round one
48 notes
·
View notes
Text
Being rendered helpless (PANOPTICON)
• Rita Ora's thumb (Encounter for aftercare following multiple organ transplant)
• Florence Welch's thumb (Laceration with foreign body of right ring finger with damage to nail)
• Winona Ryder's thumb (Secondary lacrimal gland atrophy)
• Lucy Hale's thumb (Failure in dosage during unspecified surgical and medical care)
• Conan O'Brien's thumb (Influenza due to other identified influenza virus with otitis media)
• Tyra Banks's thumb (Malignant neoplasm of overlapping sites of other and unspecified parts of mouth)
• AnnaSophia Robb's thumb (Laceration of extensor muscle, fascia and tendon of left middle finger at forearm level)
• Minka Kelly's thumb (Acute tonsillitis, unspecified)
• Djimon Hounsou's thumb (Cyst and mucocele of nose and nasal sinus)
• Forest Whitaker's thumb (Meningococcal myocarditis)
• Jimmy Buffett's thumb (Other disorders of continuity of bone, right radius)
• Kate Bosworth's thumb (Other hyperparathyroidism)
• Kristen Bell's thumb (Solitary bone cyst, left ulna and radius)
• Matt Bomer's thumb (Laceration of other muscles, fascia and tendons at shoulder and upper arm level, unspecified arm)
• Prince Harry's thumb (Laceration without foreign body of back wall of thorax without penetration into thoracic cavity)
• Avril Lavigne's thumb (Calcification and ossification of muscle)
• Demi Lovato's thumb (Nondisplaced fracture of lateral condyle of unspecified femur)
• Carmen Electra's thumb (Salter Harris Type III physeal fracture of upper end of humerus, left arm)
• Mary-Louise Parker's thumb (Atherosclerosis of other type of bypass graft(s) of the extremities with intermittent claudication, left leg)
• Vince Vaughn's thumb (Toxic effect of contact with other venomous marine animals, assault)
• Sean Lennon's thumb (Unspecified open wound of left front wall of thorax without penetration into thoracic cavity)
• Tate Donovan's thumb (Osseous and subluxation stenosis of intervertebral foramina of abdomen and other regions)
• Jennifer Aniston's thumb (Alcohol abuse with intoxication)
• Zachary Quinto's thumb (Mooren's corneal ulcer, unspecified eye)
• Tracy Morgan's thumb (Preterm labor without delivery, unspecified trimester)
• Jenna Elfman's thumb (Inflammatory polyneuropathy, unspecified)
• Kaley Cuoco-Sweeting's thumb (Perforated corneal ulcer, unspecified eye)
• DJ AM's thumb (Kaschin-Beck disease, left knee)
• Gordon Ramsay's thumb (Unspecified injury of extensor muscle, fascia and tendon of right index finger at forearm level)
• Elle Fanning's thumb (Benign neoplasm of connective and other soft tissue of unspecified upper limb, including shoulder)
• Scott Speedman's thumb (Encounter for routine postpartum follow-up)
• Curtis Stone's thumb (Swimmer's ear, left ear)
• Uma Thurman's thumb (Altered mental status, unspecified)
• Khloe Kardashian's thumb (Retinal hemorrhage, left eye)
• Maria Menounos's thumb (Passenger in three-wheeled motor vehicle injured in collision with fixed or stationary object in nontraffic accident)
• Miranda Kerr's thumb (Other combined immunodeficiencies)
• Brooklyn Decker's thumb (Atherosclerosis of other type of bypass graft(s) of the extremities with intermittent claudication, left leg)
• Ellie Goulding's thumb (Osteonecrosis in diseases classified elsewhere, thigh)
• Bethenny Frankel's thumb (Other chronic hematogenous osteomyelitis, left humerus)
• Judi Dench's thumb (Resistance to unspecified beta lactam antibiotics)
2 notes
·
View notes
Text
Pneumonia In Children And Adults

Introduction
Pneumonia stands as a prevalent respiratory infection, exerting a significant burden on global public health. Its impact extends beyond mere morbidity, contributing to substantial healthcare costs and socioeconomic consequences. This discussion aims to elucidate the general nature of pneumonia, encompassing its pathophysiology, clinical presentation, diagnostic modalities, treatment strategies, complications, and preventive measures. By indulging into these factors, we aim to provide a better understanding of pneumonia’s complexity and underscore the importance of timely recognition and management.
Pathophysiology

Pneumonia ensues from the infiltration of infectious agents, including bacteria, viruses, fungi, and less commonly, parasites, into the lower respiratory tract. Upon inhalation or aspiration of these pathogens, they gain access to the alveoli, where they incite an inflammatory response. This inflammatory cascade triggers the release of pro-inflammatory cytokines and chemokines, recruiting immune cells to the site of infection. Neutrophils, macrophages, and lymphocytes converge to eradicate the invading pathogens, leading to the characteristic consolidation and exudate formation within the affected lung tissue. As the infection progresses, alveolar edema, impaired gas exchange, and parenchymal damage ensue, culminating in the clinical manifestations of pneumonia.
Clinical Presentation

The clinical presentation of pneumonia encompasses a spectrum of symptoms, ranging from mild respiratory complaints to life-threatening respiratory failure. Common symptoms include cough, productive sputum production, fever, chills, pleuritic chest pain, dyspnea, tachypnea, and systemic manifestations such as malaise and fatigue. The severity of symptoms varies depending on factors such as the underlying pathogen, the extent of lung involvement, the host’s immune status, and comorbidities. In pediatric populations, pneumonia may present with nonspecific symptoms such as feeding difficulties, lethargy, and irritability, posing diagnostic challenges. Conversely, elderly individuals may exhibit atypical presentations characterized by confusion, hypothermia, and exacerbations of underlying chronic conditions.
Diagnostic Modalities

The diagnosis of pneumonia hinges on a comprehensive clinical assessment, augmented by various diagnostic modalities to confirm the presence of pulmonary infection and reveal its etiology. A thorough history and physical examination provide invaluable insights into the patient’s symptomatology, risk factors, and clinical trajectory. Symptomatic findings such as crackles, wheezes, and diminished breath sounds may aid in localizing the site of infection and assessing disease severity. Radiographic imaging, notably chest X-rays and computed tomography (CT) scans, serves as the cornerstone of pneumonia diagnosis, revealing characteristic radiographic findings such as airspace opacities, lobar consolidation, and interstitial infiltrates. Laboratory investigations, including complete blood count (CBC), C-reactive protein (CRP), and procalcitonin levels, may corroborate the clinical suspicion of pneumonia and guide therapeutic decisions. Additionally, microbiological testing of respiratory specimens through techniques such as sputum culture, blood cultures, and polymerase chain reaction (PCR) assays facilitates pathogen identification and antimicrobial susceptibility testing, thereby informing targeted therapy.
Treatment Strategies

The management of pneumonia hinges on prompt initiation of empiric antimicrobial therapy tailored to the likely causative pathogen(s) and disease severity. Antibiotics represent the mainstay of treatment for bacterial pneumonia, with the choice of agent dictated by factors such as local antimicrobial resistance patterns, patient age, comorbidities, and recent antibiotic exposure. Commonly prescribed antibiotics include beta-lactam agents (e.g., penicillins, cephalosporins), macrolides, fluoroquinolones, and combination regimens for severe or healthcare-associated infections. Conversely, viral pneumonia necessitates supportive care measures, given the limited efficacy of antiviral agents in most cases. Influenza-associated pneumonia may benefit from neuraminidase inhibitors such as oseltamivir, while respiratory syncytial virus (RSV) pneumonia may warrant ribavirin therapy in select cases. Adjunctive therapies such as oxygen supplementation, bronchodilators, and corticosteroids may mitigate respiratory distress and improve clinical outcomes, particularly in severe or hypoxemic patients. The duration of antimicrobial therapy varies depending on factors such as the causative pathogen, clinical response, radiographic resolution, and the presence of complications. Close monitoring of clinical parameters and serial imaging studies guide the decision-making process, enabling clinicians to tailor therapy to individual patient needs.
Complications

Pneumonia harbors the potential for various complications, ranging from mild to life-threatening sequelae, necessitating vigilant monitoring and timely intervention. Common complications include pleural effusion, empyema, lung abscess, respiratory failure, septic shock, and acute respiratory distress syndrome (ARDS). Pleural effusion denotes the accumulation of fluid within the pleural space, secondary to inflammation or impaired lymphatic drainage, manifesting as dyspnea, pleuritic chest pain, and dullness to percussion on physical examination. Empyema represents a purulent collection within the pleural cavity, often complicating bacterial pneumonia and necessitating drainage via thoracentesis or chest tube placement. Lung abscesses manifest as circumscribed cavities containing necrotic debris and pus within the lung parenchyma, triggered by persistent fever, productive cough, and hemoptysis. Respiratory failure ensues from impaired gas exchange and alveolar hypoventilation, caused by worsening hypoxemia, hypercapnia, and respiratory acidosis, necessitating mechanical ventilation and intensive care support. Septic shock represents a life-threatening complication of severe pneumonia, characterized by systemic inflammatory response syndrome (SIRS) and end-organ dysfunction, requiring aggressive fluid resuscitation, vasopressor therapy, and broad-spectrum antibiotics. ARDS denotes a severe form of acute lung injury, characterized by diffuse alveolar damage, refractory hypoxemia, and bilateral infiltrates on chest imaging, necessitating lung-protective ventilation and supportive care in the intensive care unit (ICU). The occurrence of complications portends a poor prognosis and underscores the need for early recognition and intervention to mitigate adverse outcomes.
Preventive Measures

Preventing pneumonia entails a broad approach encompassing vaccination, infection control measures, and health promotion strategies aimed at reducing the risk of respiratory infections and their sequelae. Vaccination stands as a cornerstone of pneumonia prevention, targeting common bacterial and viral pathogens implicated in pneumonia pathogenesis. Vaccines such as the pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23) confer protection against Streptococcus pneumoniae, the leading bacterial cause of pneumonia, particularly in high-risk populations such as young children, older adults, and immunocompromised individuals. Influenza vaccination remains paramount in mitigating influenza-associated pneumonia and reducing disease transmission, underscoring the importance of annual vaccination campaigns targeting vulnerable populations. Additionally, adherence to infection control measures, including hand hygiene, respiratory etiquette, and environmental sanitation, plays a pivotal role in reducing the spread of respiratory pathogens in healthcare settings and the community at large. Health promotion efforts aimed at smoking cessation, optimizing nutrition, and addressing underlying comorbidities such as chronic obstructive pulmonary disease (COPD), asthma, and immunodeficiency bolster immune resilience and mitigate pneumonia risk. Furthermore, early identification and management of predisposing factors such as malnutrition, homelessness, and overcrowded living conditions attenuate pneumonia susceptibility and enhance overall health outcomes.
Conclusion
In conclusion, pneumonia emerges as a formidable respiratory infection, posing significant challenges to global public health. Its diverse etiology, clinical manifestations, diagnostic modalities, treatment modalities, complications, and preventive measures underscore the nature of pneumonia management. Timely recognition and intervention are imperative in mitigating the morbidity and mortality associated with pneumonia, necessitating a collaborative approach among healthcare providers, public health authorities, and policymakers. By fostering a comprehensive understanding of pneumonia’s manifest and implementing evidence-based strategies, we can strive towards reducing its burden and improving patient outcomes. Through ongoing research, education, and advocacy efforts, we can envision a future where pneumonia-related morbidity and mortality are substantially diminished, paving the way for enhanced respiratory health and well-being worldwide.
In managing pneumonia, compassion, empathy, and a holistic approach are essential alongside clinical expertise. Striving for excellence in knowledge and practice allows us to enhance respiratory medicine and patient outcomes.
As we address pneumonia and broader cardiovascular health complexities, let’s remain committed to optimal patient care. Together, we can impact lives positively and foster a healthier future.
Medical students encounter significant academic challenges during their studies, balancing coursework, clinical rotations, research, and personal commitments. Expert Academic Assignment Help offers tailored assistance to meet their needs, providing study materials, tutoring, assignment help, and exam preparation. Beyond academics, it fosters a supportive environment for mentorship and guidance. In essence, Expert Academic Assignment Help is a valuable resource for medical students, empowering them to excel academically and develop into competent healthcare professionals. Contact us at [email protected] for professional assistance
#assignment help#medical students#healthcare#nursing school#nursing student#medicine#medical help#academic assignments#university student#medical university#university life#university#student#student life#study blog#study inspiration#studyblr community#studyblr#study motivation#medication#medical student#medical school#medicare#writing#writers on tumblr#writerscommunity#writeblr#online writing#academic writing
2 notes
·
View notes
Text
Leukocytes or nitrites present 75% sensitivity and 82% specificity; all you need for simple cystitis
Culture positive if >10^5 CFU or 10^2 CFUs with symptoms; necessary for pyelonephritis/complicated UTI
CT will show obstruction, calculi, gas-forming infections
Men: STI, prostatitis, urethritis
Tx: Macrobid 100 mg x5 days, Bactrim DS bid x3 days
Pyelo: FQs, CTX, zosyn, cefepime if low risk for MDR
Meropenem, IV FQs, CTX, zosyn, cefepime for high risk MDR
Recurrent UTIs: pyridium, post coital abxs, urogyn referral, daily ppx with Bactrim, Macrobid, or Keflex
Pyelo f/u: PRN, urology, or urogyn f/u
Highest prevalence among uncircumcised males <3 months
In peds pts, enterococcus is not a contaminant in the urine culture as it typically is in adults
You want to avoid renal scarring; any other organism other than E.coli has increased risk of causing renal scarring in kids
US recommended in kids <2 years with first febrile UTI, any age with recurrent UTI, any age with fam hx of kidney/uro disease, poor growth, HTN, failure to respond to tx
Voiding cystourethrogram for anatomical eval and for reasons listed above
Simple cystitis tx:
Infants: cephalosporin; Keflex 50-100 mg/kg qd divided bid x5 days. Allergy? Can use Bactrim, Augmentin, rarely ciprofloxacin
Pts 1 month to 2 years: IV CTX, gentamicin; cefdinir 14 mg/kg qd divided bid x10 days
If no improvement in 48-72 hours, change abxs and pursue imaging
For infants, you need to do f/u imaging if not done in hospital
Pregnant women have acute cystitis, not simple cystitis because pregnant women are not simple
Abxs in pregnancy: beta lactam, Macrobid (not in first trimester), Fosfomycin; duration of therapy is 5-7 days
Bactrim avoided during pregnancy. Cefpodoxime is another one safe in pregnancy.
Pyelo in pregnancy: consider intraamniotic infection and placental abruption; it’s not an indication for delivery. Can tx with IV CTX or zosyn.
You have to recheck UA after treatment of asymptomatic bacteriuria in pregnant pts; 30% don’t clear it
Macrobid and Bactrim should be avoided in pregnancy; Macrobid more so in the first trimester; avoid Bactrim throughout pregnancy
Febrile neonate: tachypnea, irritability, cyanosis, poor feeding; <1% of term infants have UTIs. Limited data for preterm infants.
Term infants tend to get E. coli. In preterm infants, coagulase neg staph and Klebsiella are more common; really small premies can have candida.
Hematogenous spread can occur in premies.
Neonates: UA, culture, blood culture, lumbar puncture; imaging, voiding cystourethrogram
Broad spectrum abxs in babies: Ampicillin and gentamicin for 10 to 14 days
CTX can increase serum free bilirubinà increased jaundice
Kids can have impaired renal growth that resolved
Catheter Associated UTI = CAUTI; no need to screen unless pt is symptomatic
Pyuria is not enough to diagnose UTI in pts with indwelling catheters; you need a culture, which you compare to previous culture. Percutaneous nephrostomy tubes, stents – get urology involved. Tx with broad spectrum abxs until you get culture results. Tx 7-14 days. Levofloxacin x5 days if not severely ill; 3 days for pts under 65 w/o upper UTI sxs
For transplant pts, there’s more resistance to cipro and Bactrim
For transplant pts with simple cystitis: FQs, 3rd gen cephalosporins x10-14 days; zosyn, meropenem, cefepime if complicated and call ID
Do not screen (these are guidelines, not what we always do): peds pts, functionally impaired adults, long term care facility pts, diabetics, pts w/ renal transplants, pts with spinal cord injuries
4 notes
·
View notes
Text
Antibiotics

What are they?
Antibiotics are medicines that benefits in preventing infections affected by bacteria. It works by fighting the germs or by stopping them from recreating or reproducing.
The term antibiotic generally means “against life.” Any medicine that destroys bacteria in your body is technically an antibiotic. However majority people utilize the word when they’re speakingabout drugthat is intended to kill germs.
How they work
There are various types of antibiotics, which work in their different method. Though, the two key antibiotics work include:
A bactericidal antibiotic, such as penicillin, destroys the germs in the body. These medicines generallydisturb either the creation of the bacterial cell wall or its cell contents.
A bacteriostatic prevent bacteria from growing.
It might consume some hours or days after consuming the first dose before people feel recover or their symptoms enhanced.
Types
There are different classes or sets of antibiotics, which based on their chemical structure. few classes of antibiotics consist the following:
Penicillin’s- ex: amoxicillin (Amoxil)
Macrolides- ex: azithromycin (Zithromax) and erythromycin (Ery-Tab)
Cephalosporin’s- ex: cephalexin (Keflex) and cefdinir (Omnicef)
Fluoroquinolones- ex: ciprofloxacin (Cipro) and levofloxacin (Levaquin)
Beta-lactams with increased activity- ex: amoxicillin/clavulanate (Augmentin)
Urinary anti-infective- ex: nitrofurantoin (Macrobid)
Lincosamides- ex: clindamycin (Cleocin)
This list is not complete — other classes and brand names are also existing. In accumulation, penicillin, cephalosporin, and other antibiotics can be considered as subclasses of beta-lactam medicines.
When to take
Professionalssuggest that antibiotics should consume only when you are in actual need. This is to certify that the germs are destroyed and is incapable to grow and extent to other portion of the body.
Also, antibiotic consumption can occasionally be allied with side effects and antibiotic resistance.
Resistance
Antibiotics are a dominant bacteria-killing tool when consumedwisely and safely. However up to one-half of all antibiotic consumption isn’t required. Overdo has headed to antibacterial resistance. Bacteria adjust with time and turn out to be “super bacteria” or “superbugs.” They convert so that antibiotics no longer act on them. They pose a hugedanger, as there isn’t any drug to slaughter them.
The best technique to supportin decelerating the growth of super bacteria is by being clever with antibiotics. Here’s how:
Belief your doctor if they tell you don’t require them.
Don’t consume antibiotic for a viral infection.
Only consume those antibiotic your doctor has approved for you.
consume them as suggested by doctor.
Don’t miss dosages.
consume them for the complete number of days your doctor recommends.
Don’t save them for afterwards.
What they treat
Majority of germs that are present in your body are harmless. Some of them are even useful. though, bacteria can affectnearly any body part. Luckily, antibiotics can generally benefit in treating.
These are the types of infections that can be treated with antibiotics medicine:
few ear and sinus infections
Dental infections
Skin infections
Meningitis (bulge of the brain and spinal cord)
Strep throat
Bladder and kidney infections
Bacterial pneumonias
Whooping cough
Clostridioides difficile
Only bacterial infections can be treated with antibiotics medicines.
Side effects
Antibiotics generally leads to the following side effects:
diarrhoea
sickness
vomiting
rash
upset stomach
sensitivity to sunlight, when consuming tetracycline
with some antibiotics or persistent use, fungal infections of the mouth, gastric tract, and vagina side-effects can be occurred.
Some rare side effects of antibiotics comprise:
lower platelet count, when consuming cephalosporins, and penicillins, among others
serious aches and pains, when consuming fluoroquinolones
hearing loss, when consuming macrolides or aminoglycosides
lower granulocyte — a type of WBC — count, if consumed penicillin
development of kidney stones, if consume disulfonyl amides
few people — particularly older adults — can grow C. difficile infection. They might feel bowel inflammation, which may cause to serious, bloody diarrhea.
Allergy
Each year, there are more than 140,000 emergency sector visits for allergic reaction to antibiotics. Nearly four out of five emergency sector visits for antibiotic-linked side effects are owed to an allergic reaction. These reactions may range from minor rashes and itching to severe burning skin reactions, swelling of the face and throat, and breathing difficulties. Lessening needless antibiotic consumption is the best technique to decrease the danger of side effects from antibiotics. You must talk to your doctor about any previous medicinal reactions or allergies.
Interactions
Antibiotics may interact with other medicines you consume, building those drugs or the antibiotics less operative. Severalmedicineblends can degrade the side effects of the antibiotic or other medication. General side effects of antibiotics contain nausea, diarrhoea, and stomach pain. Occasionally these symptoms may cause dehydration and other complications. Ask your doctor about medicine interactions and probable side effects of antibiotics. Inform your doctor instantly if you face any side effects from antibiotics you are consuming
How to use
People generally consume antibiotics by mouth. Though, doctors can manage them by injection or smear them directly to the portion of the body with infection.
Most antibiotics can start acting within some hours. Doctors guide people to consume the whole course of medicine to stop the reoccurrence of the infection.
Discontinuing the medicines before the course has overrises the danger that the bacteria will developtough to future treatments. The ones that last will have had some contact to the antibiotic and canaccordingly develop fighting to it.
A person is required to complete the course of antibiotic treatment even after they see an enhancement in symptoms.
Doctors and the leaflet offered with the medicine provide preciseguidelines on how to take the medicineproperly.
individual can follow fewguidelines for consuming antibiotics effectually, such as:
don’t drink alcohol when consuming metronidazole.
Avoiding dairy products when consuming tetracyclines, as these mayinterruptthe absorption of the medicine.
consuming the medicines at the same time, or at fixed times in the day — this based on how many times a day a person required to consume the medicine.
#pharmrxpro#pharmrxpromedicines#erectile dysfunction#medication information#antibiotics#healthylifestyle#Healthcare#painkiller
2 notes
·
View notes
Text
Antibiotics Market: Combating Bacterial Infections in a New Era of Medicine
The Antibiotics market plays a crucial role in global healthcare by providing treatments that combat bacterial infections. As antibiotic resistance rises, the market is witnessing both challenges and innovations, making it essential for healthcare providers and decision-makers to stay informed. This article explores the latest trends, market segmentation, key growth drivers, and leading companies in the antibiotics industry.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/antibiotics-market
Market Overview
According to SkyQuest’s Antibiotics Market report, the global antibiotics market is valued at USD 41.4 billion in 2023 and is projected to grow at a CAGR of 2.5% during the forecast period. This growth is propelled by the increasing incidence of infectious diseases, the rising global population, and advancements in antibiotic development.
Market Segmentation
By Product Type:
Beta-Lactam and Beta-Lactamase Inhibitors: Widely used for treating a variety of bacterial infections, including respiratory and urinary tract infections.
Quinolones: Effective against a broad spectrum of bacterial pathogens, commonly used for respiratory infections.
Macrolides: Used primarily for respiratory infections and soft tissue infections.
Aminoglycosides: Target severe bacterial infections, especially in hospital settings.
Tetracyclines: Commonly used for skin infections, respiratory infections, and sexually transmitted diseases.
Others: Includes specialized antibiotics for niche infections or resistant bacterial strains.
By Spectrum of Activity:
Broad-Spectrum Antibiotics: Effective against a wide variety of bacteria, frequently prescribed for undiagnosed infections.
Narrow-Spectrum Antibiotics: Target specific bacteria, reducing the risk of resistance and minimizing side effects.
By Route of Administration:
Oral Antibiotics: Convenient for outpatient care and commonly prescribed for less severe infections.
Injectable Antibiotics: Typically used in hospitals for serious infections or when rapid treatment is needed.
Topical Antibiotics: Applied directly to the skin to treat local infections or prevent wound infections.
By End-User:
Hospitals & Clinics: Major users of antibiotics, especially for severe infections requiring immediate attention.
Pharmacies: Provide antibiotics for outpatient care, over-the-counter purchases, or prescription-based sales.
Research Laboratories: Focus on the development of new antibiotics and testing their efficacy against resistant bacteria.
Get more info at: — https://www.skyquestt.com/report/antibiotics-market
Key Growth Drivers
Rising Prevalence of Infectious Diseases: The continuous rise in bacterial infections worldwide, especially in developing regions, drives the demand for antibiotics.
Antibiotic Resistance: The emergence of drug-resistant bacteria necessitates the development of new antibiotics, boosting market growth.
Government Support and Initiatives: Various global health organizations and governments are actively funding research to combat antibiotic resistance.
Technological Advancements: Progress in biotechnology and genetic research is aiding the development of novel antibiotics.
Leading Companies in the Market
SkyQuest’s report highlights several key players in the Antibiotics market, including:
Merck & Co., Inc., Allergan plc (AbbVie), GlaxoSmithKline plc., Pfizer Inc., Novartis AG, Sanofi S.A., AstraZeneca plc, Johnson & Johnson, Teva Pharmaceutical Industries Ltd., Mylan N.V., Eli Lilly and Company, Bayer AG, Bristol-Myers Squibb Company, AbbVie Inc., Astellas Pharma Inc., Boehringer Ingelheim GmbH, Daiichi Sankyo Company, Limited, Roche Holding AG, Sun Pharmaceutical Industries Ltd., Takeda Pharmaceutical Company Limited.
Challenges and Opportunities
The antibiotics market faces significant challenges, including antibiotic resistance and stringent regulatory approval processes. However, these challenges also create opportunities for innovation in developing next-generation antibiotics and alternative therapies.
Future Outlook
The future of the antibiotics market is shaped by ongoing efforts to combat antibiotic resistance and the development of advanced, targeted therapies. As companies invest in research and innovation, the market is expected to see steady growth. For more detailed insights and strategic recommendations, refer to SkyQuest’s in-depth Antibiotics Market report.
The Antibiotics market remains a cornerstone of global healthcare, with ongoing advancements aimed at tackling bacterial infections and resistance. Decision-makers who focus on innovation and the development of new antibiotics will stay at the forefront of this critical healthcare sector. For more in-depth insights and emerging trends, consult SkyQuest's Antibiotics Market report.
0 notes
Text
Introduction of Faropenem and Potassium Clavulanate Tablets
Bacterial infections can range from mild to severe, often requiring a tailored antibiotic regimen for effective treatment. Faropenem and Potassium Clavulanate Tablets combine two powerful agents to combat a broad spectrum of bacterial pathogens. This combination therapy is particularly useful against infections caused by beta-lactamase-producing bacteria, which can resist many common antibiotics. In this article, we’ll explore the uses, dosage, benefits, and potential side effects of Faropenem and Potassium Clavulanate Tablets. Understanding how this medication works can help you make informed decisions about your healthcare.
What are Faropenem and Potassium Clavulanate Tablets?
Faropenem and Potassium Clavulanate are combined in a single formulation to enhance the antibacterial efficacy of Faropenem.
Faropenem: A beta-lactam antibiotic belonging to the penem class, Faropenem is effective against a wide range of gram-positive and gram-negative bacteria. It works by inhibiting the synthesis of the bacterial cell wall, leading to bacterial cell death.
Potassium Clavulanate: A beta-lactamase inhibitor, Potassium Clavulanate extends the spectrum of Faropenem by inhibiting the beta-lactamase enzymes produced by bacteria. These enzymes can break down beta-lactam antibiotics, rendering them ineffective.
Together, this combination offers a robust defense against resistant bacterial strains, making it a valuable option for treating complex infections.
#aingopharma#antibiotics#pcd pharma#pcd company#pcd franchise#Faropenem#Potassium Clavulanate#manufacturing
0 notes
Text
Microorganisms, Vol. 12, Pages 1703: Tracing Acinetobacter baumannii’s Journey from Hospitals to Aquatic Ecosystems
Background: This study provides a comprehensive analysis of Acinetobacter baumannii in aquatic environments and fish microbiota by integrating culture-dependent methods, 16S metagenomics, and antibiotic resistance profiling. Methods: A total of 83 A. baumannii isolates were recovered using culture-dependent methods from intra-hospital infections (IHI) and wastewater (WW) and surface water (SW) samples from two southern Romanian cities in August 2022. The antibiotic susceptibility was screened using disc diffusion, microdilution, PCR, and Whole Genome Sequencing assays. Results: The highest microbial load in the analyzed samples was found in Glina, Bucharest, for both WW and SW samples across all investigated phenotypes. For Bucharest isolates, the resistance levels corresponded to fluoroquinolones > aminoglycosides > β-lactam antibiotics. In contrast, A. baumannii from upstream SW samples in Târgoviște showed the highest resistance to aminoglycosides. The blaOXA-23 gene was frequently detected in IHI, WW, and SW isolates in Bucharest, but was absent in Târgoviște. Molecular phylogeny revealed the presence of ST10 in Târgoviște isolates and ST2 in Bucharest isolates, while other minor STs were not specifically correlated with a sampling point. Using 16S #rRNA sequencing, significant differences in microbial populations between the two locations was identified. The low abundance of Alphaproteobacteria and Actinobacteria in both locations suggests environmental pressures or contamination events. Conclusions: These findings indicate significant fecal contamination and potential public health risks, emphasizing the need for improved water quality monitoring and management. https://www.mdpi.com/2076-2607/12/8/1703?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
The global demand for Antibacterial Drugs was valued at USD 51,512.50 Million in 2023 and is expected to reach USD 87,029.28 Million in 2032, growing at a CAGR of 6.00% between 2024 and 2032.The antibacterial drugs market, a vital segment of the global pharmaceutical industry, has seen substantial growth and transformation over recent years. This market plays a crucial role in combating bacterial infections, which remain a significant public health concern worldwide. With the rise of antibiotic resistance and the constant emergence of new bacterial strains, the demand for effective antibacterial drugs continues to grow, driving innovation and investment in this sector.
Browse the full report at https://www.credenceresearch.com/report/antibacterial-drugs-market
Market Dynamics
The antibacterial drugs market is driven by several key factors. One of the primary drivers is the increasing prevalence of bacterial infections. Conditions such as pneumonia, tuberculosis, and sepsis are prevalent globally, necessitating the widespread use of antibacterial drugs. Additionally, the rise in hospital-acquired infections and the growing number of surgeries also contribute to the demand for these medications.
Another significant factor is the increasing awareness and diagnosis of bacterial infections. Improved diagnostic techniques have enabled earlier and more accurate detection of bacterial infections, leading to timely and appropriate use of antibacterial drugs. Furthermore, public health initiatives aimed at reducing the burden of infectious diseases have also bolstered the market.
However, the market faces considerable challenges. The most pressing issue is antibiotic resistance, which occurs when bacteria evolve mechanisms to resist the effects of antibiotics. This phenomenon has led to a reduction in the efficacy of existing drugs, necessitating the development of new and more potent antibacterial agents. The high cost of research and development (R&D) for new drugs and the regulatory hurdles associated with their approval also pose significant challenges.
Market Segmentation
The antibacterial drugs market can be segmented based on drug class, route of administration, and region.
1. By Drug Class:
- Beta-Lactams: Including penicillins and cephalosporins, these are among the most commonly prescribed antibiotics.
- Macrolides: Used to treat respiratory and soft tissue infections.
- Quinolones: Effective against a broad range of bacteria.
- Aminoglycosides: Often used in severe infections caused by Gram-negative bacteria.
- Tetracyclines: Broad-spectrum antibiotics used for various infections.
- Others: Includes sulfonamides, glycopeptides, and more.
2. By Route of Administration:
- Oral: Tablets, capsules, and suspensions.
- Parenteral: Injections and intravenous infusions.
- Topical: Creams, ointments, and drops.
3. By Region:
- North America: The largest market due to advanced healthcare infrastructure and high R&D investment.
- Europe: Significant market share with a strong focus on combating antibiotic resistance.
- Asia-Pacific: Rapidly growing due to increasing healthcare expenditure and high prevalence of infectious diseases.
- Latin America: Emerging market with growing awareness and healthcare access.
- Middle East & Africa: Developing market with increasing focus on healthcare improvements.
Key Players and Competitive Landscape
The antibacterial drugs market is highly competitive, with numerous pharmaceutical companies vying for market share. Some of the leading players include:
- Pfizer Inc.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Novartis AG
- Johnson & Johnson
- Sanofi
- Bayer AG
These companies invest heavily in R&D to develop new antibacterial agents and improve existing ones. Strategic collaborations, mergers, and acquisitions are common as companies seek to enhance their product portfolios and expand their market presence.
Future Prospects
The future of the antibacterial drugs market looks promising, with several trends shaping its trajectory. The development of novel antibiotics, particularly those targeting multi-drug resistant bacteria, is a primary focus. Additionally, the use of advanced technologies such as artificial intelligence (AI) and machine learning (ML) in drug discovery is expected to accelerate the development of new antibacterial agents.
The growing emphasis on antimicrobial stewardship programs aims to optimize the use of antibiotics, reducing the risk of resistance and preserving the efficacy of existing drugs. Furthermore, increased funding and incentives from governments and non-profit organizations for antibiotic R&D are anticipated to drive innovation in this field.
Key Players
Spero Therapeutics
Allecra Therapeutics
R-Pharm Group
Melinta Therapeutics LLC
MicuRx
TenNor Therapeutics Ltd
Venatorx Pharmaceuticals, Inc.
GlaxoSmithKline plc.
AstraZeneca
Bayer AG
Johnson & Johnson
Bristol-Myers Squibb Company
Merck & Co., Inc.
Eli Lilly and Company
AbbVie Inc.
Novartis AG
Pfizer Inc.
Sanofi
Others
Segmentation
By Drug Class
Beta-Lactams
Penicillins
Cephalosporins
Carbapenems
Quinolones
Macrolides
Tetracyclines
Aminoglycosides
Sulfonamides
Others
By Route of Administration
Oral Antibiotics
Injectable Antibiotics
By Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
By Indication
Respiratory Tract Infections
Urinary Tract Infections
Skin Infections
Ear Infections
Sexually Transmitted Infections (STIs)
Gastrointestinal Infections
Others
By Patient Age Group
Adults
Pediatrics
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/antibacterial-drugs-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Link
1 note
·
View note
Text
Fwd: Postdoc: WageningenU.ExpermentalEvolution
Begin forwarded message:
> From: [email protected]
> Subject: Postdoc: WageningenU.ExpermentalEvolution
> Date: 3 May 2024 at 05:42:21 BST
> To: [email protected]
>
>
>
> Postdoc position: Experimental evolution of antibiotic resistance in
> spatially structured environments
>
> Laboratory of Genetics, Wageningen University, the Netherlands
>
> — Job description —
>
> A fully funded postdoc position (three years when full-time
> employed) is available in our group for an experimental evolutionary
> biologist/microbiologist to work as part of an interdisciplinary project
> studying how spatial environmental structure affects the evolution
> of antibiotic resistance (supervised by prof. Arjan de Visser and
> dr. Hilje Doekes; full project proposal available upon request). You will
> construct bacterial strains with common beta-lactam resistance mechanisms
> and use these in fitness assays and evolution experiments, to help
> develop spatially explicit models of bacterial growth, competition and
> evolution under beta-lactam pressure. Throughout the project, you will
> collaborate closely with a PhD student who will develop these analytic
> and computational models.
>
> Your duties and responsibilities will include:
> * Construct bacterial strains with common resistance mechanisms;
> * Perform experiments with these strains to measure fitness and
> estimate relevant parameters (e.g. kill rate, antibiotic
> degradation rate);
> * Perform and analyse evolution experiments with constructed strains;
> * Engage in frequent model-experiment feedback;
> * Communicate your results by delivering talks at (inter)national
> conferences and prepare scientific publications;
> * Participate in supervising BSc and MSc thesis students.
>
> — Qualifications —
>
> You are a highly motivated researcher with a PhD in molecular
> evolutionary biology, microbial genetics, or a related field. You
> have solid experience in microbiological and molecular genetics and
> expertise in genome analyses and are familiar with evolutionary theory
> and quantitative models. Further, you have good communication skills
> to operate in a multidisciplinary team and interact closely with the
> theoretical PhD student.
>
> — Offer —
>
> Wageningen University & Research offers excellent terms of employment
> (https://ift.tt/5Q67gPt).
> Your salary will be between € 3.226 and € 5.090 gross per month for
> a full-time working week of 38 hours, depending on work experience. A
> contract for 0.8 FTE can be discussed. Initially, we offer you a one-year
> contract, which will then be extended to the full period if there is
> mutual enthusiasm.
>
>
> — More info —
>
> If you have questions about this position, please contact Arjan de
> Visser: [email protected].
>
> Applications are welcome until 27 May. For more info or to apply:
> https://ift.tt/FeSzKRk
>
> "Visser, Arjan de"
0 notes
Text
Demystifying the Beta-lactam and Beta-lactamase Inhibitors Market: Trends and Insights

The Beta-lactam and Beta-lactamase Inhibitors Market represents a critical sector within the broader pharmaceutical industry, addressing the pressing global challenge of antimicrobial resistance (AMR).
According to the study by Next Move Strategy Consulting, the global Beta-lactam and Beta-lactamase Inhibitors Market size is predicted to reach USD 34.20 billion with a CAGR of 1.9% by 2030.
Request a FREE sample, here: https://www.nextmsc.com/beta-lactam-beta-lactamase-inhibitors-market/request-sample
Trends in the Beta-lactam and Beta-lactamase Inhibitors Market
Rising Prevalence of Antibiotic Resistance: Antibiotic resistance has emerged as a global public health crisis, with bacteria developing resistance to multiple classes of antibiotics. The overuse and misuse of antibiotics in human medicine, agriculture, and animal husbandry have accelerated the proliferation of resistant bacteria. In particular, the widespread dissemination of extended-spectrum beta-lactamases (ESBLs) and carbapenemases poses a significant threat to healthcare systems worldwide.
Technological Advancements in Drug Discovery: Advances in biotechnology, genomics, and computational biology are driving innovation in antimicrobial drug discovery. High-throughput screening techniques, structural biology, and virtual screening methods enable rapid identification and optimization of novel beta-lactamase inhibitors. Furthermore, the application of CRISPR-Cas9 technology facilitates the precise modification of bacterial genomes to study antimicrobial resistance mechanisms and develop novel therapeutic strategies.
Regulatory Initiatives and Antimicrobial Stewardship: Regulatory agencies are increasingly prioritizing antimicrobial stewardship and promoting the responsible use of antibiotics to mitigate the spread of resistant bacteria. In the United States, the Food and Drug Administration (FDA) has implemented guidelines for the development of antimicrobial drugs, emphasizing the importance of demonstrating clinical efficacy and safety in treating resistant infections. Similarly, the European Medicines Agency (EMA) has established regulatory frameworks to incentivize the development of new antibiotics through market exclusivity and streamlined approval processes.
Market Dynamics and Competitive Landscape: The Beta-lactam and Beta-lactamase Inhibitors Market is characterized by intense competition among pharmaceutical companies, biotechnology firms, and academic research institutions. Established players such as Pfizer, Merck & Co., and GlaxoSmithKline dominate the market with a diverse portfolio of beta-lactam antibiotics and inhibitors. However, emerging biotech startups and small to medium-sized enterprises (SMEs) are gaining traction by focusing on niche therapeutic areas, innovative drug delivery platforms, and strategic partnerships.
Collaborative Research and Development: Collaborative research consortia, public-private partnerships, and academic-industry collaborations are driving preclinical and clinical research in the field of antimicrobial drug discovery. Initiatives such as the Innovative Medicines Initiative (IMI) in Europe and the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) in the United States provide funding, expertise, and infrastructure to accelerate the development of novel antibiotics and beta-lactamase inhibitors.
Insights into Market Segmentation and Therapeutic Applications
The Beta-lactam and Beta-lactamase Inhibitors Market can be segmented based on product type, mechanism of action, route of administration, and therapeutic indication. Key product categories include beta-lactam antibiotics (e.g., penicillins, cephalosporins, carbapenems) and beta-lactamase inhibitors (e.g., clavulanic acid, sulbactam, tazobactam). Mechanistically, beta-lactamase inhibitors can be classified as competitive or suicide inhibitors, depending on their mode of enzyme inhibition. Furthermore, beta-lactam and beta-lactamase inhibitors can be administered via various routes, including oral, intravenous, and intramuscular routes, depending on the severity and site of infection.
Therapeutically, beta-lactam and beta-lactamase inhibitors are indicated for the treatment of a wide range of bacterial infections, including respiratory tract infections, urinary tract infections, skin and soft tissue infections, and intra-abdominal infections. Additionally, combination therapy with beta-lactam antibiotics and inhibitors has demonstrated efficacy against multidrug-resistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, and carbapenem-resistant Enterobacteriaceae (CRE).
Future Perspectives and Challenges
Despite significant progress in antimicrobial drug discovery, the Beta-lactam and Beta-lactamase Inhibitors Market faces several challenges and uncertainties. These include:
Antibiotic Resistance: The continued evolution and dissemination of antibiotic-resistant bacteria pose a formidable challenge to the effectiveness of beta-lactam antibiotics and inhibitors. Strategies to combat resistance include the development of novel antibiotics with alternative mechanisms of action, combination therapy approaches, and the implementation of infection prevention and control measures.
Regulatory Hurdles: Regulatory agencies require robust clinical evidence to support the approval and marketing of new antibiotics and beta-lactamase inhibitors. Clinical trial design, patient recruitment, and endpoint selection present logistical and ethical challenges, particularly in the context of rare and multidrug-resistant infections.
Inquire before buying, here: https://www.nextmsc.com/beta-lactam-beta-lactamase-inhibitors-market/inquire-before-buying
Economic Considerations: The economic viability of antibiotic development remains a concern for pharmaceutical companies, given the high costs and uncertain returns associated with antibiotic R&D. Market incentives, reimbursement policies, and public-private partnerships are essential to incentivize investment in antibiotic innovation and ensure access to effective antimicrobial therapies.
Global Health Equity: Access to essential antibiotics and beta-lactamase inhibitors remains uneven across different regions and socioeconomic groups. Disparities in healthcare infrastructure, affordability, and antibiotic stewardship practices contribute to the persistence of infectious diseases and antimicrobial resistance in low- and middle-income countries. Addressing these disparities requires a comprehensive approach involving governments, healthcare providers, and international organizations.
Research and Development Incentives: Encouraging investment in research and development (R&D) for new antibiotics and beta-lactamase inhibitors is crucial to address gaps in the current antimicrobial pipeline. Governments, philanthropic organizations, and private-sector stakeholders can provide financial incentives, grants, and tax credits to stimulate innovation in antimicrobial drug discovery. Additionally, initiatives such as priority review vouchers and market exclusivity extensions can incentivize pharmaceutical companies to prioritize antibiotic R&D and bring new treatments to market.
Antibiotic Stewardship Programs: Implementing comprehensive antibiotic stewardship programs is essential to promote rational antibiotic use, reduce unnecessary prescribing, and mitigate the emergence and spread of antibiotic-resistant bacteria. Healthcare facilities, including hospitals, clinics, and long-term care facilities, can establish multidisciplinary teams to develop and implement evidence-based guidelines for antibiotic prescribing, monitoring, and surveillance. Moreover, education and training initiatives for healthcare providers, patients, and the public are essential to raise awareness about the risks of antibiotic misuse and the importance of responsible antibiotic use.
Surveillance and Monitoring: Strengthening global surveillance systems and monitoring mechanisms is critical to track the epidemiology of antibiotic-resistant infections, detect emerging resistance trends, and inform public health interventions. National and international surveillance networks, such as the Centers for Disease Control and Prevention (CDC) in the United States and the European Centre for Disease Prevention and Control (ECDC) in Europe, play a vital role in collecting, analyzing, and disseminating data on antimicrobial resistance patterns. Collaborative efforts to harmonize surveillance methodologies, share data, and facilitate information exchange across borders are essential to enhance global preparedness and response to antibiotic-resistant threats.
Public Awareness and Education: Promoting public awareness and education about antimicrobial resistance is key to fostering behavioral change, empowering individuals to make informed decisions about antibiotic use, and reducing demand for unnecessary antibiotics. Public health campaigns, educational materials, and social media initiatives can raise awareness about the risks of antibiotic resistance, the importance of completing antibiotic courses as prescribed, and the role of individuals in preventing the spread of resistant bacteria. Furthermore, incorporating antimicrobial resistance education into school curricula and healthcare provider training programs can cultivate a culture of responsible antibiotic stewardship from an early age.
Conclusion
The Beta-lactam and Beta-lactamase Inhibitors Market plays a vital role in addressing the global threat of antimicrobial resistance and ensuring effective treatment options for bacterial infections. By understanding the key trends, insights, and challenges within this dynamic market, stakeholders can navigate regulatory, scientific, and economic complexities to drive innovation and improve patient outcomes. Collaboration across sectors, disciplines, and geographic regions is essential to develop sustainable solutions that preserve the efficacy of beta-lactam antibiotics and safeguard public health for future generations.
#market research#market trends#global market#beta lactam#beta lactamase#antibiotics#healthcare industry
0 notes
Text
Unveiling the Healing Power of Mox (Amoxicillin) Capsules A Comprehensive Guide
In the realm of medicine, antibiotics play a vital role in combating bacterial infections and restoring health. Among these, Mox (Amoxicillin) capsules stand out as a widely prescribed antibiotic renowned for its broad-spectrum efficacy and safety profile. In this comprehensive guide, we delve into the specifics of Mox capsules, exploring their composition, indications, dosage, potential side effects, and more, to shed light on this indispensable medication to the mox amoxycillin capsules
Understanding Mox (Amoxicillin) Capsules: Mox capsules belong to the penicillin class of antibiotics and contain amoxicillin as the active ingredient. Amoxicillin is a bactericidal agent that works by inhibiting the synthesis of bacterial cell walls, leading to the destruction of susceptible bacteria. Mox capsules are available in various strengths, typically ranging from 250mg to 500mg, and are administered orally.
Indications: Mox capsules are prescribed for the treatment of a wide range of bacterial infections, including:
Respiratory Infections: Such as bronchitis, pneumonia, sinusitis, and tonsillitis.
Ear, Nose, and Throat Infections: Including otitis media (ear infection), pharyngitis (sore throat), and sinus infections.
Urinary Tract Infections: Such as cystitis and pyelonephritis.
Skin and Soft Tissue Infections: Including cellulitis and impetigo.
Gastrointestinal Infections: Such as Helicobacter pylori eradication in combination with other medications for the treatment of peptic ulcers.
Dosage and Administration: The dosage of Mox capsules varies depending on the severity of the infection, the patient's age, weight, and renal function, as well as the specific bacterial strain causing the infection. However, a typical dosage regimen may include:
Adults: The usual dose for adults is 250mg to 500mg every 8 hours or 500mg to 875mg every 12 hours, depending on the severity of the infection.
Children: The dosage for children is based on their weight, typically ranging from 20mg to 45mg per kilogram of body weight per day, divided into two to three doses.
Mox capsules should be taken with a full glass of water, preferably on an empty stomach, at least one hour before or two hours after meals, to enhance absorption.
Potential Side Effects: While Mox capsules are generally well-tolerated, they may cause side effects in some individuals. Common side effects may include:
Gastrointestinal Disturbances: Such as nausea, vomiting, diarrhea, and abdominal pain.
Allergic Reactions: Including skin rashes, itching, hives, and, rarely, severe allergic reactions such as anaphylaxis.
Yeast Infections: Due to disruption of the normal microbial flora, Mox capsules may increase the risk of developing yeast infections, particularly in women.
It is essential to seek medical attention if any adverse reactions occur while taking Mox capsules.
Precautions and Contraindications: Before taking Mox capsules, it is crucial to inform your healthcare provider about any allergies, medical conditions, or medications you are taking, including over-the-counter supplements and herbal remedies. Mox capsules should be used with caution in individuals with a history of allergic reactions to penicillin or other beta-lactam antibiotics.
Conclusion: Mox (Amoxicillin) capsules are indispensable antibiotics used in the treatment of various bacterial infections. With their broad-spectrum efficacy, convenient oral administration, and relatively low cost, Mox capsules are a cornerstone of antibiotic therapy worldwide. However, it is essential to use them responsibly, following the guidance of a healthcare professional, to minimize the risk of side effects and antibiotic resistance. By understanding the indications, dosage, potential side effects, and precautions associated with Mox capsules, patients and healthcare providers can ensure safe and effective use of this essential medication in the management of bacterial infections.
0 notes
Photo

●Fluoroquinolone-based regimens – For patients who have no contraindications to fluoroquinolones and are at low personal risk for a fluoroquinolone-resistant isolate (table 2), we suggest an oral fluoroquinolone for empiric therapy. Appropriate regimens include ciprofloxacin 500 mg twice daily, ciprofloxacin 1000 mg extended release once daily, or levofloxacin 750 mg once daily [38-42]. Fluoroquinolones are given for five to seven days.
In the case that community prevalence of E. coli fluoroquinolone resistance is known to be higher than 10 percent, we suggest a single dose of a long-acting parenteral agent prior to administering the fluoroquinolone [43]. We prefer ceftriaxone (1 g IV or intramuscular [IM] once) because of its safety, efficacy, and microbial spectrum. Ertapenem (1 g IV or IM once) is an alternative for patients with an allergy that precludes ceftriaxone use or expected resistance to ceftriaxone, and aminoglycosides (gentamicin or tobramycin 5 mg per kg IV or IM once) are reserved for patients who cannot use the other two. Since timely use of an agent with in vitro activity is essential to treat acute complicated UTI and minimize progression of infection, the threshold for selecting an antimicrobial for empiric broad-spectrum therapy should be set at a relatively low resistance prevalence. For fluoroquinolones, a resistance prevalence of 10 percent has been suggested based on expert opinion [1].
The benefits of fluoroquinolones are thought to outweigh their risks for acute complicated UTI, but patients should be advised about the uncommon but potentially serious musculoskeletal and neurologic adverse effects associated with fluoroquinolones. (See "Fluoroquinolones", section on 'Adverse effects'.)
●Fluoroquinolone-sparing regimens – For patients who have contraindications to fluoroquinolones or other concerns about fluoroquinolone use, our approach depends on the relative severity of illness. For those with mild infection, we use a single dose of a long-acting parenteral agent followed by a non-fluoroquinolone oral agent [43].
As above, we prefer ceftriaxone (1 g IV or IM once) as a long-acting parenteral agent because of its safety, efficacy, and microbial spectrum. Ertapenem (1 g IV or IM once) is an alternative for patients with an allergy that precludes ceftriaxone use or expected resistance to ceftriaxone, and aminoglycosides (gentamicin or tobramycin 5 mg per kg IV or IM once) are reserved for patients who cannot use the other two.
Following the dose of the parenteral agent, options include the following:
•Trimethoprim-sulfamethoxazole – one double-strength (160 mg/800 mg) tablet orally twice daily for 7 to 10 days
•Amoxicillin-clavulanate – 875 mg orally twice daily for 7 to 10 days
•Cefpodoxime – 200 mg orally twice daily for 7 to 10 days
•Cefdinir – 300 mg orally twice daily for 7 to 10 days
•Cefadroxil – 1 g orally twice daily for 7 to 10 days
For outpatients who are systemically ill or are at risk for more severe illness, we favor continuing the parenteral therapy until culture and susceptibility testing results can guide selection of an appropriate oral agent.
Results of urine culture and susceptibility testing should be followed to ensure that the chosen empiric antimicrobial regimen is appropriate and to guide modification of the regimen, if necessary. The durations of therapy listed above assume that the patient is appropriately responding to antibiotic therapy; longer durations or re-evaluation may be warranted in those who are slow to respond. These issues are discussed in detail elsewhere. (See 'Directed antimicrobial therapy' below.)
In some cases, results of urine culture and susceptibility testing may be known at the time of treatment initiation. For those with mild illness caused by a susceptible pathogen, oral therapy with trimethoprim-sulfamethoxazole without initial parenteral therapy is reasonable. If trimethoprim-sulfamethoxazole is not an option, an oral beta-lactam is an alternative (if the organism is susceptible), but based on data from cystitis studies, it is generally associated with lower efficacy rates for UTI.
2 notes
·
View notes
Text
"an ESBL expressing strain of E. coli that was resistant not only to beta-lactam antibiotics but also to fluoroquinolones, gentamicin, tetracycline and Bactrim. It was sensitive only to carbapenems."
To me this means I tried at least 2 fluoroquinolones and beta-lactam antibiotics because they pluralize those words, if it was just 1 drug in those categories, they would have named the drug.
Also there are at least 7 carbapenema, i tried at least 5 different carbapenems before the PICC line IV infusions of ertapenem, which ultimately solved the problem. Google says there's a 12-41% mortality rate, glad I didn't have those numbers at the time lol!
0 notes