#equivalence point titration
Text
Alchemy 410, Chapter 4: Catalysis
SUMMARY: Illyth Arabana and Gale Dekarios can’t be in the same room without wanting to throttle each other. Can they survive being lab partners in their fourth year alchemy class?
Gale and Illyth are slogging through the most tedious part of their project but Illyth can barely stay awake.
RATING: M
PAIRING: Gale/OC
TAGS: Enemies to lovers, enemies to friends to lovers, pre-canon, academia, alchemy, lab partners, slow burn, height difference, eventual smut, a couple of nerds fall in love
WORD COUNT: 1.3k
Drip.
Drip.
Drip.
“Gods, this is taking forever,” Illyth groaned, rubbing the bridge of her nose as she watched the pale pink fluid drip through the buret and into a flask below. The titration was a tedious, painstaking process. It required consistent attention, as there was no room for error. There was a chemical indicator in the fluid dripping through the buret tube that would turn the solution a faint robin’s egg blue when chemical equilibrium was reached. If the titration went on for a moment too long, the potion would be ruined and the process would need to be restarted.
The pair were in Gale’s dorm room once again. It was their third attempt that month to successfully titrate the potion to its equivalence point, having failed twice already. The two of them squabbled about whose fault it was that prior attempts were unsuccessful, but culpability did not change the fact that if they could manage to get the titration correct, they would still need to reproduce the results twice after that.
“Why haven’t we found a more efficient way to do this?” Illyth complained. “Who in the hells has time to sit here like this?”
“You do, apparently,” Gale snorted. He was leaning against the foot of his bed, squinting at an Alzhedo language textbook and scribbling notes onto a piece of parchment next to him.
“Clearly,” Illyth replied, trying to stifle her ire. Her patience was running thinner than usual and she was struggling to stay awake. With midterms approaching, she was operating on a serious sleep deficit. Because of her and Gale’s respective schedules, the only time they could meet was later in the evenings when Illyth’s fatigue was more difficult to ignore. She drew a deep breath and sighed, blinking a couple of times to clear the haze of exhaustion.
Behind her, Gale muttered under his breath as he flipped back and forth between the parchment and the textbook.
Illyth smirked to herself. “Having some trouble over there, prodigy?”
“Shut up,” Gale sneered, shooting an irate glance at Illyth, who chuckled in response. She turned the stopper on the buret and looked back over her shoulder at him.
“May I take a look?” she asked, turning to face Gale. “As a student of arcane linguistics, I believe I may be able to assist.”
Gale huffed in a mixture of embarrassment and resignation. “Fine.”
Illyth blinked hard, trying to refocus her bleary eyes. She scooted over next to Gale and peered down at the assignment in front of him. Her eyes flicked between the textbook and the parchment. The book was about the history of the Genasi in the Old Empires, written by a sage from Calimport. Illyth was familiar with the author after taking this class last term.
“Gods, this is my least favorite book,” she grumbled. “Its grammatical structures are so awkward.”
Gale watched as Illyth ran her delicate fingers along each line on the page, tracing the sentences and muttering under her breath.
“Ah, yes, so,” she began. “Assuming you have Professor K’han’ar, which I would imagine you do since he’s the only one who insists on making people read this, he wants you to provide a timeline of the events leading up to and following the Spellplague their impact on Genasi society, but this is a trick question of sorts because Genasi lived in isolation until the Spellplague.”
She turned her attention to the notes Gale scribbled on the parchment beside him. She hummed in recognition as she scanned the page. “Your tenses are all over the place,” she remarked. “May I make a couple of suggestions?”
Gale shrugged and gestured passively to the parchment. “Be my guest.”
Illyth smiled softly. This was a rare opportunity for her to showcase her skills. She picked up the quill and sketched a chart at the top of the page that outlined the different tenses.
“Luckily, Alzhedo doesn’t have irregular verbs, unlike Elvish or Draconic,” Illyth commented. “This chart might help, though. I made it last term.”
Gale picked up the parchment and nodded approvingly. When Illyth laid the information out so plainly, the tense differences were much clearer to him. “This might be quite useful,” he said. His deep brown eyes met Illyth’s wine-red eyes and he smiled earnestly. “Thank you.”
“Mm-hm,” Illyth hummed, returning to the titration. She opened up the stopper once again.
Drip.
Drip.
Drip.
The rhythmic dripping had a hypnotic effect on Illyth, whose fatigue was only worsening. Her eyelids began to sag and, despite her best efforts to stay awake, she was beginning to doze off.
Drip.
Drip.
Drip.
Her neck slackened and her head lolled to the side. She jolted awake with a gasp, attempting to regain control over her exhaustion. Worriedly, she looked to the titration and sighed in relief, seeing that the robin’s egg blue neutrality indicator had yet to develop.
“Allow me to take watch,” Gale said. “If you go on much longer like that, the potion will be ruined. Again.”
Illyth snorted under her breath, considering arguing back to insist that she was fine.
“Alright,” she relented. Illyth scooted away from the alchemy instrumentation and traded places with Gale to lean against the foot of the bed. It felt like an admission of weakness and failure to allow Gale to take over, but there was no concealing that she was too tired to continue.
Her eyelids fluttered shut once more, but Illyth was too exhausted to realize that she was dozing off.
Drip.
Drip.
Drip.
Gale could hear Illyth’s soft snoring behind him as he watched the titration. She’d fallen asleep immediately. For all of her toughness and resolve, she was vulnerable now. Her posture was soft and at ease and her lips parted as she slept. Gale smiled to himself and shook his head at the obstinance of his lab partner. Even in her exhaustion, Illyth still felt the need to prove herself, to show him how capable she was. Gale couldn’t deny that their intellects complemented each other, but Gods she was acerbic. Nobody challenged him in the way she did, a trait that intrigued and irritated Gale in equal measure.
The potion took on a faint blue tinge and Gale immediately stopped the titration. He examined the liquid in the flask and swirled it around, appreciating the perfection he’d achieved. He took note of the changes in volume between the buret and the flask and recorded the measurements on the data sheet that Illyth made.
Gale sighed contentedly. They were only a third of the way done with the potion brews, but he felt a sense of satisfaction in finally achieving a perfectly neutral solution suitable for consumption. Gale eyed the instrumentation and glassware in front of him, pursing his lips in determination. There had to be a more efficient way to produce this potion. Perhaps if he toyed with the process a little longer, he could find a way. Even though his eyelids were growing heavy with sleep, the challenge of discovery was too compelling to ignore.
Gale lit another candle and started the next potion brew.
⭐️⭐️⭐️⭐️⭐️⭐️⭐️⭐️⭐️⭐️
Illyth awoke with a start at the sound of soft mumbling nearby. Early morning sunlight filtered through the windows, covering Gale’s dorm room in a soft, warm glow. She blinked several times, trying to clear the bleariness from her eyes.
She scanned the room, trying to get a sense of where she was. She didn’t recall having fallen asleep in Gale’s dorm room, but she did recall changing places with Gale to let him watch over the potion titration. Yet, there she was on the floor with a blanket wrapped around her shoulders. She assumed that Gale laid the blanket over her when he realized that she’d fallen asleep. Illyth smiled to herself, taking a moment to appreciate his gesture.
Three pale blue potion bottles sat on the makeshift lab table, corked and ready to consume. Illyth reasoned that Gale must have stayed up until the wee hours of the morning to finish the potions.
Gale was curled up with his head resting on the table, snoring and muttering in his sleep. He didn’t stir when Illyth draped the blanket over his shoulders before she left.
Whatever he was dreaming of, it was making him smile.
#bg3#gale dekarios#bg3 gale#gale of waterdeep#baldur's gate 3#bg3 fic#baldur's gate#Gale#gale baldurs gate 3#gale fic#gale romance#gale x oc
15 notes
·
View notes
Text
Thirst for Knowledge
Ectoberhaunt 2022, Side Order. Prompt: Thirst
Summary: New experiments are being done in the Fenton Basement Laboratory, and with that new data will be brought up.
0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o0o
Jack and Maddie Fenton were both working down in the lab right now, Maddie doing a chemical titration with some ectoplasm in a beaker and burette filled with a strong alkaline solution; all the while Jack was in another desk, measuring how ectoplasm refracts light using a spectrophotometer, measuring the sample in both glass cuvettes and quartz cuvettes, the samples being crystallized ectoplasm, liquid ectoplasm and gaseous ectoplasm, the last which was sealed in the cuvettes in order to avoid any leakage of it.
“How are your samples going honey?” Maddie asked not lifting her gaze from the beaker, one hand holding it and constantly mixing it with the alkaline solution that was dropping slowly from the burette, which was being controlled with her other hand so that only drops were being dropped in an interval of 3 drops per second.
“They’re great Mads, and with the help of the Fenton Spectrophotometer everything is going peachy as well. Adding that accessory for solid samples as a permanent feature was a brilliant idea” replied Jack as he put one of the crystals inside the machine, before closing it and turning it on, waiting for the results to come out.
“Oh that’s good to know dear, adding that to the machine wasn’t easy after all”
“You tell me about it, I had to rebuild the original Spectrophotometer like 5 different times, and we ended up buying a new one because that old model was too abused with my hands working on it.”
“Well, when you managed to do it at the first try on the new model we bought then you did us a big favor, those things can be expensive, and by keeping it inside of it we can make sure that it’ll never go missing.”
“That’s for sure at least. How are you doing with your titration by the way?”
“Everything’s fine here, nearing the expected equivalence point for normal ectoplasm, so I have to be extra careful now.”
“I’ll shut up now and let you focus then.”
“Thanks Ja-Oh” the sample in Maddie’s hand began turning from neon green to orange, however it soon faded back into green. “One or two more drops should do the trick.” And with that, one more drop was added. The color orange remained for a few seconds longer yet it still disappeared with time and the movement of Maddie’s hand, and as such one more drop was added to the sample, now remaining color orange after a whole minute of missing and spinning the sample. “Alright, I’m done with it.”
“That’s great Mads, I’m just putting the last sample of gaseous ectoplasm in the quartz cuvette and then I’ll be done here as well.”
“Alright, I can’t wait to read the results,” the mother stood up and began putting all of the used samples and chemicals used in a container beside the table she was working on, “I’ll begin cleaning my table meanwhile, you should start as well since you’re finishing your experiment as well.”
“Great idea Mads, we don’t want the kids nor any spook to grab any of these after all.” And with that he began cleaning his table as well. Granted his station was cleaner than Maddies on virtue of him working with minuscule samples, unlike Maddie who had to use various milliliters of samples and alkaline solutions in order to do everything. The Fenton Spectrophotometer pinged and Jack put the containers of ectoplasm back where they belonged, then checked on the results, wrote them down and finally took out the last sample before getting rid of it in the appropriate ways.
“Well then, let’s check the results already,” an excited Jack walked to the computer of the lab and began inputting all the data collected into it, letting it all load before adding the next set of results, “What were your results honey?”
“Here, let me type them for you,” Maddie positioned herself besides Jack and began typing her own results in another program to avoid mixing them up, and once both of them put them in, they began comparing them to the previously collected data.
“Mads, is it my imagination or does your ectoplasm sample seem more acidic than normal?”
“That’s not your imagination Jack, I collected those samples with the Specter Speeder from deeper in the Ghost Zone than we’re used to traveling to. There was this river of ectoplasm that I was too curious to leave alone.”
“Well that’s amazing, I wonder what was so different from that ectoplasm that was able to take the form of a river and why it was as acidic as it seems to be according to the results.”
“Not to mention that somehow it was able to stand out between all the ambient ectoplasm of the ghost zone as well.”
“Yeah, I wonder about that. One would think that it wouldn’t be able to stand out like that, but then again Ice and water can be differentiated easily enough.”
“True, but that’s usually because there’s air molecules stuck between the expanded hydrogen bonds that bond the water molecules, thus giving them the extra white inside of them, allowing them to see at a glance the difference between each of them.”
“That suggests that ectoplasm has impurities in it depending on the state of it. Oh I’m getting excited already thinking what kind of discoveries we’ll do with this information, I wonder if there’s pure ectoplasm being naturally formed in the Ghost Zone? Is the air of the Zone pure ectoplasm or would liquid or solid ectoplasm be the pure one? What about plasmic ectoplasm?”
“Well, there are many questions there, but not enough data to get answers. Shall we see your results now and compare them to the ones you’ve made before?”
“Sure thing Mads, I can’t wait to see how that crystalized sample we got last night will compare with the liquid and gaseous ectoplasm.”
“... Well, there’s certainly a difference in the refraction value given by the solid form of ectoplasm, but if that’s because it’s in the form of a crystal or because solid ectoplasm is like that we don’t know yet.”
“Yeah, this is the only sample of solid ectoplasm we’ve managed to collect thus far after all. If only we knew how that was made to replicate it and make more tests on it”
“If only, but that won’t stop us regardless, right Jack?”
“You’ve said it Mads, a Fenton never gives up after all!”
“Mmmhhh, hey Jack, have you thought of doing this experiment on some particles of crystals suspended in gaseous ectoplasm?”
“What are you thinking about honey?”
“Well, if humans can use steam and silver particles for magic tricks, then who’s to say that isn’t something ghosts do to fool our instruments?”
“MADDIE YOU’RE A GENIUS, I’LL GET ON IT RIGHT NOW,” and with that the wall of a man ran towards the crystal of ectoplasm, let it on a table and went up to the kitchen, returning with the cheese grater, ready to turn a part of the ectoplasmic crystal to dust.
#Ectoberhaunt22#EH Order#Day 11#Thirst#Danny Phantom#Jack Fenton#Maddie Fenton#Experiments on ectoplams#surprise surprise the possibility of there being different kinds of ectoplasm is very real
15 notes
·
View notes
Note
what no whos pensive anon?????? im HEART(♡) anon
SORRY HEART (♡) ANON it was a typo i promise. anyways did u know mol acid = mol base at the equivalence point in a titration Hhahahhaahagahahjja (im losing my Fucking mind ive done so much chemistry in the past hour)
2 notes
·
View notes
Text
titration lab
when i first learned about titrations
i hated them
equivalence points and pKa’s and molarity calculations
i carved S shaped curves into my mind and still couldn’t understand
what exactly made them work
i am not a patient person
i dreaded sitting there for hours watching the
drip, drip, drip,
of the strong base or acid
one drop at a time, to get an accurate result
one drop the difference between pale success and bold failure
it didn’t take me long to figure out that
one drop is nothing.
we would be here forever if we did what we were supposed to and
took our time
so i turned the stopcock and let it run too long
five mLs, ten mLs
until the solution was solid pink
and my lab report had 200% error.
when you snapped at me for the first time, it was a drop in clear solution.
i am not a patient person. i never swirled my flasks thoroughly enough. i let tens of milliliters of titrant flow out of the burette before i even thought about slowing down.
one time, my lab partner and i forgot to add the indicator
and it took us 40 mLs of sodium hydroxide before we realized something was wrong
one drop of phenolphthalein
and the whole solution bloomed bright pink
sudden and all at once and way too strong
you held a pillow to my face until i begged you to let me breathe.
one drop is nothing.
the solution stays crystal clear
until it rides the S shaped curve up
and approaches the equivalence point
but by then, it is often too late
and i haven’t swirled enough, or been vigilant enough
and instead of disappearing into clear liquid
the phenolphthalein pink stays
bright as cherry lipstick
and the mark blooming on my cheek
the day you hit me was the day i finally figured out titration curves.
weak acids buffer the pH change
when you add a strong base
and they share the same ions
but eventually, add enough base
and the delicate balance is ruined with one drop
hot pink flaring in an erlenmeyer
how much does it take?
five? ten? twenty?
how many drips before i’ve had enough?
#poetry#stream of consciousness#chemistry#my poem#poets on tumblr#original poetry#writing#spilled ink
4 notes
·
View notes
Text
Theory of Qualitative Analysis
Qualitative analysis is the determinization of the quantity of individual elements or compounds present in a substance.
Two types: Volumetric and Gravimetric.
Volumetric Analysis
General Terms
Titration: aka titrimetry, is a quantitative technique that can be used to calc. the conc. of a given analyte in a mixture
Titrant: The solution of known conc. used in titration (primarily in burette)
Analyte: The solution of unknown conc. which is being analyzed (primarily in the conical flask)
Standard Solution: A solution with known conc. of a substance/element. A known weight of solute is dissolved to make a specific volume of standard solution.
A primary standard solution can be prepared directly and has the following features: highly pure, cheaply available, very soluble in water, neither deliquescent nor hygroscopic, and very stable. Ex: Oxalic acid
Secondary standards are substances whose standard solutions can not be prepared directly. Ex: potassium permanganate.
Indicator: A readily soluble substance that can change color at different pH levels. It should not effect the actual chemical reaction occurring and only 1-2 drops of indicatory should be sufficient to produce the necessary color change.
Endpoint: The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been added to a solution. The end point is approximate to the equivalence point.
Titration Error: The difference between the end point and the equivalence point, can be either positive or negative.
Volumetric analysis measures volume of a reagent needed to react with an analyte.
Volumetric analysis can be classified into acid-base titration, redox titration, precipitation titration, and complexometric titration.
Acid-Base/Neutralization Titration
In this titration, an acid reacts with a base to neutralize completely and form a salt. This can happen when the number of equivalents of an acid/base of unknown conc. is equal to the number of equivalents of the base/acid of known conc.
Acidimetry: the given base is estimated using standard acid solution.
Alkalimetry: the given acid is estimated using standard base solution.
Common indicators used:
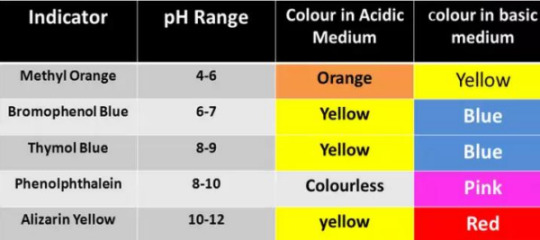
An acid-base indicator is a substance which is one color in acid and another in base. There are two theories which explain how they work: Ostwald and Quinonoid.
Ostwald Theory: An indicator is either a weak organic acid/base. The unionized compound has one color and the ion produced has another color. This is how it ionizes:
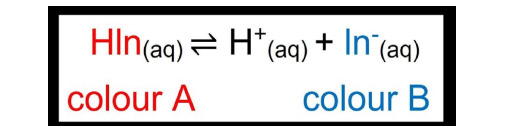
Phenolphthalein: a colorless organic compound which is a weak organic acid. It dissolves in water and dissociates slightly to form H+ ions (colorless) and Ph- ions (pink).
HPh ⇌ H+ + Ph-
i) In Acidic Medium: If the solution is made acidic, then the increase in H+ ion conc. suppresses the dissociation of phenolphthalein due to common ion effect, then the equilibrium shifts towards L.H.S. of the equation and the solution remains clear. Ex: HCl + Phenolphthalein
ii) In Basic Medium: If the solution is made basic, then the OH- ions reacts with the H+ ions to form unionized water molecules, the decrease in the H+ ion conc. shifts the equilibrium towards the R.H.S, therefore more of the indicator ionizes and the solution becomes pink. Ex: NaOH + Phenolphthalein
Methyl Orange: An organic compound which is a weak organic base. It is soluble in water and dissociates to a very small extent.
MeOH ⇌ Me+
i) In Acidic Medium: If the solution is made acidic, then the increase in H+ ions react with OH- ions to form unionized water molecules. Decrease in OH- ion conc. shifts the equilibrium towards the R.H.S., therefore the solution acquires an orange color due to Me+ ions. Ex: HCl + Methyl Orange
ii) In Basic Medium: If the solution is made basic, then the OH- ion conc. suppresses the dissociation of MeOH- due to common ion effect. The equilibrium shifts towards L.H.S and the solution remains pale yellow. Ex: NaOH + Methyl Orange.
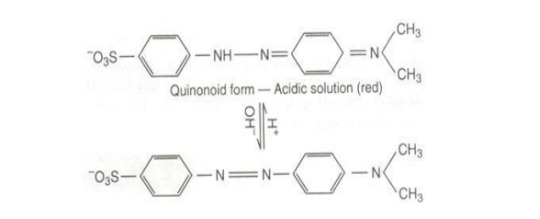
Quinonoid Theory: The color change of an acid-base indicator arises as a result of structural change. It is supposed that an indicator exists as a equilibrium mixture of two tautomeric forms, benzenoid and quinonoid forms.
One form exists in acid and the other in base.
The two forms possess two different colors and as the pH of the solution containing the indicator is changed, the solution shows a change of color.
Phenolphthalein: stable in both forms, Benzenoid - Quinonoid

Methyl Orange: stable in both forms, Quinonoid - Benzenoid
Correct detection of end point will affect the titration calc. and is very important. So it is very important to pick the correct indicator. Acid-base reactions are of 4 types:
Strong acid vs Strong base
Weak acid vs Strong base
Strong acid vs Weak base
Weak acid vs Weak base
To chose the best indicator, one must understand the pH changes in the above 4. The pH change in the vicinity of the equivalence point is the most important.
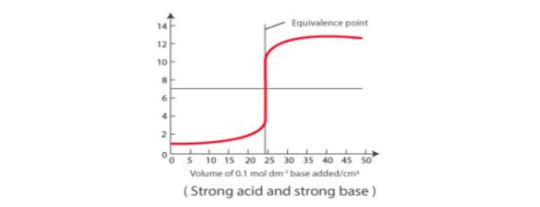
Titration Curve: the curve obtained by plotting pH as y-axis against the volume of alkali added as x-axis.
In each case below, 25ml of acid has been titrated against a standard solution of a base. Each titration curve becomes almost vertical and then bends away again.
The region of abrupt change in pH indicates the equivalence point.
The indicator should be picked so that it changes its color within vertical distance of the curve.
Strong acid vs strong base: pH curve of strong acid (HCl) and strong base (NaOH) is vertical around the pH range 4-10. So phenolphthalein, methyl red, and methyl orange, are suitable.
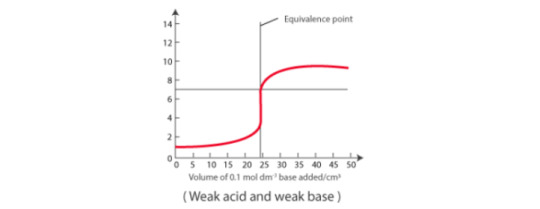
Weak acid vs strong base: pH curve of weak acid (CH3COOH) and strong base is vertical around the pH range 7-11. so phenolphthalein is okay
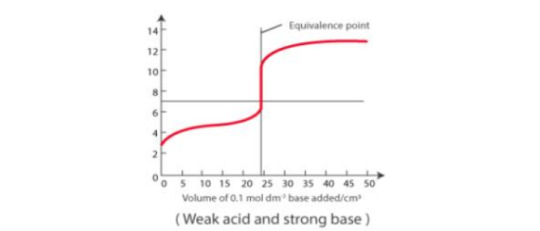
Strong acid vs weak base: pH curve of strong acid with weak base (NH4OH) is vertical around the pH range 4-7, so methyl red and methyl orange are both okay.
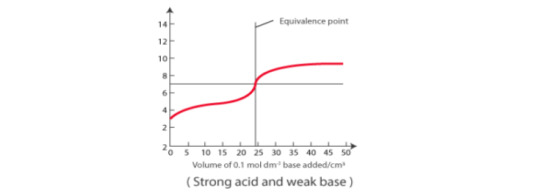
Weak acid vs weak base: pH curve of weak acid and weak base indicates that there is no vertical part, therefore no indicator can be used.
Redox Titration
Redox Titration: reactions that involve the transfer of electrons from one species to another. The species that loses electrons is oxidized while the species that gains electrons is reduced.
Oxidation: addition of O, removal of H+, loss of electrons, increase in the oxidation state of the substance.
Reduction: removal of O, addition of H+, gain of electrons, decrease in the oxidation state of the substance.
Oxidizing agent: the substance which undergoes reduction to induce oxidization. (Ex: KMnO4)
Reducing agent: the substance which undergoes oxidization to induce reduction (Ex: Mohr's salt)
Oxidimetry: Determination of strength of reducing agent with standard solution of oxidizing agent.
Reducimetry: Determination of strength of oxidizing agent with standard solution of reducing agent.
Types of Redox Titrations:
Dichrometry: Estimation which involves the use of potassium dichromate (K2Cr2O7) - primary standard
Iodometry: Estimation involves liberated iodine (I2) - CU(II)+Hypo
Permanganometry: Estimation which involves the use of potassium permanganate (KMnO4) - self indicator
Bromometry: Uses a bromine titrant (Br2)
Cerimetry: Employs cerium salts (IV)
Redox Indicators: these indicators are oxidants and or reductants. Oxidized form has one color while reduced form has another. When slight excess of oxidant is present, the indicatory changes in color and is shows as the end point of the titration. There are 3 types: self, external, and redox.
Self Indicators: The oxidized or reduced form of the titrant is self indicating (Ex: KMnO4)
External Indicator: The indicator is externally added to the solution. It does not take part in the reaction. (Ex: Potassium Ferro cyanide in the ferrous sulphate + K2Cr2O7 experiment)
Redox Indicator: A compound that changes color at specific potential differences. It must have a reduced and oxidized form with different colors and the redox process must be reversible (Ex: Diphenyl amine indicator has blue/violet color when oxidized and is colorless when reduced.
Precipitation Titration
A type of titration were a precipitate is formed during the course of the reaction.
The titrant reacts with the analyte to form an insoluble material and the titration continues till the very last amount of analyte is consumed.
Principle: the quantity of the added precipitating reagent or precipitant is equivalent to the substance being precipitated.
There are three main types based on end point detection: Mohr's, Volhard's and Fajan's Method.
Mohr's Method: This method uses chromate as an indicator. Chromate forms a precipitate with Ag+ but this ppt has a greater solubility than that of AgCl in NaCl vs AgNO3 titrations
Therefore, AgCl is formed first and after all Cl- is consumed, the first drop of excess Ag+ will react with the chromate indicator to give a red ppt.
Volhard's Method: Standard potassium tricyanate is titrated against Ag+ solution containing Fe3+ (Ferric alum indicator)
The excess Ag+ is then titrated with standard SCN- solution till a red color is obtained.
This method is widely used because it can detect the end point very well.
Fajan's Method: Fluorescein and its derivatives are adsorbed to the surface of colloidal AgCl (Reddish tinge on white ppt)
After all chloride is used, the first drop of Ag+ will react with fluorescein (FI-) to form a red color.
Complexometric Titration
Principle: Based on complex formation between the metal ion and ligand.
Particularly useful for determination of a mixture of different metal ions in solution.
An indicator with a marked color change is usually used
EDTA: Ethylene diamine tetra acetic acid, has 4 carboxyl groups and two amine groups that act as electron pair donors.
The ability of EDTA to potentially donate six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand.
However in practice, EDTA is usually only partly ionized and thus forms fewer that six coordinate covalent bonds with metal cations.
EDTA forms stable complex with various metal ions.
The complexation occurs in a single step: there is a sharp change in the metal ion conc. at the equivalence point, the M-EDTA complex is water soluble, and the stoichiometry for all metal ions is the same 1:1 irrespective of charge.
Metal Ion Indicators: Compounds that change color when bonded to metal.
Requirements:
1. Color must be intense enough
2. Color contrast between the indicator and metal-indicator complex must be distinct
3. M-indicator complex should be stable enough to ensure color change but less stable than EDTA.
4. The change in equilibrium bust me rapid and sharp.
5. The color reaction should be selective
6. The indicator must be very sensitive to metal ions.
7. The indicator must be stable in the titration medium.
8. The indicator must me stable in storage too.
9. All of the above must be fulfilled in the pH range of the reaction
10. It should be commercially available and pure.
Examples of metal ion indicators are:
Eriochrome Black T/EBT
Murexide
Xylenol orange
Pyridylazonaphthol/PAN
Types of EDTA Titrations:
Direct
Back
Substitution
Alkalimetric
Indirect
Gravimetric Analysis
A method of analytical chemistry to determine the quantity of analyte based on the mass of a solid.
Principle: The mass of an ion in a pure compound can be determined and used to find the mass percent of that same ion in a known quantity of an impure compound.
Certain conditions must be met: the ion being analyzed must be completely precipitated, the precipitate must be a pure compound, and the precipitate must be easy to filter.
The 7 steps of gravimetry are:
1. Prep. of sample solution
2. Precipitation
3. Digestion
4. Filtration
5. Washing
6. Dying and Ignition
8. Weighing and Calculation
Preparation of Sample Solution
When sample solution is prepared, dilute solution is generally preferred.
Volume is adjusted to suit the amount of precipitating agent.
pH of the solution is adjusted for precipitation to occur.
The desired property of the solution is maintained for precipitation to occur.
Precipitation
The analyte is converted into a soluble precipitate.
Nucleation: Individual particles group together to form a larger colloidal particle called a nuclei. As nucleation increases, a larger number of nuclei appear, leading to more adsorption of impurity.
The initial nucleus grows by the deposition of their precipitate particles to form a crystal or geometric shape.
The greater the super saturation, the more rapid crustal growth rate and colloidal precipitate form.
Increase in the growth rate increases chance of imperfection in the crystal and surface area of precipitate increases, which leads to easy trapping of impurities.
Precipitate Contamination is of two types:
1. Co-Precipitation: Precipitation where soluble compounds in a solution are removed during the course of precipitation. It is of 2 subtypes: surface adsorption and occlusion.
Surface adsorption: Trapping of impurities on the surface of the crystal
Occlusion: Trapping of impurities within the crystal.
2. Post-Precipitation: Where the precipitation of undesirable compound occurs after the formation of the precipitate of the desirable compound, secondary ppt after primary ppt is already formed.
Ex: Ca oxalate ppt in presence of Mg ion. After some time, Mg oxalate forms deposit on Ca oxalate surface.
Digestion
The ppt is left hot, just below boiling point, for up to an hour to digest the particles.
Digestion involves dissolution of small particles and re-precipitation on larger ones when slowly cooled, this results in larger particles.
This process is called Oswald Ripening, it is very useful for colloidal precipitation.
Filtration
Ppt is physically separated from mother liquid.
The filtration method depends on the nature of ppt, the cost of media, and heating temp required for drying.
Examples of filtration mediums are:
Filter paper
Filter pump
Filter mats
Crucible fitted with porus plate.
Crucible to be used at high temps.
Washing
Co-precipitate impurities may be washed off the surface of the ppt even after filtering.
Excess mother liquid on the surface of the ppt can be washed off too.
Many ppts cannot be washed with pure water because peptization would occur.
Ex: NHO3 can be used to wash off AgCl ppt.
Drying and Ignition
The ppt must be pure, stable, and of known composition.
Drying and ignition allow the ppt to be in suitable condition for weighing.
It removes water efficiently.
Appropriate chemical changes occur during heating
Ex: AgCl is dried at 100°-130°C to remove physically bound water.
A higher temp is necessary if the water is chemically bonded, trapped in the crystals, or to ensure appropriate chemical change.
Weighing and Calculation
After ignition, ppt is taken out into a clean crucible and is weighed accuracy on an analytical balance.
Calculations are done.
0 notes
Text
Automatic Potentiometric Titrator

An automatic potentiometric titrator is a sophisticated instrument used in analytical chemistry for performing titrations automatically. The titrator uses a potentiometric method, where the potential difference between a reference electrode and a measuring electrode (usually a pH electrode or a redox electrode) is measured. This potential difference changes as the titrant is added to the sample, and it reaches a minimum or maximum at the equivalence point of the reaction.
0 notes
Text

Potentiometric Titrator
Potentiometric titrator is a touch screen instrument that displays the titration methods and curves in detail. The device can save up to 100 sets of GLP compliant data, also has 100 user-defined methods and 10 user-defined shortcuts for easy operation. It comprises four titration modes Dynamic endpoint titration (DET), Monotone equivalent point titration (MET), Preset endpoint titration (SET), and Manual titration (MAT). Auto calculation and the formula are pre-installed which provides time-efficient work.
Potentiometric titrator provides results which are based on analysis methods that are highly important in the field of electrochemistry and are commonly used for the determination of different organic and inorganic ions in various areas such as process control and environmental, industrial, agricultural analysis and medicinal drug analysis.
#potentiometrictitrator, #titrator, #titrationmethods
0 notes
Text
Phenolphthalein is therefore a nearly perfect indicator for the titration of a weak acid by a strong base, where the pH at the equivalence point is on the basic side (figure 11.19).
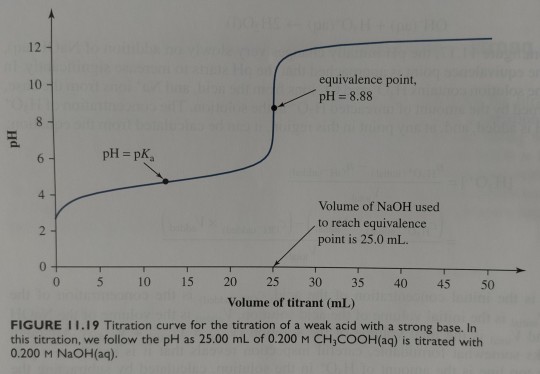
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#phenolphthalein#indicator#titration#acid#base#ph#basic#chemical reactions
0 notes
Text
Figure 11.17 shows a plot of pH versus volume of titrant added for the titration of 25.00 mL of 0.200 M HCl(aq) with 0.200 M NaOH(aq). (...) As you can see from figure 11.17, the pH initially changes very slowly on addition of NaOH(aq), and it is not until the equivalence point is approached that the pH starts to increase significantly. (...) For example, in the titration of HCl with NaOH described earlier (figure 11.17), the pH just before the equivalence point (when 24.97 mL of the base has been added) is 3.92.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#chemical reactions#acid#base#titration#hydrochloric acid#sodium hydroxide
0 notes
Text
Karl Fischer Titration And Its Compounds
Karl Fischer titration is a traditional chemical analysis titration method that uses coulometric or volumetric titration to quantify trace levels of water in a sample. Karl Fischer, a German scientist, created it in 1935. Titration is now performed using automated procedures and a variety of substances like a Karl Fischer reagent.
The Karl Fischer titration is popular due to its various practical benefits over other moisture assessment methods, including accuracy, rapidity, and selectivity. Karl Fischer's titration is water-selective because the titration process consumes water.
Measuring mass loss on drying will identify the loss of any volatile material. On the other hand, the strong redox chemistry (SO2/I2) implies that redox-active sample components may react with the reagents. As a result, Karl Fischer titration is inappropriate for solutions containing, for example, dimethyl sulfoxide.
Karl Fischer Titration is utilized in a variety of technical items such as polymers, oils, and gasses. It is found in pharmaceuticals, present in cosmetics and employed in industries.
Karl Fischer Titration has the following advantages:
It is suitable for determining water in gases, liquids, and solids.
A coulometric titrator detects free water, dissolved water, and emulsified water.
It is a quick procedure that requires little sample preparation.
This method is quite accurate.
Karl Fischer titration offers high accuracy and precision, often within 1% of available water; for example, 3.00% appears as 2.97-3.03%. Even though Karl Fischer titration is a destructive analysis, the sample quantity is minimal and is normally restricted by weighing accuracy.
For example, to achieve a 1% accuracy using a scale with a normal precision of 0.2 mg, the sample must include 20 mg water, equivalent to 200 mg for a 10% water sample. Coulometers have a measurement range of 1-5 ppm to roughly 5%.
Volumetric Karl Fischer titration measures samples up to 100% accurately but need impractically enormous volumes of sample for analytes containing less than 0.05% water. The titration response of Karl Fischer is linear. As a result, single-point calibration using a calibrated 1% water standard is adequate, and calibration curves are not required.
Karl Fischer titration may be used to measure liquids and, with specific apparatus, gases. The main drawback of solids is that water must be freely available and introduced into a methanol solution.
Many common substances, particularly delicacies like chocolate, release water slowly and with difficulty, necessitating extra efforts to bring the complete water content into contact with the Karl Fischer reagents. A high-shear mixer, for example, might be added to the cell to break the sample.
Because Karl Fischer titration has issues with chemicals that have significant water binding, such as lithium chloride, it is inappropriate for the specific solvent LiCl/DMAc.The reagents are solvent alcohol (ROH), a specified concentration of iodine (I2), a base (RN), and sulphur dioxide (SO2).
In an aqueous setting, the Bunsen reaction between iodine and sulphur dioxide is the foundation for Karl Fischer Reagent reactions. Finar's R&D department has created an in-house technology that is suited for both manual and automation equipment, assuring a constant factor as leading lab chemical suppliers.
Finar also offers Aquamicron Coulometric Karl Fischer Reagents from Japan. If you are looking for the best laboratory chemicals manufacturers in India then go for Finar.
0 notes
Text
Free iphone simulator online

Free iphone simulator online install#
Free iphone simulator online android#
Free iphone simulator online software#
Free iphone simulator online simulator#
Keep in mind that iPhone emulators can only be used for iPhone OS devices. If you are someone who wants a realistic feeling while playing a car driving simulating game for iOS, then Real Racing 3, Car Parking and Driving Simulator, and Grid AUTOSPORT are the best games for you.
Free iphone simulator online android#
Please feel free to contact me if you have any question or require any help. So these are the paid as well as the free Best Driving Simulation Games for iOS and Android 2022.
Free iphone simulator online simulator#
IOS simulator downloads are also available for downloading to help you get started with your own ios applications. My site contains detailed information on all types of iPhone Emulators. If you wish, you can modify the settings by choosing the options available in the "Settings" section of the iPhone Simulator.įor more information about iPhone Simulator, visit my blog on iPhone Application Development. To start using the iPhone Simulator, first, launch iPhone Emulator and then select "Emulator" option to enter the simulator world.
Free iphone simulator online install#
iPhone Simulator application is easy to install and run, and does not require any technical knowledge. IPhone Simulator allows you to browse through and select from a large number of games, simulates different features of iPhones like text messaging, browsing, emailing, playing audio/video and many more. As an iPhone Simulator, the user can view his/her iPhone through a virtual interface that resembles that of the real iPhone. It emulates hardware based on the actual hardware of the iPhone, including the gyroscope, accelerometer, camera and screen. The iPhone SDK, previously the iPhone SDK, is an application development kit developed by Apple Inc to allow the creation of applications for the Apple's iOS and iPod touch operating systems.
Free iphone simulator online software#
Or, the answer will be provided if you press ‘Show Answer’.The iPhone Simulator, also known as the iPhone Emulator, is the software development package for the iPhone, originally the iPhone SDK. Follow along on paper and try to locate the equivalence point by checking the center of the vertical line, and calculate the concentration of the unknown acid. Y-axis is pH, and x-axis is volume of added NaOH. After the simulation stops, you are given the chance to see the titration curve. The acid will turn pink overtime, you need to keep adding NaOH until it turns fully pink (then the simulation will stop). For either acid, watch the fluctuation on the pH bar to regulate your droplet volume. Tip: If using HCOOH, you can use a higher droplet volumes first, then lower it. Please use smaller concentrations, or else the pH will rise very quickly and you will miss the equivalence point. Tip: If using HCl, note that the acid will be neutralized very quickly. Press Tap faster to add more NaOH (this simulates leaving the stopcock open for longer.) 6. iPhone Simulator allows you to browse through and select from a large number of games, simulates different features of iPhones like text messaging, browsing, emailing, playing audio/video and many more. The pH indicator bar on the right shows the raising of the pH. As an iPhone Simulator, the user can view his/her iPhone through a virtual interface that resembles that of the real iPhone. The volume of NaOH added and pH of current solution are indicated on screen. Please select what volume of droplet you would like to add to the acid in the beaker (0.2, 0.5, 1.0, 2.0 or 5.0 mL) 3. You will be given NaOH in the burette at the appropriate concentration. On the home screen, choose HCl or HCOOH to start the strong acid or strong base respectively. Switches to pH via pOH calculation when beyond equivalent point. Calculates initial pH condition (no NaOH added), does not assume is zero. Uses the Henderson-Hasselbach equation for the weak acid/ strong base combination. Suitable for first-year undergraduate or senior high school level students. Use this app to practice your titration skills, for when you need it in the lab. Try to calculate the unknown concentration yourself, or click to Show Answer. dynamic phenolpthalein visual indicator, along with pH and volume status - dynamic droplet indicator - adjust volume droplet between (0.2mL to 5.0mL), or simply tap faster - Graph of pH vs volume of NaOH added shown at the end. This is a good way to do a dry run through a real titration experiment, before trying it at school! Features: - Simulate titration of strong acid (HCl) with strong base (NaOH), or weak acid (HCOOH) with strong base (NaOH). The concentration of the acid will be unknown to you to simulate a lab experiment, but the volume will be fixed at 50mL. You will be titrating of a strong acid (HCl) with a strong base (0.1M NaOH), or a weak acid (HCOOH) with strong base (1.0 M NaOH). This app uses the Henderson- Hasselbach equation and concepts in Acid-Base equilibria to do a simulation of a titration to determine concentration.

0 notes
Text
titration is just edging for chemists except there’s no good outcome possible
#science bastard dot tumblr dot com posts bullshit. more at 7.#chemistry#science#sciencecore#man idk how to tag this#i mean yeah the pink is great and all but what about the titration curve? the actual math? ruined#how the fuck am i supposed to find the equivalence point now#lab records#science memes
138 notes
·
View notes
Text
Peter Parker x Reader
Summary - Peter is helping the reader study for chemistry and things start to get heated.
Warnings - Smut!, makeout scenes, handjob, hinting towards oral
Word Count - 1,912
Authors Note - Confession time! This is the first smutty thing I had ever wrote. Ever! So if this is absolutely trash? I apologize. But I love Peter and so I had to give it a shot.

If it weren’t for Peter then I would have failed chemistry a long, long time ago.
This was a fact that I was reminded of every Friday night when the two of us would hole up in his room with a collection of all of the Parker families best snacks and way more flashcards than anyone would ever want to see.
Peter had essentially made it his own personal mission to help me pass chemistry after listening to me whine about getting an F on the first test of the semester. That was honestly just the kind of person he was, overly caring and always willing to do anything he could to be the most helpful person in the room. That was one of the many things that I loved about my boyfriend. However, the one thing I hated? Was having to study for several long and painful hours with very few breaks.
“Pete!” I groaned, throwing my head back dramatically, “We’ve been going for like, two hours straight now! And I still just barely understand what you’re talking about. Can we please stop for a second?”
Peter frowned at me from the desk chair where he sat, still holding the flashcard up. “C’mon Y/N! You know this!” he encouraged. “You just gotta tell me at what pH the equivalence point lies for a weak strong-acid base titration!”
I stared blankly at him, my brain not even comprehending a single word that had just left his mouth. “7?”
“Wha-7? What? No. The answer is slightly basic.” The confused look taking over his features was enough to make me smile. “Where did you even get 7?”
I shrugged my shoulders at him, using my hands to push myself up off the ground where I had been sitting this whole time, stretching slightly. “I don’t know Pete! I mean, my brain is literally rotting. So that? Was the only answer I could come up with.”
He just jokingly rolled his eyes in response, turning slightly in the chair so that he could place the flashcards face down on his desk.
One of the few good things that had come from me failing chemistry and being forced to suffer through these study sessions was all the extra time I got to spend with him. While I would have preferred doing anything other than studying, I still was just happy to take all of the quality time that I could get with him. Ever since he got bit by that spider our alone time had been cut down significantly, so if studying was one of the only times I could enjoy his presence then I would happily take it.
“Ten minutes, ok? Then we really need to get back to it. Don’t forget about your test next week.” Peter reminded me, pointing his index finger in my face and pretending to scold me.
“Okay, dad.”
“So what do you want to do with your break then?” He asked as he absentmindedly swiveled his chair around, his eyes focusing in on the bag of gummy bears laying on the ground. “Snack?”
I shook my head at him, a playful grin now pulling at my lips. I walked towards him, placing my hands on the arms of his chair and leaning in until I was barely an inch away from his face. “I think I’ve got a better idea.” My voice came out low, my lips just slightly brushing against his as I spoke.
Peter swallowed, quickly nodding his head, “I think I like this idea.”
“Thought you would.”
Carefully, I positioned myself in Peter’s lap, his hands resting on my hips and helping to guide me into place. He slowly reached up and brushed my hair out of my face, tucking it gently behind my ear and letting his fingers fall down the side of my neck, fingertips lightly tracing against my skin as he leaned in. His nose bumped against mine, and with both of us still inexperienced and used to making these little mistakes we just let out a small laugh before his lips finally found their way to mine.
Kissing Peter was something that I never got tired of. A familiar warmth blooming in my chest as I felt him run his hand down my back, finally coming to a stop as it lightly grazed against my butt, a bit of confidence eventually kicking in as he began to grip it in his hand, a low moan escaping his lips.
Butterflies would still flutter in my stomach as he kissed me, his lips so incredibly soft against my own and his tongue shyly tracing against my bottom lip, begging for me to part them. His kiss tasted slightly sour, remnants of the candy he had been eating during studying still prominent, but I didn’t mind as his tongue slipped inside my mouth.
I brought my hands up to his head, my fingers instantly becoming tangled in his dark hair. I tightened my grip on it, tugging at it just a bit, knowing that the sensation was always enough to really start to drive him crazy. And it definitely did the trick, his own hands now moving to my hips and dragging me down even further against his own, slowly moving me so that I was grinding against him.
As I felt something begin to press against the side of my thigh I broke away from him, “Thought we needed to get back to studying?” I teased.
“Shut up.” his head fell onto my shoulder so his lips were brushing against my ear as he spoke. “It can wait a little longer.”
Satisfied with his answer I let one of my hands fall from his hair, placing my hand over the bulge in his jeans, earning a small whine from him at the feeling. He began peppering kisses along the side of my neck, occasionally running his tongue over the skin, sucking gently as I moved my fingers up to undo his jeans.
Peter lifted us both slightly, allowing me just enough room to be able to wiggle his jeans and boxers down just a touch, just enough to be able to give him the sort of physical touch he wanted right now, wrapping my hand around his dick and watching as his head instinctively fell back, his teeth digging into his bottom lip.
It had been several weeks since we had sex, every moment constantly being interrupted by either schoolwork, his aunt knocking on the door, or Spider-Man duties, meaning that the both of us were very touch starved at this point and I knew that he was just as desperate as I was.
I pulled my hand away from him for just a moment--much to Peter’s dismay--as I licked my hand, giving just enough moisture to be able to slide it up and down his length with ease. A low groan escaped his throat at the new feeling of my hand tightly wrapped around his dick, my thumb occasionally circling around the tip as I watched him clench his eyes shut even tighter.
After just a few short minutes of me stroking his dick, he roughly grabbed my wrist, stopping me from moving it any more. “Off.” his other hand tugged at my shorts. I happily obliged with his demands, lifting myself off his lap enough for him to help me remove the articles of clothing despite the rather awkward position.
Quickies were not a common thing for the two of us.
Peter preferred to take his time when it came to sex--usually stopping to worship each and every inch of my body, his lips coming into contact with every bit of skin they could find. And of course I loved the way it felt, his tongue mapping out my entire body until he eventually found his way to the one place we both needed him to be, but right now? I knew I wouldn’t be able to sit through all of that and neither would he.
I needed Peter Parker and I needed him right now.
His hand was guiding his length, slowly dragging it back and forth along my slit, taking in the sight in front of him. His gaze met mine, just barely pressing against my entrance, waiting for confirmation that it was ok to keep going. My hands rested on his shoulders, slowly sinking down on his cock, moaning rather loudly at the sensation.
“Quiet princess,” Peter remarked, “wouldn’t want May to hear and interrupt, yeah?”
The use of the nickname certainly wasn’t helping me stay quiet and he knew that--knowing that the moment he used it I would be absolute putty in his hands. He gripped my hips tightly, slowly helping to guide me up and down his length, a series of quiet curses leaving his beautiful lips.
Peter and I had been together for a while now and we had been having sex for months, and yet I still wasn’t used to just how amazing he felt. He stretched me out perfectly, always managing to hit all of the right spots without even trying. It honestly wasn’t shocking--everything about him was perfect, so of course his dick was just as fucking amazing.
As I settled into a rhythm with riding him he moved one of his hands from my hips, letting it venture underneath my shirt--which we hadn’t removed yet--and began squeezing at my tits and pinching at my nipples.
“You feel so good baby...” His breathing was quick, his words coming out rather strangled, and I knew that he was struggling to hold on after going so long without, trying desperately to hold on and help me reach my own orgasm.
I placed my hand under his chin, gently lifting it so that his eyes were looking into mine. “It’s ok-” I assured him, not wanting to make him wait any longer. “I just want you to cum inside of me, Pete. Please.”
The sight of me begging from on top of him while asking for him to cum inside of me was obviously enough to drive him over the edge. His thrusts became quicker, sloppier, his hands still trying to guide me so that I was meeting his hips with each thrust.
“Fuck y/n.” Pete breathed out, burying his face in the crook of my neck once more, biting down on the sensitive skin as he emptied himself inside of me, various ungodly noises escaping his lips as he continued to move my hips against him.
I ran my fingers through his hair, my other hand lightly brushing against his arm as he came down.
“You’re amazing, you know that?” Peter asked me with a light chuckle, still out of breath as he leaned back to look at me.
I kissed his forehead in response, watching as his cheeks turned a light shade of pink. Here we were, sitting almost fully naked in his desk chair with his dick inside of me and yet that is what managed to make him blush. Typical Peter.
“Guess it’s time to get back to studying, huh?” I reminded him jokingly.
Peter licked his lips, wrapping his arms around me before picking me up and moving us to his bed, laying m down on the mattress. “I think I’ve got a better idea.” He mocked before sinking down between my thighs. Maybe study dates weren’t so bad.
#peter parker imagine#tasm peter parker#peter parker one shot#peter parker smut#peter parker fluff#peter parker fluff imagines#peter parker imagines#tasm imagine#tasm fanfiction#tasm peter x reader#peter parker x reader#the amazing spiderman#the amazing spiderman imagines#spiderman imagines#spiderman one shot#spiderman fluff#spiderman smut#andrew garfield imagine#andrew garfield one shot#peter parker x you#peter parker x y/n
1K notes
·
View notes
Note
forgive me for being dumb, but what is a titration ?
a titration is a procedure where u try to determine the concentration of solution A by reacting it w solution B (known concentration and volume) in measured amounts until u reach the equivalence point !
#today was pretty simple we were just using HCl and NaOH#making NaCl and water etc etc#anons my beloved#asks!
7 notes
·
View notes
Photo

[Reactions of potassium amide
With halobenzenes in ammonia
Via benzyne intermediates occur
Bergstrom and associates did report,
Based on two-component competition runs,
Bromobenzene the fastest to react,
By iodobenzene closely followed,
The chloro compound lagging far behind,
And fluorobenzene to be quite inert
At reflux (-33°).
Reactions with para-dihalobenzenes,
In which the halogens were not the same,
The same order of mobility revealed,
But differences in reactivity
Were somewhat less in magnitude.
The irregular mobility rank
Explanation finds in the mechanism
Whereby arynes are formed. There are two steps:
Abstraction of the ortho proton
And the expulsion of the halogen
From the anion intermediate.
In Scheme I the mechanism is set forth.
Here proton removal is favored, in rate
And in respect to equilibrium,
By high electronegativity
Of halogen. But the expulsion step
Is faster in the opposite order.
According to the evidence, for both
Iodine and bromine step 1 limits rate.
But on the other hand, the setting free
Of halogen determines total rate
For chlorine and fluorine atoms on the ring.
We have repeated the experiments
With dihalobenzenes of Bergstrom’s group.
They are extended to the isomers
Meta and ortho, and to the action
Of potassium anilide reagent.
Throughout, halide ions have been determined
By potentiometric titration
In which end points for diverse halide ions
Are discrete, and easy to recognize.
Nitrogenous products were not assayed.
Results
Data for reactions of all nine mixed
Dihalobenzenes (excluding fluorine)
With four equivalents of amide base
Are set forth in Table I. Reactions
With the same base in deficiency
Appear, for six substrates, in Table II.
In Table I, more than one halide ion
Is set free from each dihalobenzene
Molecule. This suggests the possibility
That maybe haloanilines too react
With potassium amide. In Table III [...]]
We also note footnote 2, which reads:
[Note from Editor—Although we are open to new styles and formats for scientific publication, we must admit to surprise upon receiving this paper. However, we find the paper to be novel in its chemistry, and readable in its verse. Because of the somewhat increased space requirements and possible difficulty to some of our nonpoetically inclined readers, manuscripts in this format face an uncertain future in this office. However, we take this opportunity to encourage readers and authors to examine carefully a new format represented by the articles on pages 3591–3646 and the Editor’s Notice in the November 1970 issue of this journal.]
Bunnett and Kearley (1971)
213 notes
·
View notes
Text
Does anyone know how to calculate the equivalence point and buffer region of acid-base titration?

#biochemistry#chemistry#colledge#titration#studyblr#study tips#study space#study hard#studyspo#someone help#pls help#send help#please help#questions#request
1 note
·
View note