#oxidized stainless
Text



#jewelry#rings#halloween#coffin#coffin jewelry#goth#goth jewelry#stainless#oxidized stainless#zombie#skeleton#halloween jewelry#halloween accessories#creepy#ring#goth rings
1 note
·
View note
Photo

Study examines oxide growth in additively manufactured metals in a supercritical carbon dioxide environment
A new joint study by Southwest Research Institute and Sandia National Laboratories examines the differences in oxide film growth on additively manufactured (AM) metals and wrought stainless steel in a supercritical carbon dioxide (sCO2) environment.
sCO2 is carbon dioxide held above a critical temperature and pressure, which causes it to combine the properties of gas and liquid. Current power plants typically use water as a thermal medium in power cycles. Replacing water with sCO2 increases efficiency by as much as 10 percent, which also allows for considerably smaller turbomachinery and a smaller footprint. Its supercritical state makes sCO2 a highly efficient fluid to generate power because small changes in temperature or pressure cause significant shifts in its density.
SwRI is a leader in sCO2 power cycles. The Institute has received numerous Department of Energy and industry-funded projects to implement pilot-scale sCO2 power cycle components and system-level equipment in addition to the 10 MWe Supercritical Transformational Electric Power (STEP) Pilot Plant under construction at SwRI.
Read more.
27 notes
·
View notes
Text


After a bit of research and a lot of trial & error, I managed to get all the forge scale (the discoloration in the metal from oxidation) off, straighten out the majority of the wiggles in the band, heat harden it, and give it a good polish. At this point, if I do anything else to it I’m just making more work for myself, and I can’t think of anything that could be improved that wouldn’t also involve me starting a new ring.
#mine#me#hobbies#jewelry making#metal working#puzzle ring#silversmithing#argentiumsilver#for anyone who wanted to know#the pickle recipe that I used to get rid of the forge scale is just 7 or 8 grams of citric acid dissolved in a cup of water and heated until#it steamed#you could also use a cup of lemon juice and a teaspoon of salt (again heated until it steamed) to get the same results#just soak the ring in that solution until it’s slightly cloudy and all the oxidation is gone and then you can start polishing#don’t use iron or aluminum in this process at all cause things can get weird. A stainless steel pot and wooden chopsticks will work well#once you’re done with the pickle keep dumping baking soda into it until it stops reacting (stirring it in as you go) and you can dump it#down the drain
12 notes
·
View notes
Text
Because F2 is an extremely powerful oxidising agent (E° = +2.87 V), the element cannot be prepared by chemical oxidation of F- and therefore obtained by electrolysis of KF·2HF, as depicted in figure 14.15; here, KF acts as an electrolyte.
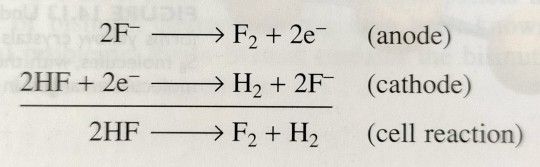
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#fluorine#oxidation#electrolysis#schematic#electrolytic cell#redox#reduction#cathode#anode#carbon#stainless steel
1 note
·
View note
Text

0 notes
Photo

Enclosed Kitchen (Boston)
#Inspiration for a large transitional u-shaped medium tone wood floor and brown floor enclosed kitchen remodel with a single-bowl sink#shaker cabinets#gray cabinets#quartz countertops#gray backsplash#stainless steel appliances#an island and white countertops enclosed#dekton counter#trillium#whites#kitchen#aspect cabinets#oxidized metal
0 notes
Text

#Maxima Triply Stainless Steel Water Bottle with stylish design and mirror finish is a best hydrating companion for you following are the fea#•Insulation type: Single wall; Temperature retention: No; Leak proof: Yes. Superior rust-proof steel. The bottle is made of premium food-gr#•Best Usage: Office/School/College/Gym/Picnic/Home/Fridge. The bottle has multiple uses#right from numerous outdoor activities like hiking#and yoga exercises to using it for your office and work-from-home setup as well.#•The taste and nutritive value of the drinks remain intact. The water bottle is fully resistant to oxidation and rust and never leaves a me#•The bottle lid is sealed by a silicone ring that makes it completely leakproof when it's laid on its side or even shaken. Ideal for storin#iced tea#juices#shakes#and more.#•It is simple to use and easy to clean with inner and outer steel that is convenient to wash. This bottle is easy to clean and maintain#Use a mild detergent or dishwashing liquid and clean on the inside with a bottle brush.#best quality stainless steel cookware#https://www.maximaworld.in/#stainless steel kadai#stainless steel pressure cooker#stainless steel cookware#triply pressure cooker#best kitchenware#triply kadai#steel feeding bottle
0 notes
Photo

Hex Nuts and Bolts
Click here to see more of this product
#hex nuts and bolts#hex nut bolt#hex nut screw#allen bolts and nuts#hex head nut#allen nut bolt#hex bolt nut#hexagonal bolt and nut#m10 stainless steel nuts#m6 stainless steel nuts#black oxide hex bolts and nuts#hex head bolts and nuts#stainless steel hex bolts and nuts#m16 stainless steel nuts#hex screw nut#hexagonal nut bolt#black hex bolts and nuts#hex bolt & nut#hex bolt nut washer#hexagon bolt nut#buy hex nuts
0 notes
Text
"Ever since I was a little boy, I've been fascinated by teeth. So when the American Dental Association's scouts came to my high school and saw me doing dentistry, they couldn't help but make me an offer," rambled my dentist.
"No shit?" I mumbled around a stainless steel poking tool being repeatedly thrust into my gums.
This was uncomfortable for more than two reasons. An exact number is not available at this time. I wanted to tell him a story about going to art school. About how a chance encounter with a sculptor when I was an impressionable teenager taught me that a beautiful spark of the divine existed inside me. Also that I could make things out of clay that looked kind of like a tiger if you squinted. None of this was possible while I was stuck in the dentist's chair.
Now, you might be surprised that I would waste my time and money on a dentist. Surely, I can do my own work for cheaper, right? Not so. For specialty work, especially specialty work that you carry around in your mouth, it always makes sense to consult a professional. Their expertise is worth every penny.
"Now, let's get you hooked up to the nitrous oxide for the next part of your dental work," he said, and turned his back.
Working quickly, I snaked the vacuum tube up the collar of my shirt and into the mask. By the end of today, I'd have saved about six dollars in nitrous-bottle-refill charges at the speed shop, and all it would cost me is approximately $900 on some other guy's credit card. Plus, I'd have a nice-looking smile as I get the hole shot, at long last, on that base-model Toyota Sienna on my commute.
125 notes
·
View notes
Text
Do you know? One of my personal Ninjago headcanons is that Kai and Jay are experts in almost everything metalworking.
Just think about it, Kai is a blacksmith so it comes with the job to know about metals, their alloys, the care, what he is for each and type of metal he needs to create 'such a thing.' An example of this is that you cannot use aluminum instead of stainless steel or surgical steel to make a sword, but you can use aluminum to make pots and cover it with an anti-oxidant. While Jay is an inventor/mechanic, so he would know which metals are better conductors, heat absorbent or lightweight in case of making a shell for a machine.
I also imagine them both talking about it and asking each other for advice, or things like they both decided that every time they have to buy something it is Jay who goes since he is precise asking about the metals they need such as inches, thickness and the type, while Kai makes sure that they are of good quality and don't have too many impurities and things like that.
I don't know, I like that they both talk about each other in some way other than just joking and this is something they have in common, even though it's more than just work.
110 notes
·
View notes
Text


woagh! rare wip wednesday. i finally found some words and im making... progress? on the other half of mithridatism, aka monster trio poison immunity angst pt. 2, aka zoro's perspective (the parts sanji isnt there for), aka [[a really good title i prommy]].
anyway, thank u @asexualzoro for the funniest thing to happen to me all week, although it is only (as previously mentioned) wednesday. theres still time for comedy. i’m keeping my options open.
text under the cut! as always, keep in mind this is really just a draft…
Then, without another word, he lifts his left hand—fingers splayed—and Zoro feels the fucked up, unnatural buzz of Law’s power blanketing the room like a thousand tiny pinpricks to his senses. He opens his mouth, already halfway to cursing when Law snaps D and A—and suddenly there, in the center of the cold stainless steel operating table, is a jar.
It’s an unassuming thing—thick purple-red visible through clear surgical-grade glass etched and labeled with a clinical sterility, such a contrast from the repurposed, hand-sealed rows in the Cook’s pantry that Zoro laughs—a half-formed chuckle of disbelief huffed out into the beat of stillness that falls between them. The viscous liquid sits in heavy contrast to the bright, terrible gleam of the room itself; Zoro can’t take his eye off it. Can’t stop staring, like his left lid has been peeled open and taped back, his neck trapped in a vice, his feet nailed to the floor.
“You’re insane,” Zoro sneers, and in his peripheral vision, he sees Law shrug.
“This is the New World. I’m not stupid enough to waste valuable resources,” Law replies, unaware or simply uncaring. “The opportunity to study something so potent only rarely—if ever—surfaces. The opportunity to study something resistant to it—well.” Law shrugs again, and Zoro hears the metal edge of the surgical table creak under his own grip. Something in the room snarls, but Law’s expression doesn’t change. “Really, Zoro-ya, you’re being dramatic.”
“You kept his blood,” Zoro spits, and it’s not a question. There’s a sick kind of shine through the glass, an illness to the color that’s not just oxidation but something worse, maybe—because Zoro knows blood. Knows it intimately, deeply, religiously—knows it better than sweat and sake and seawater, and that—
“Oh, I kept more than that,” Law replies. “But two years is a long time, and storage space on a submarine is inherently limited.”
“You’re fucked in the head.”
Law raises an eyebrow, unmoved. “Like I said,” he hums, “pragmatist.”
“We fought for you,” Zoro seethes, “and the whole time Luffy was trying to keep you from killing yourself on Doflamingo’s doorstep, you had this in your cabinets like some kind of fucked-up vampire.”
“Do you think he would care?” Law asks, and Zoro grits his teeth, silenced, because no, actually. He knows full-well Luffy wouldn’t give a shit if he were even aware of the theft—both because he trusts Law (probably picked him, Zoro knows, the moment the Polar Tang surfaced next to Marineford’s battlefield) and because Luffy would genuinely, honestly, wholeheartedly believe in punching his way through whatever risk a rival Captain’s unrestrained study of his physiology might bring. And Zoro doesn’t doubt he could.
(Law seems to feel the same—he still hasn’t denied Luffy’s own ability to kill him with a little time and effort, after all.)
#we're really getting into the zolu with this one 🤌 no (implied) tag here babbeeyyy#which may not be a good thing--considering the subject matter.#whats that spotify? play mitskis 'im your man' one more time? why yes. dont mind if i do#lolll sorry lew this was just way too fucking funny#gyro.odt
46 notes
·
View notes
Text
WD-40
I had a neighbor who bought a new pickup.
I got up very early one Sunday morning and saw that someone had spray painted red all around the sides of this beige truck (for some unknown reason).
I went over, woke him up, and told him the bad news.
He was very upset and was trying to figure out what to do... probably nothing until Monday morning, since nothing was open.
Another neighbor came out and told him to get his WD-40 and clean it off.
It removed the unwanted paint beautifully and did not harm his paint job that was on the truck.
I was impressed!WD-40 who knew?
"Water Displacement #40".
The product began from a search for a rust preventative solvent and degreaser to protect missile parts.
WD-40 was created in 1953, by three technicians at the San Diego Rocket Chemical Company.
Its name comes from the project that was to find a 'Water Displacement' Compound.
They were finally successful for a formulation, with their fortieth attempt, thus WD-40.
The 'Convair Company' bought it in bulk to protect their atlas missile parts. Ken East (one of the original founders) says there is nothing in WD-40 that would hurt you.
When you read the 'shower door' part, try it. It's the first thing that has ever cleaned that spotty shower door. If yours is plastic, it works just as well as on glass.
Then try it on your stove-top. It's now shinier than it's ever been. You'll be amazed.
WD-40 Uses:
Protects silver from tarnishing.
Removes road tar and grime from cars.
Cleans and lubricates guitar strings.
Gives floor that 'just-waxed' sheen without making them slippery.
Keeps the flies off of Cows, Horses, and other Farm Critters, as well.
Restores and cleans chalkboards.
Removes lipstick stains.
Loosens stubborn zippers.
Untangles jewelry chains.
Removes stains from stainless steel sinks.
Removes dirt and grime from the barbecue grill.
Keeps ceramic/terracotta garden pots from oxidizing.
Removes tomato stains from clothing.
Keeps glass shower doors free of water spots.
Camouflages scratches in ceramic and marble floors.
Keeps scissors working smoothly.
Lubricates noisy door hinges on both home and vehicles doors.
Removes that nasty tar and scuff marks from the kitchen flooring. It doesn't seem to harm the finish and you won't have to scrub nearly as hard to get them off. Just remember to open some windows if you have a lot of marks.
Removes those nasty bug guts that will eat away the finish on your car if not removed quickly!
Gives a children's playground gym slide a shine for a super fast slide.
Lubricates gearshift and mower deck lever for ease of handling on riding mowers.
Rids kids rocking chair and swings of squeaky noises.
Lubricates tracks in sticking home windows and makes them easier to open.
Spraying an umbrella stem makes it easier to open and close.
Restores and cleans padded leather dashboards in vehicles, as well as vinyl bumpers.
Restores and cleans roof racks on vehicles.
Lubricates and stops squeaks in electric fans.
Lubricates wheel sprockets on tricycles, wagons, and bicycles for easy handling.
Lubricates drive belts on washers and dryers and keeps them running smoothly.
Keeps rust from forming on saws and saw blades, and other tools.
Removes grease splatters from stove-tops.
Keeps bathroom mirror from fogging.
Lubricates prosthetic limbs.
Keeps pigeons off the balcony (they hate the smell).
Removes all traces of duct tape.
Folks even spray it on their arms, hands, and knees to relieve arthritis pain.
Florida's favorite use is: 'cleans and removes love bugs from grills and bumpers.'
The favorite use in the state of New York, it protects the Statue of Liberty from the elements.
Attracts fish. Spray a little on live bait or lures and you will be catching the big one in no time.
Use it for fire ant bites. It takes the sting away immediately and stops the itch.
It is great for removing crayon from walls. Spray it on the marks and wipe with a clean rag.
Also, if you've discovered that your teenage daughter has washed and dried a tube of lipstick with a load of laundry, saturate the lipstick spots with WD-40 and rewash. Presto! The lipstick is gone!
If you spray it inside a wet distributor cap, it will displace the moisture, allowing the engine to start.
And FYI - the main Ingredient is - FISH OIL.
1K notes
·
View notes
Text

Armor for steel: New method could enable advances in energy, electronics and aerospace
Researchers have demonstrated that stainless steel and other metal alloys coated with hexagonal boron nitride, or hBN, exhibit non-stick or low-friction qualities along with improved long-term protection against harsh corrosion and high-temperature oxidation in air. The work has been published in Advanced Materials Interfaces.
Metal alloys—mixtures of two or more metals—are created to be strong, durable and resistant to corrosion or oxidation. By adding coatings, or "armor," to make those materials even tougher, scientists could enhance existing products and enable the creation of new, innovative ones.
For example, armoring may boost the ability of solar panels to conduct heat and resist environmental factors. Additionally, it allows semiconductors to maintain proper operating temperature, and aerospace turbine blades to guard against wear, reduce friction and withstand hot conditions.
Read more.
27 notes
·
View notes
Text
Principles of the RBMK Reactor

The RBMK-1000 Boiling Water Reactor is a Soviet-designed nuclear reactor capable of generating 1,000 megawatts of electricity. The core of the reactor is a short, wide cylinder. The active zone is contained inside a large metal drum, known as the core shroud. The reactor assembly is supported by a large metal disk known as the Lower Biological Shield. This sits on top of a larger metal cross labeled “Structure S”. On top of all this rests the 2,000 ton Upper Biological Shield of the reactor, known as "Structure E". The reactor sits in a large reinforced concrete shell which provides structural support and shields plant personnel from radiation.
The core region of the reactor is a large pile of graphite 14.52m × 9.7m. This pile is composed of graphite blocks 25cm by 25cm, with a height of between 20 and 60cm depending on its location in the reactor. Drilled through these blocks is a 11.4cm diameter hole, through which a zirconium alloy tube (known as a ‘technological channel’) is inserted. These contain either a fuel assembly, a control rod, or reactor monitoring equipment. These channels can be opened in situ or removed completely to replace any fuel or equipment inside them. Zirconium is used due to its high melting point and because it allows the neurons that produce the fission reaction in the core to pass through it far easier than other alloys such as stainless steel.
These metal technological channels have water pumped into them from the bottom by the Main Circulation Pumps. The entire reactor vessel is pressurized with a helium-nitrogen mixture, to prevent the oxidization of the graphite. Graphite is flammable in oxygen, but removed from it it can become quite an efficient thermodynamic conductor.
Below: A photo of RBMK technological channels at Chernobyl Unit 2. The length of these gives a good idea as to how massive the core of the RBMK is.
This picture is a screencap from this video.

The fuel of an RBMK is small uranium oxide pellets, stacked into small metal pipes and bundled together into fuel assemblies. Uranium oxide is a ceramic material composed of Uranium 235. This element, under special conditions, can create a nuclear chain reaction which generates heat. The RBMK has three primary components that help create these special conditions to create the controlled fission reactions in the core. These are graphite, water, and boron.
Graphite is used in the core of an RBMK as a moderator. Basically, it slows down the neutrons discarded by U-236 atoms (a U 235 atom which a neutron has collided with) when they split apart. When they are released they are travelling at a tremendous speed, and have very little chance of coming into contact with another atom of uranium. Slowing them down, however, creates a higher chance of the neutrons coming into contact with an atom of U-235, creating the unstable U-236 and then pulling itself apart, thereby creating more neutrons (as well as several other elements) and sustaining a nuclear chain reaction. This sustained reaction is what creates the heat in the core of a nuclear reactor. The more neutrons there are in the core, the more reactivity (and therefore heat) is created. It should be noted that graphite is combustible at high temperatures. The core contained 1,700 tons of graphite.
Water in the core of an RBMK serves as a coolant. Because the core of a nuclear reactor gets extremely hot, it becomes necessary to cool its components if you wish to avoid destructive melting within the core region. Water is the most common coolant in nuclear reactors, as it is cheap and abundant. The water is pumped in under high pressure at about 265 C by the Main Circulation Pumps from the bottom of the reactor up into the technological channels containing the fuel and other components of the reactor. After passing through the channels and heating up to about 284 C, the water is piped out of the top of the reactor. Some of the coolant water heats up so much that it forms into steam bubbles inside the reactor. When the water is pumped out of the core it is then sent into four steam separator drums, where the steam is separated from the water. The water is then pumped back into the reactor, while the steam is sent to the turbine generators of the plant to create electricity. After this, the steam is condensed back into water using cool water from the plant cooling pond and recirculated into the cooling system.
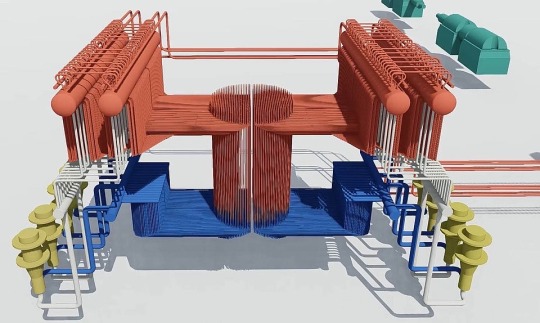
Below: A model showing the circulation system of an RBMK-1000 reactor. Coolant water is in blue and hot water/steam is in red. The yellow structures are the main cooling pumps, and the green structures are steam turbines. This model is spatially to scale, essentially what you would see if you removed every part of the reactor except for the coolant circuit.
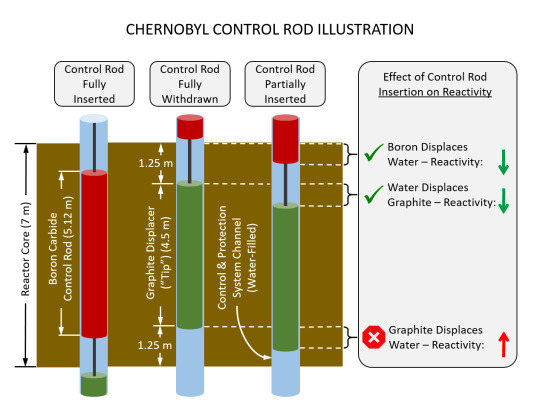
Some of the channels in the RBMK contain control rods (large boron carbide rods) that move up and down in the channel as necessary to keep the reactor within operational limits. Boron is a neutron sponge. It absorbs neutrons and can effectively eliminate a chain reaction. It functions as the brakes on a human made nuclear reaction, useful both in making sure a chain reaction does not become a runaway criticality and also in being the off switch on a nuclear reactor. The RBMK has 211 of these control rods, some of which are under operator control and some of which are under the control of a computer. A design quirk of the RBMK is that at the end of each standard control rod was a 14ft 9in graphite displacer. When a control rod was withdrawn out of the core it left behind a space that would be filled with water, a neutron absorber. Since more water in the core would kill reactivity, the designers of the reactor hung this displacer from the control rods to replace the space left by the control rod with something that would increase reactivity rather than kill it. This was a sound design choice, but it was a major factor in the events of the accident at Chernobyl.
Below: An illustration of the control rod displacers in an RBMK.
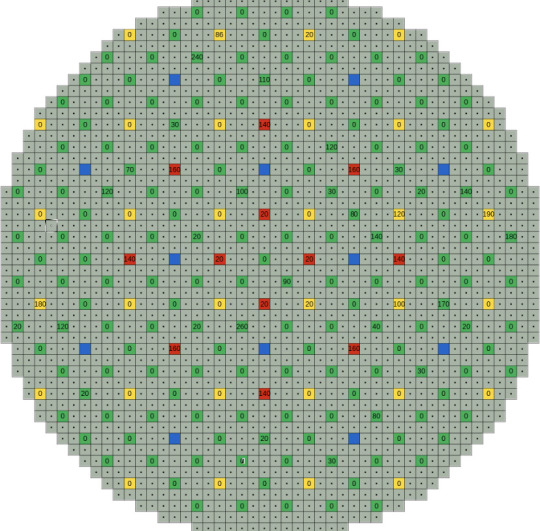
Below: A top down view of the channels of an RBMK. You can see the layout of the control rods (green), neutron detectors (blue), shortened control rods inserted from below the reactor (yellow), automatic control rods (red), and the fuel channels (grey). The number on the green, yellow, and red squares are the last recorded insertion depths of control rods in Chernobyl Unit 4 1m 30s before the explosion. Only one is fully inserted.
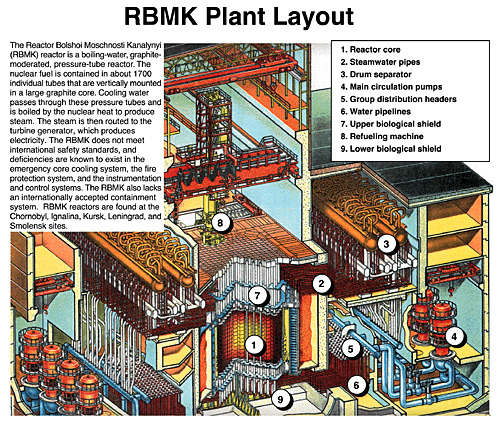
Below: A cutaway of the RBMK system layout.
Two additional factors also come into play regarding the water. Water is naturally a neutron absorber, albeit a far less effective one than boron. The more water in the core, the less neutrons are present and therefore the lower the reactivity. However, when transformed into steam, water loses nearly all of its neutron absorbing properties. The more steam in the core, the higher reactivity is. This is called a ‘positive void coefficient’, and it was a known quirk of the RBMK and indeed several other reactor designs. However, the RBMK had a much higher level of this effect in its core due to its design. This is important to the accident sequence.
It is also important to note that the RBMK is an enormous construction. It is temperamental, unstable unless operating at full power, and requires constant monitoring and guidance from its operators. It requires three operators just to run it normally, and it was notoriously difficult to operate. The core region is so large that the equipment used to monitor it could not accurately read a large portion of it, and hotspots of reactivity would often form resulting in alarming and unexplained jumps in power output and temperature. While in theory not a bad design, the RBMK was a deeply flawed machine.
An enormous thank you is owed to @nicotinebeige , who was extremely helpful in the creation of this post. If you like film photography, you should check out their blog!
This is a technical explanation of the RBMK design. For a history of the RBMK, check out this post. Apologies for any mistakes! I’m most definitely not an expert on nuclear physics, and if anything is unclear you should absolutely check out other sources for more info. As always, thank you for your interest!
#chernobyl#nuclear reactor#history#radiation#accidents and disasters#nuclear power#autism#nuclear#reactor#chnpp#rbmk#rbmk 1000
121 notes
·
View notes
Note
You can't wash a cybertruck in sunlight because the metal isn't coated (that's a bonus feature not a standard thing) so it'll rust. Adamsomething has a video from a few weeks ago going through the design if you're curious! (It gets worse than this)
Huh. I had no idea sunlight accelerated oxidation of stainless steel.
12 notes
·
View notes