#porphyria Treatment
Text
The porphyria therapies market is projected to grow at an annualized rate of 10.97% during the period 2021-2030
Several novel and innovative therapeutic approaches, both small molecule and biologics, are being currently investigated at various phases of development in order to avoid the beginning of disease-induced attacks and other long-term effects of porphyria
Roots Analysis has announced the addition of “Porphyria Targeting Therapies Market” report to its list of offerings.
Porphyria is a rare disorder that is characterized by excessive accumulation of porphyrin, a compound that aids in the formation of heme (an essential part of hemoglobin that helps carry oxygen in blood). Any anomaly caused by genetic or acquired abnormalities in heme biosynthesis (produced majorly in bone marrow and liver) can result in toxicity. It is worth highlighting that, till date, more than 1,000 mutations that can cause porphyria have been identified. However, prevalence of porphyria still remains unknown. Several treatment options such as gene therapy, proteasome inhibition and pharmacologic chaperones are currently being investigated among various other targeted treatment options.
To order this 130+ slides report, which features 90+ figures, please visit https://www.rootsanalysis.com/reports/porphyria-pipline-review.htmlhttps://www.rootsanalysis.com/reports/dna-damage-response-targeting-therapeutics-market.html.
Key Market Insights
15+ therapies have been / are being developed for the different type of porphyria
Treatment.
More than 70% of the aforementioned candidates are currently under clinical evaluation. Further, three therapies, namely Panhematin™, GIVLAARI® and SCENESSE®, have already been approved for the treatment of different types of porphyria.
Around 50% of the therapeutics are being developed as biologics
Majority (over 65%) of the abovementioned biologic drugs have been / are being designed for administration via the intravenous route. Furthermore, majority of the drugs (37%) have been / are being targeting acute intermittent porphyria.
Over 30% of the therapies have been / are being developed for erythropoietic protoporphyria
More than 65% the abovementioned therapies are currently being evaluated in clinical phases. Further, around 60% of the aforementioned therapy candidates are being developed as small molecules.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/reports/porphyria-pipline-review/request-sample.html
Key Questions Answered
§ What are the prevalent R&D trends related to Porphyria?
§ What are the key challenges faced by stakeholders engaged in this domain?
§ What are the principal therapies developed by the companies in this domain?
§ Who are the leading industry and non-industry players in this market?
§ What are the key geographies where research on porphyria is actively being conducted?
§ Who are the key investors in this domain?
§ Who are the key opinion leaders / experts in this field?
§ What kind of partnership models are commonly adopted by industry stakeholders?
§ What are the factors that are likely to influence the evolution of this upcoming market?
§ How is the current and future market opportunity likely to be distributed across key market segments?
For additional details, please visit https://www.rootsanalysis.com/reports/porphyria-pipline-review.html mailto:or email [email protected]
You may also be interested in the following titles:
1. Progressive Supranuclear Palsy: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2030
2. Soft Tissue Sarcoma: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
4 notes
·
View notes
Text
Porphyria Treatment Market Size Growth Rate by Application 2021 Analysis, Share, Manufacturers, Growth Factor and Forecast to 2026
A series of diseases known as porphyria occur when the body builds up the chemical porphyrin, which can impact the neurological system, the skin, and even cause stomach pain. The activity of haemoglobin depends on the presence of porphyrin. Porphyria Treatment Market and cutaneous porphyrias are the two main kinds of porphyria. Seizures, respiratory issues, elevated blood pressure, constipation, and severe stomach discomfort are all signs of acute porphyrias.

One important aspect that is likely to boost the industry is the emergence of novel medicines. Measurements of chemicals including porphyrin precursors and porphyrins in blood plasma, red blood cells, faeces, and urine are the most popular tests used to diagnose porphyria. It is helpful for confirmation and family studies to measure DNA mutations and cellular enzymes. Although there is no known cure for porphyria, each variety of the disease can be treated.
Read more @ https://creativeedge16.blogspot.com/2022/08/porphyria-treatment-market-modern.html
#Porphyria Treatment Market#Porphyria Treatment#Porphyria Treatment Market Demand#Pharmaceutical#Coherent Market Insights
0 notes
Text
Acute Hepatic Porphyria Treatment Market Growth Strategies, Quality Assessment, and Trends by 2024-2031

The "Acute Hepatic Porphyria Treatment Market" is a dynamic and rapidly evolving sector, with significant advancements and growth anticipated by 2031. Comprehensive market research reveals a detailed analysis of market size, share, and trends, providing valuable insights into its expansion. This report delves into segmentation and definition, offering a clear understanding of market components and drivers. Employing SWOT and PESTEL analyses, the study evaluates the market's strengths, weaknesses, opportunities, and threats, alongside political, economic, social, technological, environmental, and legal factors. Expert opinions and recent developments highlight the geographical distribution and forecast the market's trajectory, ensuring a robust foundation for strategic planning and investment.
What is the projected market size & growth rate of the Acute Hepatic Porphyria Treatment Market?
Market Analysis and Size
As per the NCBI, the global occurrence of acute hepatic porphyria is anticipated to vary from one in 500 to one in 50,000 individuals. Acute hepatic porphyria (AHP) is one of the types of hepatic porphyria. The incidence of clinical acute hepatic porphyria is stated to be around 5 to 10 per 100,000 individuals. It is witnessed that the persistent increase in research grants and R&D expenditures from government and other pharma companies to boost the development of rare disease drugs that would change the scenario of porphyrias treatment.
Data Bridge Market Research analyses a growth rate in acute hepatic porphyria treatment market in the forecast period 2023-2030. The expected CAGR of acute hepatic porphyria treatment market is tend to be around 5% in the mentioned forecast period. The market was valued at USD 3.72 million in 2022, and it would grow upto USD 5.5 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Browse Detailed TOC, Tables and Figures with Charts which is spread across 350 Pages that provides exclusive data, information, vital statistics, trends, and competitive landscape details in this niche sector.
This research report is the result of an extensive primary and secondary research effort into the Acute Hepatic Porphyria Treatment market. It provides a thorough overview of the market's current and future objectives, along with a competitive analysis of the industry, broken down by application, type and regional trends. It also provides a dashboard overview of the past and present performance of leading companies. A variety of methodologies and analyses are used in the research to ensure accurate and comprehensive information about the Acute Hepatic Porphyria Treatment Market.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-acute-hepatic-porphyria-treatment-market
Which are the driving factors of the Acute Hepatic Porphyria Treatment market?
The driving factors of the Acute Hepatic Porphyria Treatment market include technological advancements that enhance product efficiency and user experience, increasing consumer demand driven by changing lifestyle preferences, and favorable government regulations and policies that support market growth. Additionally, rising investment in research and development and the expanding application scope of Acute Hepatic Porphyria Treatment across various industries further propel market expansion.
Acute Hepatic Porphyria Treatment Market - Competitive and Segmentation Analysis:
Global Acute Hepatic Porphyria Treatment Market, By Types (Acute Intermittent Porphyria (AIP), Hereditary Coproporphyria (HCP) and Others), Treatment (Medications, Caloric Deprivation, Dehydration and Others), Route of Administration (Oral, Parenteral and Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) – Industry Trends and Forecast to 2031.
How do you determine the list of the key players included in the report?
With the aim of clearly revealing the competitive situation of the industry, we concretely analyze not only the leading enterprises that have a voice on a global scale, but also the regional small and medium-sized companies that play key roles and have plenty of potential growth.
Which are the top companies operating in the Acute Hepatic Porphyria Treatment market?
Key players operating in the acute hepatic porphyria treatment market include:
Teva Pharmaceutical Industries Ltd (Israel)
Apotex Inc (Canada)
Pfizer Inc (U.S.)
H. Lundbeck A/S (Denmark)
Lilly (U.S.)
F. Hoffmann-La Roche Ltd. (Switzerland)
Mylan N.V. (U.S.)
Siemens Healthcare GmbH (Germany)
ACON Laboratories, Inc. (U.S.)
Danaher. (U.S.)
ARKRAY, Inc (Japan)
Abbott (U.S.)
Alnylam Pharmaceuticals, Inc (U.S.)
Boston Scientific Corporation (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
Recordati Rare Diseases Inc (Italy)
Short Description About Acute Hepatic Porphyria Treatment Market:
The Global Acute Hepatic Porphyria Treatment market is anticipated to rise at a considerable rate during the forecast period, between 2024 and 2031. In 2023, the market is growing at a steady rate and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
North America, especially The United States, will still play an important role which can not be ignored. Any changes from United States might affect the development trend of Acute Hepatic Porphyria Treatment. The market in North America is expected to grow considerably during the forecast period. The high adoption of advanced technology and the presence of large players in this region are likely to create ample growth opportunities for the market.
Europe also play important roles in global market, with a magnificent growth in CAGR During the Forecast period 2024-2031.
Acute Hepatic Porphyria Treatment Market size is projected to reach Multimillion USD by 2031, In comparison to 2024, at unexpected CAGR during 2024-2031.
Despite the presence of intense competition, due to the global recovery trend is clear, investors are still optimistic about this area, and it will still be more new investments entering the field in the future.
This report focuses on the Acute Hepatic Porphyria Treatment in global market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This report categorizes the market based on manufacturers, regions, type and application.
Get a Sample Copy of the Acute Hepatic Porphyria Treatment Report 2024
What are your main data sources?
Both Primary and Secondary data sources are being used while compiling the report. Primary sources include extensive interviews of key opinion leaders and industry experts (such as experienced front-line staff, directors, CEOs, and marketing executives), downstream distributors, as well as end-users. Secondary sources include the research of the annual and financial reports of the top companies, public files, new journals, etc. We also cooperate with some third-party databases.
Geographically, the detailed analysis of consumption, revenue, market share and growth rate, historical data and forecast (2024-2031) of the following regions are covered in Chapters
What are the key regions in the global Acute Hepatic Porphyria Treatment market?
North America (United States, Canada and Mexico)
Europe (Germany, UK, France, Italy, Russia and Turkey etc.)
Asia-Pacific (China, Japan, Korea, India, Australia, Indonesia, Thailand, Philippines, Malaysia and Vietnam)
South America (Brazil, Argentina, Columbia etc.)
Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa)
This Acute Hepatic Porphyria Treatment Market Research/Analysis Report Contains Answers to your following Questions
What are the global trends in the Acute Hepatic Porphyria Treatment market?
Would the market witness an increase or decline in the demand in the coming years?
What is the estimated demand for different types of products in Acute Hepatic Porphyria Treatment?
What are the upcoming industry applications and trends for Acute Hepatic Porphyria Treatment market?
What Are Projections of Global Acute Hepatic Porphyria Treatment Industry Considering Capacity, Production and Production Value? What Will Be the Estimation of Cost and Profit? What Will Be Market Share, Supply and Consumption? What about Import and Export?
Where will the strategic developments take the industry in the mid to long-term?
What are the factors contributing to the final price of Acute Hepatic Porphyria Treatment?
What are the raw materials used for Acute Hepatic Porphyria Treatment manufacturing?
How big is the opportunity for the Acute Hepatic Porphyria Treatment market?
How will the increasing adoption of Acute Hepatic Porphyria Treatment for mining impact the growth rate of the overall market?
How much is the global Acute Hepatic Porphyria Treatment market worth? What was the value of the market In 2020?
Who are the major players operating in the Acute Hepatic Porphyria Treatment market? Which companies are the front runners?
Which are the recent industry trends that can be implemented to generate additional revenue streams?
What Should Be Entry Strategies, Countermeasures to Economic Impact, and Marketing Channels for Acute Hepatic Porphyria Treatment Industry?
Customization of the Report
Can I modify the scope of the report and customize it to suit my requirements? Yes. Customized requirements of multi-dimensional, deep-level and high-quality can help our customers precisely grasp market opportunities, effortlessly confront market challenges, properly formulate market strategies and act promptly, thus to win them sufficient time and space for market competition.
Inquire more and share questions if any before the purchase on this report at - https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-acute-hepatic-porphyria-treatment-market
Detailed TOC of Global Acute Hepatic Porphyria Treatment Market Insights and Forecast to 2031
Introduction
Market Segmentation
Executive Summary
Premium Insights
Market Overview
Acute Hepatic Porphyria Treatment Market By Type
Acute Hepatic Porphyria Treatment Market By Function
Acute Hepatic Porphyria Treatment Market By Material
Acute Hepatic Porphyria Treatment Market By End User
Acute Hepatic Porphyria Treatment Market By Region
Acute Hepatic Porphyria Treatment Market: Company Landscape
SWOT Analysis
Company Profiles
Continued...
Purchase this report – https://www.databridgemarketresearch.com/checkout/buy/singleuser/global-acute-hepatic-porphyria-treatment-market
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975
Email:- [email protected]
Browse More Reports:
Global Molybdenum Market – Industry Trends and Forecast to 2029
Global Endoscope Reprocessing Market – Industry Trends and Forecast to 2028
Global Bone Marrow-Derived Stem Cells (BMSCS) Market – Industry Trends and Forecast to 2029
Global Oxygen Therapy Market – Industry Trends and Forecast to 2029
Global Acute Hepatic Porphyria Treatment Market – Industry Trends and Forecast to 2030
#Acute Hepatic Porphyria Treatment Market#Acute Hepatic Porphyria Treatment Market Size#Acute Hepatic Porphyria Treatment Market Share#Acute Hepatic Porphyria Treatment Market Trends#Acute Hepatic Porphyria Treatment Market Growth#Acute Hepatic Porphyria Treatment Market Analysis#Acute Hepatic Porphyria Treatment Market Scope & Opportunity#Acute Hepatic Porphyria Treatment Market Challenges#Acute Hepatic Porphyria Treatment Market Dynamics & Opportunities
0 notes
Text
The Role of Human Hemin (Normosang) in Managing Acute Hepatic Porphyria
Acute hepatic porphyria refers to a group of rare metabolic disorders characterized by deficiencies in enzymes involved in the biosynthesis of heme, a component of hemoglobin. These deficiencies lead to the accumulation of porphyrins and their precursors, causing symptoms such as severe abdominal pain, nausea, vomiting, and neurological manifestations.
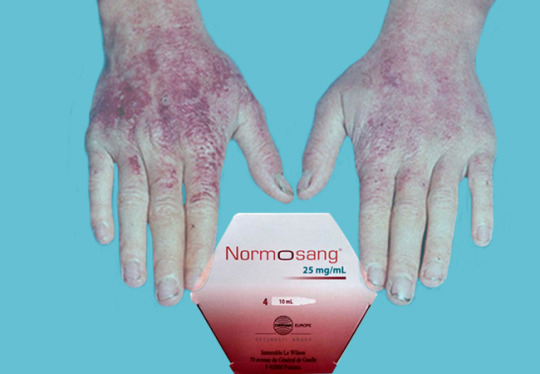
Understanding Acute Hepatic Porphyria:
AHP encompasses four main types: acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP), and ALA dehydratase deficiency porphyria (ADP). These disorders manifest primarily through neurovisceral symptoms such as severe abdominal pain, nausea, vomiting, and neurological manifestations like seizures and neuropathy. Cutaneous symptoms may also occur, ranging from photosensitivity to blistering skin lesions.
The cornerstone of managing AHP involves minimizing triggers such as certain medicines, hormonal changes, and dietary factors, coupled with symptomatic relief during acute attacks. Historically, therapies like intravenous glucose, hemin, and pain management have been employed, but they often fall short in providing long-term solutions and may have limitations in certain patient populations.
Human Hemin: A Game-Changer in AHP Treatment:
Human hemin (supplied under the brand Normosang), a novel therapeutic option, has emerged as a promising breakthrough in AHP management. It is a formulation of heme arginate, a stable heme molecule that directly addresses the enzymatic defects underlying AHP. By providing exogenous heme, Human hemin replenishes the deficient enzyme, thereby restoring heme biosynthesis and mitigating the accumulation of toxic porphyrin intermediates.
The efficacy of Human hemin has been demonstrated in clinical trials, with notable reductions in the frequency and severity of acute attacks among AHP patients. Furthermore, its convenient subcutaneous administration and favourable safety profile make it an attractive option for both healthcare providers and patients.
In addition to Human Hemin, other treatments for acute porphyria attacks may include medications to manage symptoms such as pain and nausea, as well as measures to prevent future attacks, such as avoiding triggers like certain medications, alcohol, and fasting.
#Human Hemin (Normosang)#(Normosang) price in India#Human Hemin in India#Acute Hepatic Porphyria#Acute Hepatic Porphyria treatment
0 notes
Text

#Porphyria Targeting Therapies Market#porphyria treatment market#porphyria treatment market size#Porphyrias Treatment
0 notes
Note
My question is why are you such an asshole, Lich? Who hurt you?
You're not the first to ask that question. There really isn't one specific answer. Lich's asshole behavior can be blamed on Irken society in general. Lich was born taller than average and was given special treatment/ privileges the average smeet was not growing up.
His aggressive, prejudiced, perfectionist attitude and behavior was encouraged by his coddle drones/ superiors throughout his entire life.
Lich also suffered from a chronic illness, similar to porphyria in humans. He experienced debilitating stomach pain, food allergies, extreme light sensitivity among several other forms of sensory sensitivity, and even cravings for consuming Irken blood. These medical issues he kept private as much as possible.
His issues with his predecessor, tallest Pepperoncini really began because of this incident.
7 notes
·
View notes
Text
"I am your king!"
It looks like Shonda is in the bipolar disorder camp when it comes to George's illness and she's starting it already. Interesting. I think most modern historians agree with her. I'll be honest, I was hoping she'd be in the arsenic-induced porphyria camp.
Here's hoping we won't see the horrific "treatment" the real George III was subjected to by his "doctor" late in life.
18 notes
·
View notes
Text
Full Details About Methoxsalen
Methoxsalen is a naturally occurring furocoumarin compound found in several plants, such as Ammi majus (Umbelliferae), and in smaller amounts in other plants like celery, parsley, and figs. It is commonly used in combination with ultraviolet A (UVA) light therapy to treat various skin conditions.
Methoxsalen USP
Here are the full details about methoxsalen:
Medical Uses: Methoxsalen is primarily used for its photoactive properties in a treatment known as PUVA therapy (psoralen plus ultraviolet A). It is used to treat certain skin disorders, including:
Psoriasis: A chronic autoimmune skin condition characterized by red, scaly patches of skin.
Vitiligo: A disorder where the skin loses its natural color in patches.
Cutaneous T-cell lymphoma: A type of non-Hodgkin's lymphoma that affects the skin.
Mechanism of Action: Methoxsalen works by becoming activated when exposed to UVA light. Upon absorption of UVA light, it forms covalent crosslinks with DNA, which interferes with DNA replication and inhibits cell division in the skin. This process is thought to help control the abnormal skin cell growth seen in conditions like psoriasis and vitiligo.
Forms and Administration: Methoxsalen is available in various forms for administration, including:
Oral Tablets: Methoxsalen tablets are taken orally, usually one to two hours before UVA exposure. The dosage and treatment schedule depend on the individual's condition and response to therapy.
Topical Formulations: Some formulations of methoxsalen can be applied topically to the affected areas of the skin before UVA exposure.
PUVA Therapy: PUVA therapy involves the administration of methoxsalen followed by exposure to UVA light. The procedure is usually performed in a medical setting under the supervision of healthcare professionals. The UVA light source can be a specialized device or a light booth.
Side Effects: While PUVA therapy can be effective in treating skin conditions, it can also cause side effects, including:
Nausea and Dizziness: Some people may experience nausea and dizziness after taking methoxsalen orally.
Photosensitivity: Methoxsalen makes the skin sensitive to light, so patients need to avoid sunlight and use protective measures for several hours after treatment.
Skin Irritation: Topical methoxsalen may cause skin irritation in some individuals.
Eye Irritation: Protective eye shields should be worn during UVA exposure to prevent eye irritation.
Contraindications: Methoxsalen is contraindicated in certain conditions, including:
Hypersensitivity: Individuals with a known hypersensitivity to methoxsalen or related compounds should avoid its use.
Porphyria: A group of rare disorders that affect the production of heme, a component of red blood cells.
Pregnancy and Breastfeeding: Methoxsalen is generally avoided during pregnancy and breastfeeding due to potential risks to the fetus or infant.
2 notes
·
View notes
Text
Acute porphyria why don’t you have a treatment where I exist fuck you bitch.
Stop looking like I have a pain medication addiction I have a doctor saying what I have right here excuse me for not having a “pain face”. Fuck you too ableist doctor a psychiatrist doesn’t treat this shit.
#cursing cw#I am very angry#sorry the language is not typical on my blog#excuse me#now I will leave#it’s been three weeks#chronic illness#chronic pain#disability#ableism
5 notes
·
View notes
Text
0 notes
Text
LEEAYZH. pronunciation of the word liege.
hey, what's goodie? name is kelly. she/her. taurus. i was born in 1981; you can do the math yourself. also happen to be a roleplay veteran, been at this since 1997. this is a regency era original character. 85-90% of the time i'm mobile; i've also got a full time job and outside responsibilities. i'm going to be keeping this real simple. don't be a dick and we'll get along just fine.
work schedule; sundays [ 2:00 pm through 7:00 pm EST ], mondays through thursdays [ 8:00 am through 3:00 pm EST ].
blogroll; @stcllata, @galcttica, @bykokoro, @nottc.
information is under the read more.
— FULL NAME: darius (darijo) graves.
— BIRTHDATE / AGE: december 21, 1791 / 32.
— SPECIES: human.
— OCCUPATION: the current lord wraxall (his father and grandfather preceded him). since 1812.
— FACE CLAIM: taylor zakhar perez.
— DEFAULT VERSE: regency era / bridgerton.
— ALT VERSE(S):
— BIOGRAPHY: little is known about darius prior to his adoption into the graves family. it is simply said that he was discovered by the couple and they were infatuated almost instantly. they wouldn't know what they were in for.
darius was always a quiet child, well mannered and respectful. for a time, things were blissful, until the week of his tenth birthday. he was beginning to show painful leisons more and more often.
unfortunately, he was diagnosed with porphyria, specifically porphyria cutanea tarda. he would be limited on being exposed to sunlight (the cause of the lesions), though alternating symptoms would persist (fevers, seizures, abdominal and chest pain, hallucinations) for days/weeks at a time.
thankfully, his parents were gungho from the beginning. they poured their lives and souls into seeking out the very best treatment for him. even if in his later years, came to regret it. he's always felt they wasted far too much of their livelihoods for his sake.
he entered the ton for three seasons (15 through 17), socially it did him wonders, but there was little along the marriage lines. as he was honest with all those showing favor. he wouldn't dream of shouldering them with his burden.
it would be around this time that his mother would discover herself to be pregnant. it was a miracle, as they had been told long ago that conceiving was not possible. it wouldn't be long before they welcomed a little girl into their lives.
in the beginning of 1812, his father announced that he would be retiring and that darius would be assuming the duties therein. honestly, he was glad for it. the life consumed him almost wholly, leaving time for little else but devotion to it.
now, as he graces another decade; he does find himself disliking the silence. though he won't deflect from his duties for the sake of pleasure.
1 note
·
View note
Text
Taking RNAi from interesting science to impactful new treatments
New Post has been published on https://thedigitalinsider.com/taking-rnai-from-interesting-science-to-impactful-new-treatments/
Taking RNAi from interesting science to impactful new treatments


There are many hurdles to clear before a research discovery becomes a life-changing treatment for patients. That’s especially true when the treatments being developed represent an entirely new class of medicines. But overcoming those obstacles can revolutionize our ability to treat diseases.
Few companies exemplify that process better than Alnylam Pharmaceuticals. Alnylam was founded by a group of MIT-affiliated researchers who believed in the promise of a technology — RNA interference, or RNAi.
The researchers had done foundational work to understand how RNAi, which is a naturally occurring process, works to silence genes through the degradation of messenger RNA. But it was their decision to found Alnylam in 2002 that attracted the funding and expertise necessary to turn their discoveries into a new class of medicines. Since that decision, Alnylam has made remarkable progress taking RNAi from an interesting scientific discovery to an impactful new treatment pathway.
Today Alnylam has five medicines approved by the U.S. Food and Drug Administration (one Alnylam-discovered RNAi therapeutic is licensed to Novartis) and a rapidly expanding clinical pipeline. The company’s approved medicines are for debilitating, sometimes fatal conditions that many patients have grappled with for decades with few other options.
The company estimates its treatments helped more than 5,000 patients in 2023 alone. Behind that number are patient stories that illustrate how Alnylam has changed lives. A mother of three says Alnylam’s treatments helped her take back control of her life after being bed-ridden with attacks associated with the rare genetic disease acute intermittent porphyria (AIP). Another patient reported that one of the company’s treatments helped her attend her daughter’s wedding. A third patient, who had left college due to frequent AIP attacks, was able to return to school.
These days Alnylam is not the only company developing RNAi-based medicines. But it is still a pioneer in the field, and the company’s founders — MIT Institute Professor Phil Sharp, Professor David Bartel, Professor Emeritus Paul Schimmel, and former MIT postdocs Thomas Tuschl and Phillip Zamore — see Alnylam as a champion for the field more broadly.
“Alnylam has published more than 250 scientific papers over 20 years,” says Sharp, who currently serves as chair of Alnylam’s scientific advisory board. “Not only did we do the science, not only did we translate it to benefit patients, but we also described every step. We established this as a modality to treat patients, and I’m very proud of that record.”
Pioneering RNAi development
MIT’s involvement in RNAi dates back to its discovery. Before Andrew Fire PhD ’83 shared a Nobel Prize for the discovery of RNAi in 1998, he worked on understanding how DNA was transcribed into RNA, as a graduate student in Sharp’s lab.
After leaving MIT, Fire and collaborators showed that double-stranded RNA could be used to silence specific genes in worms. But the biochemical mechanisms that allowed double-stranded RNA to work were unknown until MIT professors Sharp, Bartel, and Ruth Lehmann, along with Zamore and Tuschl, published foundational papers explaining the process. The researchers developed a system for studying RNAi and showed how RNAi can be controlled using different genetic sequences. Soon after Tuschl left MIT, he showed that a similar process could also be used to silence specific genes in human cells, opening up a new frontier in studying genes and ultimately treating diseases.
“Tom showed you could synthesize these small RNAs, transfect them into cells, and get a very specific knockdown of the gene that corresponded to that the small RNAs,” Bartel explains. “That discovery transformed biological research. The ability to specifically knockdown a mammalian gene was huge. You could suddenly study the function of any gene you were interested in by knocking it down and seeing what happens. … The research community immediately started using that approach to study the function of their favorite genes in mammalian cells.”
Beyond illuminating gene function, another application came to mind.
“Because almost all diseases are related to genes, could we take these small RNAs and silence genes to treat patients?” Sharp remembers wondering.
To answer the question, the researchers founded Alnylam in 2002. (They recruited Schimmel, a biotech veteran, around the same time.) But there was a lot of work to be done before the technology could be tried in patients. The main challenge was getting RNAi into the cytoplasm of the patients’ cells.
“Through work in Dave Bartel and Phil Sharp’s lab, among others, it became evident that to make RNAi into therapies, there were three problems to solve: delivery, delivery, and delivery,” says Alnylam Chief Scientific Officer Kevin Fitzgerald, who has been with the company since 2005.
Early on, Alnylam collaborated with MIT drug delivery expert and Institute Professor Bob Langer. Eventually, Alnylam developed the first lipid nanoparticles (LNPs) that could be used to encase RNA and deliver it into patient cells. LNPs were later used in the mRNA vaccines for Covid-19.
“Alnylam has invested over 20 years and more than $4 billion in RNAi to develop these new therapeutics,” Sharp says. “That is the means by which innovations can be translated to the benefit of society.”
From scientific breakthrough to patient bedside
Alnylam received its first FDA approval in 2018 for treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis, a rare and fatal disease. It doubled as the first RNAi therapeutic to reach the market and the first drug approved to treat that condition in the United States.
“What I keep in mind is, at the end of the day for certain patients, two months is everything,” Fitzgerald says. “The diseases that we’re trying to treat progress month by month, day by day, and patients can get to a point where nothing is helping them. If you can move their disease by a stage, that’s huge.”
Since that first treatment, Alnylam has updated its RNAi delivery system — including by conjugating small interfering RNAs to molecules that help them gain entry to cells — and earned approvals to treat other rare genetic diseases along with high cholesterol (the treatment licensed to Novartis). All of those treatments primarily work by silencing genes that encode for the production of proteins in the liver, which has proven to be the easiest place to deliver RNAi molecules. But Alnylam’s team is confident they can deliver RNAi to other areas of the body, which would unlock a new world of treatment possibilities. The company has reported promising early results in the central nervous system and says a phase one study last year was the first RNAi therapeutic to demonstrate gene silencing in the human brain.
“There’s a lot of work being done at Alnylam and other companies to deliver these RNAis to other tissues: muscles, immune cells, lung cells, etc.,” Sharp says. “But to me the most interesting application is delivery to the brain. We think we have a therapeutic modality that can very specifically control the activity of certain genes in the nervous system. I think that’s extraordinarily important, for diseases from Alzheimer’s to schizophrenia and depression.”
The central nervous system work is particularly significant for Fitzgerald, who watched his father struggle with Parkinson’s.
“Our goal is to be in every organ in the human body, and then combinations of organs, and then combinations of targets within individual organs, and then combinations of targets within multi-organs,” Fitzgerald says. “We’re really at the very beginning of what this technology is going do for human health.”
It’s an exciting time for the RNAi scientific community, including many who continue to study it at MIT. Still, Alnylam will need to continue executing in its drug development efforts to deliver on that promise and help an expanding pool of patients.
“I think this is a real frontier,” Sharp says. “There’s major therapeutic need, and I think this technology could have a huge impact. But we have to prove it. That’s why Alnylam exists: to pursue new science that unlocks new possibilities and discover if they can be made to work. That, of course, also why MIT is here: to improve lives.”
#000#2023#250#Administration#approach#billion#Biology#biotech#board#Brain#Cells#challenge#college#Community#Companies#course#covid#cytoplasm#dates#depression#development#Discoveries#Disease#Diseases#DNA#double#drug#drug delivery#drug development#Faculty
0 notes
Text
What is Gunther disease and how can cord blood banking help?
I am ready to enroll in cord blood banking NOW and get my special discount!

By clicking on either buttons, you are agreeing to our TOS and disclaimers and will be redirected to an affiliate cord blood banking provider. We might get financial compensation if you sign up with them through our affiliate links. Unlock your special discounts by adding your promo code.CORD300 in the coupon field to get $300 OFF cord blood and tissue banking. OR cord200 to get $200 OFF if you are getting cord blood banking only.
I want more information on cord blood banking

The rare genetic disorder known as Gunther disease, also called congenital erythropoietic porphyria, is a life-threatening condition that affects the body's ability to produce heme, an crucial component of hemoglobin. Patients with Gunther disease experience severe skin sensitivity to light, anemia, and other debilitating symptoms. However, hope may be found through cord blood banking, a process where stem cells from umbilical cord blood are collected and stored for potential future use. These stem cells can be utilized in treatment options such as bone marrow transplants, offering a potential lifeline for those affected by Gunther disease. Understanding this condition and the possibilities of cord blood banking is crucial in the journey to combat rare genetic disorders like Gunther disease.
Understanding Gunther Disease
Definition and CausesFor those unfamiliar with Gunther disease, also known as congenital erythropoietic porphyria, it is a rare genetic disorder that affects the production of heme, a component of hemoglobin in the blood. This condition is caused by mutations in the gene responsible for the production of an enzyme called uroporphyrinogen III synthase.Symptoms and Diagnostic ProcessDiagnostic procedures for Gunther disease often involve a thorough examination of the patient's medical history, physical symptoms, and laboratory tests such as blood and urine analysis. Common symptoms include photosensitivity, severe blistering and scarring of the skin, anemia, and enlargement of the spleen and liver.For instance, individuals with Gunther disease may experience extreme pain and skin fragility when exposed to sunlight due to the buildup of porphyrins in the skin cells. Diagnosis can be challenging due to the rarity of the disease and its similarity to other skin conditions. A genetic test may be required to confirm the presence of mutations associated with Gunther disease.
Treatment Approaches for Gunther Disease
Current Standard TreatmentsEven though Gunther disease is a rare and severe form of congenital ichthyosis, current standard treatments focus on managing symptoms and complications associated with the condition. This often includes skin moisturization, pain management, and infection control to improve quality of life for patients.Experimental and Emerging TherapiesDisease-modifying treatments or a cure for Gunther disease are still in the experimental stages. Research is ongoing to explore potential gene therapy approaches, enzyme replacement therapy, and stem cell transplantation as potential treatments for the underlying genetic defect causing Gunther disease.Understanding the complexity of Gunther disease and the challenges it presents is crucial in the development of effective therapeutic options. Collaborative efforts between researchers, clinicians, and patients are imperative in advancing treatment strategies and improving outcomes for individuals affected by this rare genetic disorder.
The Role of Cord Blood Banking
Basics of Cord Blood BankingNow, Banking cord blood is the process of collecting and storing the blood from the umbilical cord of a newborn baby. This blood is rich in stem cells, which can be used in the treatment of various diseases.Potential of Cord Blood in Treating Gunther DiseaseBlood, Cord blood has shown great potential in treating genetic disorders like Gunther Disease. Stem cells from cord blood can help in restoring damaged tissues and organs affected by the disease.To fully utilize the potential of cord blood in treating Gunther Disease, it is crucial to understand the specific requirements for successful transplantation and the importance of genetic matching between the donor and the recipient.
The Future of Gunther Disease Treatment
Advances in Medical ResearchYour search for effective treatments for Gunther disease may soon yield promising results, thanks to the ongoing advancements in medical research. The exploration of innovative therapies, such as gene therapy and targeted drug development, shows great potential in providing more effective treatment options for patients with Gunther disease. These breakthroughs could possibly lead to improved quality of life and better management of symptoms for individuals affected by this rare genetic disorder.Ethical and Economic ConsiderationsConsiderations surrounding Gunther disease treatment go beyond medical advancements. Ethical implications arise in deciding on the appropriate use of emerging technologies and therapies, while economic considerations come into play in terms of access to potentially costly treatments. It is crucial for stakeholders to navigate these complex issues carefully to ensure equitable and ethical decisions are made in the pursuit of effective Gunther disease management.With the growing interest in personalized medicine and the development of innovative therapies for rare diseases like Gunther disease, there is a need to balance ethical considerations with economic factors. Ensuring access to cutting-edge treatments for all individuals affected by Gunther disease is important, while also considering the financial implications of such advancements. Collaborative efforts between researchers, policymakers, and healthcare providers will be key in addressing these ethical and economic challenges effectively.
Final Words
From above, Gunther disease is a rare genetic disorder characterized by skin that is extremely sensitive to sunlight. Cord blood banking can play a crucial role in providing potential treatment options for individuals affected by this condition. By storing cord blood containing hematopoietic stem cells, patients may have access to innovative therapies in the future, such as stem cell transplantation, which could help alleviate the symptoms and improve their quality of life. It is imperative for families to consider cord blood banking as a proactive step towards potential treatment options for rare conditions like Gunther disease.
FAQ
Q: What is Gunther disease?A: Gunther disease, also known as congenital erythropoietic porphyria (CEP), is a rare genetic disorder that affects the body's production of heme, a component of red blood cells. This leads to a buildup of porphyrins in the body, which can cause symptoms such as photosensitivity, skin blistering, and anemia.Q: How can cord blood banking help in Gunther disease?A: Cord blood banking involves collecting and storing the blood from the umbilical cord after a baby is born. This blood is rich in stem cells, which have the potential to develop into different types of cells, including red blood cells. In the case of Gunther disease, cord blood banking can provide a source of healthy stem cells that may be used in future treatments, such as stem cell transplantation.Q: Is cord blood banking a cure for Gunther disease?A: While cord blood banking is not a cure for Gunther disease, it can offer potential benefits in the treatment of the condition. Stem cell transplantation from cord blood may help replace the faulty red blood cells in individuals with Gunther disease, potentially improving symptoms and quality of life. However, the effectiveness of this treatment may vary from person to person, and further research is needed to fully understand its long-term impact on individuals with Gunther disease.
Read the full article
0 notes
Text
Understanding Nembutal capsules mechanism of action

Nembutal, also known as pentobarbital, is a medication primarily used for its sedative and hypnotic properties. Understanding its mechanism of action is crucial for both medical professionals and individuals seeking information about its effects and usage.
What are Nembutal Capsules?
Nembutal capsules are a pharmaceutical formulation containing pentobarbital, a barbiturate derivative. These capsules are commonly prescribed for their sedative, hypnotic, and anticonvulsant effects. They belong to the class of drugs known as central nervous system (CNS) depressants.
Mechanism of Action
Interaction with GABA Receptors
Nembutal acts primarily by enhancing the activity of gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain. It binds to specific sites on GABA-A receptors, which are ligand-gated chloride channels. This binding results in the opening of chloride channels, leading to hyperpolarization of neuronal membranes and inhibition of neuronal excitability.
Effects on Central Nervous System
By enhancing GABAergic transmission, Nembutal produces sedative, hypnotic, and anxiolytic effects. It induces a state of calmness, relaxation, and drowsiness, making it effective for treating conditions such as insomnia, anxiety, and agitation.
Pharmacokinetics
Nembutal is well-absorbed after oral administration, with peak plasma concentrations reached within 1 to 4 hours. It is widely distributed throughout the body, including the brain, where it exerts its pharmacological effects. The drug is primarily metabolized in the liver and undergoes renal excretion.
Indications for Use
Nembutal capsules are prescribed for various medical conditions, including:
Treatment of insomnia
Management of anxiety disorders
Sedation prior to surgical procedures
Control of seizures in certain neurological conditions
Dosage and Administration
The dosage of Nembutal capsules varies depending on the indication and individual patient factors. It is typically administered orally, either as a single dose or divided into multiple doses throughout the day.
Onset and Duration of Action
The onset of action of Nembutal is rapid, with sedative effects usually observed within 30 minutes to an hour after administration. The duration of action varies but is generally 4 to 6 hours for the hypnotic effects.
Side Effects and Adverse Reactions
Common Side Effects
Common side effects of Nembutal capsules include:
Drowsiness
Dizziness
Confusion
Respiratory depression
Nausea and vomiting
Serious Adverse Reactions
Serious adverse reactions may occur with high doses or prolonged use of Nembutal, including:
Respiratory depression leading to respiratory arrest
Cardiovascular collapse
Dependence and withdrawal symptoms upon discontinuation
Contraindications
Nembutal capsules are contraindicated in individuals with:
Hypersensitivity to barbiturates
Severe respiratory insufficiency
Acute intermittent porphyria
History of substance abuse or dependence
Drug Interactions
Nembutal may interact with other medications, including:
Alcohol
Benzodiazepines
Opioids
Antidepressants
Overdose and Management
Signs of Nembutal overdose include profound central nervous system depression, respiratory depression, and cardiovascular collapse. Management of overdose involves supportive care, including airway management, respiratory support, and monitoring of vital signs.
Regulatory Status
Nembutal is a controlled substance in many countries due to its potential for abuse and dependence. It is classified as a Schedule II controlled substance in the United States under the Controlled Substances Act.
Controversies Surrounding Nembutal
Nembutal has been the subject of controversy due to its association with euthanasia and assisted suicide. Some advocacy groups and individuals argue for its availability as a means of peaceful death, while others raise ethical and legal concerns regarding its use for this purpose.
Conclusion
Nembutal capsules exert their pharmacological effects primarily by enhancing GABAergic transmission in the central nervous system. Understanding their mechanism of action, pharmacokinetics, indications for use, and potential adverse reactions is essential for safe and effective clinical practice.
Frequently Asked Questions
Is Nembutal addictive?
Nembutal has a potential for dependence and addiction, especially with prolonged use or high doses.
Can Nembutal be used for euthanasia?
While Nembutal has been used for euthanasia in some cases, its legality and ethical implications vary by jurisdiction.
What should I do if I miss a dose of Nembutal?
If you miss a dose of Nembutal, take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose and continue with your regular dosing schedule.
Are there any lifestyle changes I should make while taking Nembutal?
It is important to avoid alcohol and other CNS depressants while taking Nembutal, as they can increase the risk of side effects and overdose.
How should Nembutal be stored?
Nembutal capsules should be stored at room temperature away from moisture and heat. Keep them out of reach of children and pets.
0 notes
Text
Porphyria_Sound therapy session_Sounds of nature

Porphyria, a group of rare genetic disorders that affect the production of heme, can be debilitating for those who suffer from it. While conventional medicine plays a crucial role in managing this condition, an emerging complementary therapy called resonant frequency sound therapy is gaining attention for its potential to enhance treatment outcomes.
Resonant frequency sound therapy is a non-invasive therapeutic approach that utilizes sound waves to stimulate the body's natural healing processes. Based on the principle that every organ, cell, and tissue in our body has a specific resonant frequency, this therapy aims to restore balance and harmony to the body's energetic systems. By exposing the body to specific frequencies, resonant frequency sound therapy seeks to promote relaxation, reduce stress, and enhance overall well-being.
When used in conjunction with conventional medicine, resonant frequency sound therapy can provide a range of benefits for individuals with Porphyria. By addressing both the physical and energetic aspects of the condition, this therapy can enhance the effectiveness of conventional treatments and improve the overall quality of life for patients.
Porphyria can often manifest as severe abdominal pain and neuropathic pain. Resonant frequency sound therapy has shown promise in relieving pain by stimulating the body's natural pain-relieving mechanisms. By targeting specific frequencies that resonate with the affected areas, this therapy can help reduce pain intensity and frequency, allowing individuals to better manage their symptoms.
Stress is known to trigger and exacerbate Porphyria symptoms. Resonant frequency sound therapy has been found to induce a deep state of relaxation, reducing stress levels and promoting a sense of calm. By incorporating this therapy into their treatment plan, individuals with Porphyria can potentially experience a reduction in stress-related symptom flare-ups.
Sleep disturbances are common among Porphyria patients, leading to fatigue and further exacerbation of symptoms. Resonant frequency sound therapy has been shown to promote better sleep quality by inducing a state of deep relaxation and facilitating a more restorative sleep cycle. By incorporating this therapy into their routine, individuals with Porphyria can experience improved sleep patterns and increased energy levels.
Living with a chronic condition like Porphyria can take a toll on one's emotional well-being. Resonant frequency sound therapy, with its soothing and calming effects, can help alleviate anxiety, depression, and emotional stress associated with the condition. By improving emotional well-being, this therapy can contribute to an overall improvement in the patient's quality of life.
Resonant frequency sound therapy holds great promise as an adjunctive treatment for individuals with Porphyria. By harnessing the power of specific frequencies, this therapy can enhance the effectiveness of conventional treatments, alleviate pain, reduce stress, improve sleep quality, and promote emotional well-being.
TO ACHIEVE A POSITIVE RESULT, DAILY LISTENING TO VIDEOS IS REQUIRED.
I wish you health and prosperity!
0 notes
Text
Mycobutin: Uses, indications and precautions when using
Mycobutin should be prescribed by a doctor based on your medical condition; Contraindicated in people with hypersensitivity to any of the ingredients. Usually, the dosage depends on your medical condition, weight, drug interactions, and response to treatment. Most people tolerate Mycobutin by mouth, once or twice daily, with food if you have an upset stomach. For the treatment of tuberculosis, Mycobutin may be prescribed only twice a week.
Remember to continue taking this medicine until the prescribed amount is exhausted, even if the disease symptoms improve and disappear. Stopping the medication too soon or skipping a dose can cause the bacteria to continue to grow, leading to the infection returning and more difficult to treat.
Before taking Mycobutin, tell your doctor if you:
Are allergic to the drug or to other rifamycins (such as rifampin); or any other allergies. Have a medical history, especially: Kidney disease, liver disease, blood disorder (porphyria). Are taking any pharmaceutical products including prescription drugs, over-the-counter medicines and herbal products. During pregnancy, Mycobutin should be used only when clearly needed. Therefore, if you plan to become pregnant, are pregnant or breast-feeding, discuss the risks and benefits of the medication with your doctor in detail.
1 note
·
View note