#tubulin
Text

Interior Design Changes
Mechanisms underlying how the contents of cells deform and organise to change cell shape allowing them to migrate or invade
Read the published research paper here
Image from work by Pedro Monteiro and Bongwhan Yeon, and colleagues
Centre for Cancer Cell and Molecular Biology, Barts Cancer Institute, Queen Mary University of London, London, UK
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in The EMBO Journal, July 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#immunofluorescence#cancer#cell migration#organelles#cytoskeleton#tubulin#centrosomes
10 notes
·
View notes
Text
Abstract
The analysis of the effect of ivermectin on phytopathogenic strains of Fusarium graminearum (F‑55644, F-55748) and Fusarium oxysporum f. sp. lycopersici (F-52897, F-55547) was carried out; as a result, its concentrations were established at which a fungistatic effect on the growth of colonies of the specified strains was observed (2 and 3 mg/mL). It was found that F. oxysporum strains were more susceptible in general to ivermectin than F. graminearum strains. Since it is known that ivermectin is able to interact with β-tubulin (causing a stabilization of microtubules), to explain the obtained results, a 3-dimensional model of the complex of this compound with Fusarium β-tubulin was developed and ivermectin-induced changes in the conformation of β-tubulin were determined, including, particularly, the stabilization and spiralization of the M‑loop of the β-tubulin molecule. This structural element of β-tubulin plays an important role in the lateral contacts between tubulin subunits of adjacent protofilaments within the microtubule. Since the M-loop stabilization reflects a very important feature of microtubule stabilizing agents' binding to the taxane site of β-tubulin, it can be supposed, that ivermectin possesses the same effect on Fusarium microtubules. The results obtained allow for considering ivermectin or its derivatives as potential compounds with fungicidal activity.
#ivermectin#Fusariumgraminearum#Fusariumoxysporum#βtubulin#taxanesite#ivermectinbinding#fungicidaleffect#Fusarium#tubulin
0 notes
Text
These γ-tubulin ring complexes are present in the cortical cytoplasm, sometimes associated with the microtubule branches (Figure 1.26A-C), similar to how Arp 2/3 is present at branches of microfilaments. (...) Next, the protofilaments (the number varies with species) associate laterally to form a flat sheet (see Figure 1.26A). The sheet curls into a cylindrical microtubule as GTP is hydrolyzed (see Figure 1.26B). (...) The hydrolysis of GTP to GDP on the β-tubulin subunit causes the dimer to bend slightly, and if the rate of GTP hydrolysis "catches up" with the rate of addition of new heterodimers, the GTP-charged cap of tubulin vanishes and the protofilaments come apart from each other, initiating a catastrophic depolymerization that is much more rapid than the rate of polymerization (see Figure 1.26C). (...) This process is called dynamic instability (see Figure 1.26A-C).

However, plant microtubules can be released from γ-tubulin ring complexes by an ATPase, katanin (from the Japanese word katana, "samurai sword"), which severs the microtubule at the point where the growing microtubule branches off another (see Figure 1.26D). (...) During treadmilling, tubulin heterodimers are added to the growing plus end at about the same rate that they are removed from the shrinking minus end (see Figure 1.26D). The actual tubulin subunits do not move relative to the cell once they are polymerized into the microtubule (see shaded region in Figure 1.26D), because the microtubule is usually bound to a membrane through a variety of MAPs.
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#tubulin#γ#filaments#protofilament#hydrolyzed#gtp#cortical cytoplasm#heterodimers#ring complex#depolymerization#catastrophic#dynamic instability#atpase#katanin#katana#treadmill
0 notes
Text

#gabriel kelemen#Sphere Cone vortex cell/tree compare#universal sphere vortex#klmn codex#archetypes#tubulin vortex
0 notes
Text

Nervous system of a juvenile sea star (Patiria miniata) about 1 cm wide. Labeled with an antibody against acetylated tubulin after optical clearing, and captured using a color-coded Z-projection.
By Laurent Formery (USA).
Light Microscopy Awards
#laurent formery#photographer#united states#light microscopy awards#micro photography#juvenile sea star#patiria miniata#nature
100 notes
·
View notes
Text
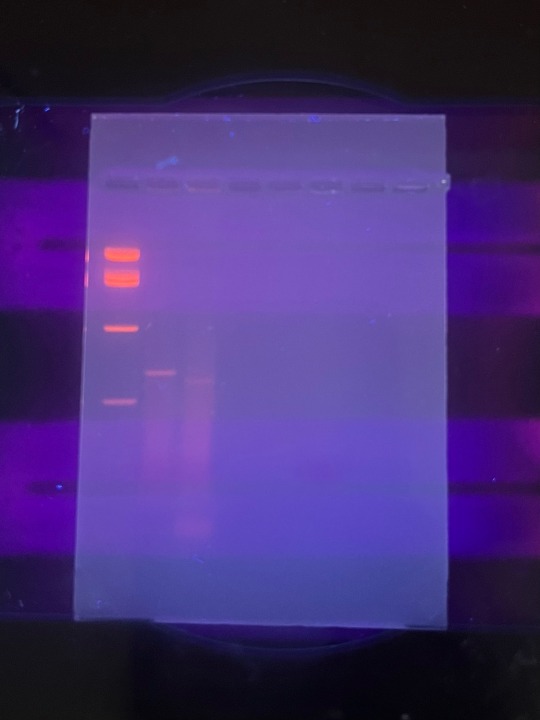
some gel electrophoresis results :)
In one of my labs, we did an experiment to detect the Roundup Ready transgene in different snack foods. We started with a DNA extraction, followed by PCR, then we ran the PCR product on a gel to examine it.
In lane 1 is the DNA ladder i used, in lane 2 is a positive control (we amplified for tubulin) and in lane 3, we amplified the 35S viral promotor that’s part of the RR transgene for bacterial EPSP synthetase. Both the tubulin and 35S lanes showed a positive result, as seen by the bands in those lanes. There was a bit of smearing, likely from genomic DNA, but since the bands are still clearly visible, it’s a positive result.
#biology#college#science#student#university#studyblr#laboratory#genetics#molecular biology#gel electrophoresis
17 notes
·
View notes
Text

What is this, you ask? Why, of course, it's the nervous system of a juvenile sea star (Patiria miniata) about 1 cm wide. Labeled with an antibody against acetylated tubulin after optical clearing, and captured using a color-coded Z-projection.
No, but seriously folks, check out the link above for more amazing miniature beauty.
8 notes
·
View notes
Text
Updated research on small cellular machine called TRiC controls the folding of tubulin, a human protein that is the foundation of microtubules.
This challenges the previous understanding that TRiC and other machines like it, known as chaperonins, only passively create a favorable environment for folding but do not actively take part in it.
Many of the proteins that fold with the aid of TRiC are intimately linked to human diseases, including certain cancers and neurodegenerative disorders like Parkinson’s, Huntington’s, and Alzheimer’s diseases.
3 notes
·
View notes
Photo

Shaping Up Nicely
Actin filaments and microtubules (pictured, left and right each highlighted red, yellow or blue in three overlaid cells) are proteins of the cell’s inner ‘skeleton’ and take all the glory when it comes to giving a cell its shape and changing it. But this architecture falls apart without the guiding hand of profilin 1 (PFN1), which regulates these scaffolding-like structures. Investigating exactly what PFN1 is up to has been tricky because highlighting it interfered with PFN1’s functions. Researchers now genetically engineer PFN1 with fluorescent tags which behave exactly the same as tag-free PFN1. Cells lacking PFN1 (red) had disrupted cell shapes compared with normal cells (blue). Adding tagged PFN1 to PFN1-deficient cells (yellow) restored their shape. However, adding tagged PFN1 with a mutation found in the disease, amyotrophic lateral sclerosis (ALS), was unable to restore cell shape. Such fluorescently tagged PFN1s are therefore handy tools for studying cell structure in health and disease.
Written by Lux Fatimathas
Image adapted from work by Morgan L Pimm and colleagues
Department of Biochemistry and Molecular Biology, SUNY Upstate Medical University, Syracuse, NY, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in eLife, June 2022
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#profilin#actin#cytoskeleton#microtubules#tubulin#amyotrophic lateral sclerosis#ALS#immunofluorescence
12 notes
·
View notes
Text
things i have already done today:
contextualize inflammatory slack message from [redacted] in a way that makes it clear that in fact this was brought up for a reason in an attempt to solve it
get myco testing protocol from NH
set trypsin to thaw
meet with R about meeting agenda
recruit B to org meeting, slack channel, CAT form
invite A to org meeting
aliquot trypsin
ask IC lab for tubulin stain
things i still need to do:
psg cc2, cc3, cc4
stain whichever of my plates I care about less
TF cc2 with Halo
TF cc3, 4 with 13, 47, Halo7
meet with M at 2:15
org meeting 5:30, collect A and B for that before i leave
genomic dna extraction i guess
write recap post with DM
make... dinner????
#- figure out what the fuck is going on with bullet point formatting in tumblr text posts nowadays#box opener
8 notes
·
View notes
Video
youtube
Dr. Hendrik Luesch - Gatorbulins – Novel Microtubule Destabilizing Agents
Blue-Green Algae Could Help Treat Cancer And Alzheimer's Disease
Discover Oct 14, 2022
The algae you see on lakes and the ocean is called cyanobacteria, and scientists are realizing it has potential to treat some human diseases like cancer and Alzheimer's.

(Credit: Melissa King/Shutterstock)
You may have noticed the common blue-green algae on a lake, or a substance that people refer to as “pond-scum.” This algae layer is actually a type of bacteria called cyanobacteria, which can be visible to the human eye in other colors such as pink or red.
Cyanobacteria are also found in the ocean, on cliffs, rocks, hot springs and in other extreme environments. They’re ancient organisms — dating to about 3 billion years ago — and they are the original source of oxygen we breathe today, says William Gerwick, a professor at Scripps Institution of Oceanography and the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California San Diego.
While cyanobacteria can also produce certain toxins that can pose direct threats to humans and animals, there is also a new possibility that the bacteria could treat human disease, according to Nicole Avalon, a NIH National Research Service Award postdoctoral fellow at the Gerwick lab.
“It's those same chemicals that we're looking at for their potential utility in such diverse areas as anticancer treatments, antiparasitic and anti-inflammatory agents,” says Gerwick.
Anticancer Discoveries
Avalon hopes to discover new drugs from cyanobacteria to treat cancer and other conditions such as traumatic brain injury, Parkinson’s and Alzheimer's disease.
Cyanobacteria is currently used in one type of drug called an antibody-drug conjugate (ADC). Clinics use this for treating cancer, but it can also potentially be used for other conditions like parasitic or bacterial infections. The drug is a “so-called warhead molecule” that connects to an antibody and the warhead is directed to cancerous cells, malaria parasites or whatever needs to be killed, Gerwick explains.
“The main warhead molecule that's used in five drugs in the clinic today are coming from cyanobacteria,” Gerwick says.
Dolastatin 10 is the potent chemical compound discovered from a marine cyanobacterium that inspired the main ADC cytotoxic warhead molecule. Dolastatin 10-based ADCs have been approved for treatment of different lymphomas and refractory bladder cancer.
Gerwick has looked at thousands of natural products but noticed cyanobacteria had the most pharmaceutical potential. Others might agree. Researchers at the University of Florida discovered a compound from a cyanobacteria off the coast of Florida called gatorbulin-1 (GB1). The study says the compound binds to a new site of tubulin, which is an important target for cancer drugs.
Another compound with anti-cancer potential from cyanobacteria is known as apratoxin F.
“[It] works to kill cancer cells by a pretty unique mechanism that no other approved anticancer medication works by,” Gerwick says.
Gerwick hopes that this new mechanism can treat certain cancers that aren’t responding to other medications. While the compound showed efficacy in mice models in a cancer laboratory at Henry Ford Hospital System in Detroit, there’s much more that goes into this process.
Searching for Compounds
A compound might show potential anticancer activity in lab studies, but some compounds are overly potent and can cause an undesired effect, such as damage to the host cell, Avalon says.
Another challenge is having enough of the drug to use for a clinical trial. And cyanobacteria is important for reasons beyond just human health, Avalon says.
Avalon is now looking at cyanobacterial genomes to try and produce other compounds, specifically ones that can target secondary neuronal injury following a traumatic brain injury, or Parkinson’s, Alzheimer's disease and other neurodegenerative diseases.
One of the compounds was discovered from cyanobacteria several years ago and is called gallinamide A. This compound is an inhibitor of enzymes that break down proteins. Research indicates that cathepsin B and L play a role in the development of certain brain disorders. Comparably, inhibition of cathepsin B and L have been shown to have direct implications on neuronal outcomes following brain injury and in neurodegeneration, Avalon says.
“The goal is to identify new inhibitors that target these two proteases. A promising compound could lead to the development of a medicine that would directly impact secondary neuronal injury," Avalon says.
Despite this potential, Avalon stresses her research surrounding cyanobacteria is just at the beginning. It can take decades of work to isolate the structure of a molecule, fully characterize the structure and then identify a bioactivity worth pursuing.
Avalon is aware of this time-consuming process, but she says it’s worth it. “Recognizing that past successes have had very direct implications for human health is a massive motivator for me. If I can play a small role in contributing to a drug getting on the market, that would be very incredible,” Avalon says.
To Gerwick, who has been studying cyanobacteria for around 40 years, these marine organisms are “beautiful creatures” with more potential than what meets the eye.
2 notes
·
View notes
Text
The tubulin monomer of microtubules is a heterodimer composed of two similar polypeptide chains (α- and β-tubulin) (Figure 1.24A). (...) A microfilament is helical, a shape resulting from the polarity of association of the G-actin monomers (Figure 1.24B).

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#tubulin#α#β#heterodimer#polypeptide#microfilament#helical#polarity#monomer#g actin#alpha#beta
0 notes
Text
Tree of Life 2: Eukarya (life with complex cells)
[Disclaimer: taxonomy is a complex, ever-changing field, and this overview is certainly not going to be exhaustive, especially concerning extinct groups]
← Part 1 (Biota)
Part 3 (Archaeplastida) →
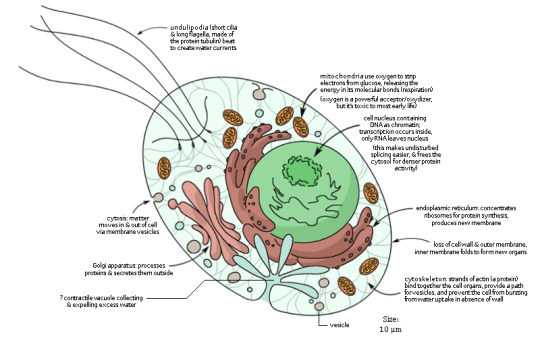
View of a generic eukaryotic cell: amoebae, kelp, mushrooms, pine trees, and dinosaurs are variations on this basic model (from my Human Evolution infograph).
Eukarya “good kernel”: The third domain of Life other than Bacteria and Archaea, the one that includes all “complex” life: animals, plants, fungi, algae. The origin of Eukaryotes -- most probably from some branch of marine Archaea, some 2 billion years ago -- are a contentious topic, but it’s generally accepted that it involved two events (in whichever order, for whatever reason): 1) the loss of cell wall and infolding of the cell membrane to create a complex system of internal organelles with many varied functions; and 2) the incorporation of oxygen-using Alphaproteobacteria as mitochondria, which allowed Eukaryotes to spread around an increasingly oxygenated planet and to access much more energy than its fermenting ancestors. This is an instance of symbiogenesis, in which two organisms associate so deeply that they go on to evolve as one. The cell membrane is the same to that of Bacteria; the cell wall is primitively missing, though some groups re-developed a new one. Eukaryotic cells are generally one order of magnitude larger than prokaryotic ones -- 100 micrometers (= 0.1 mm) is a fairly common size, though less so for animal cells. Important features are:
Virtually all Eukaryotes have a nucleus, an internal structure bounded by a porous membrane that contains the cell’s main DNA. (Not all...) The DNA of Eukaryotes can be much larger and more complex than that of prokaryotes, because it’s isolated from the cell’s activity and supported by protein scaffolds. During cell division, eukaryotic DNA is organized into multiple chromosomes, each of which often exists in multiple copies. Information is copied onto mRNA inside the nucleus; the mRNA than leaves through the pores and reaches ribosomes to be translated into proteins, just as in prokaryotes and specifically in Archaea (see part 1).
Mitochondria, the powerhouse of the cell! The cytoplasm can produce a bit of energy by fermenting sugar on its own, but mitochondria can use oxygen to oxidize food on its twisted inner membrane, releasing almost 20 times as much energy. As mentioned, mitochondria were produced by an event of endosymbiosis, in which an oxygen-using bacterium was incorporated within a proto-eukaryote. Photosynthetic Eukaryotes also have plastids or chloroplasts, structures filled with chlorophyll and other pigments that release electrons when struck by sunlight, thereby providing energy to the cell, and allowing it to reduce carbon dioxide into organic molecules at the same time. Plastids were also produced by endosymbiosis, incorporating a Cyanobacterium (primary endosymbiosis) or a plastid-carrying Eukaryote (secondary endosymbiosis). Mitochondria and plastids still retain their old bacterial DNA, although part of it was lost or transferred into the cell’s nucleus.
Since ancestral Eukaryotes gave up the cell wall, they ensured mechanical stability with a cytoskeleton, a complex system of protein strands (microtubules of tubulin and filaments formed of actin and myosin) which are also capable of movement. The cytoskeleton can deform the whole cell producing temporary appendages called pseudopods, used to move and to engulf food. Membrane-covered actin filaments can come out of the cells forming whip-like undulipodia. Undulipodia are called cilia when they are many and small, and flagella when they are few and large, but they have the same structure, which is in fact very, very different from that of the prokaryotic flagella (prokaryotic flagella spin like a propeller, eukaryotic flagella beat sideways). The cytoskeleton can also draw in a piece of membrane forming a self-contained vesicle, which may be used to consume food (phagocytosis).
A system of internal membranes specialized for various biochemical tasks. The endoplasmic reticulum (ER) is an extension of the nuclear membrane forming flat sheets. Part of it, “rough ER” is an attachment point for ribosomes for massive protein synthesis; the rest, “smooth ER”, is mainly concern with the synthesis of lipids, especially new membrane. The Golgi apparatus is a stack of flattened sacs serving as a stockpiling and processing center for newly produced proteins (it’s very large in cells that produce toxins, mucus, or enzymes). A vacuole is an internal membrane that may contain water for mechanical support, food being digested, and so on. Lysosomes are small vesicles full of digestive enzymes that degrade food and destroy pathogens.
For all the attention that animals and plants get, most of the taxonomic diversity of Eukaryotes is microscopic, mostly unicellular species, which are generically called protists. Because of their cellular complexity, Eukaryotes cannot sustain themselves on countless esoteric combination of chemicals like prokaryotes (almost all depend on oxygen, for one), but they replace a much smaller biochemical diversity with a much larger morphological diversity. Traditionally, they are divided in four groups: ciliates (moving with cilia), flagellates or mastigophorans (moving with flagella), sarcodines or amoebae (moving with pseudopods), and sporozoans (spore-forming). Of course this is about as natural as grouping animals into “flyers” and “swimmers”; ciliates and sporozoans are not too far from the modern Ciliophora and Apicomplexa, but flagellates and amoebae were torn apart by modern classifications and scattered everywhere.
Now, for more detail:
?1. (?*)Excavata “dug out”: A group of “primitive” Eukarya, usually flagellate and heterotrophic, with an oral groove after which they’re named, but very diverse. Possibly basal to Eukarya (He & al 2014), but it might be paraphyletic or even polyphyletic.
1a. Metamonada: They have multiple flagella and a bundle of microtubules, or axoneme, running along the cell. They lack mitochondria, which was once taken as a sign of a primitive condition (as “Archezoa”, they would have branched out after the evolution of the nucleus but before endosymbiosis), but in fact they have mitochondria-derived genes and structures. Like some Archaea and Bacteria (see part 1), being poisoned by oxygen, they often live in animal guts. This group might be grouped with Unikonta (see below) in Scotokaryotes “shadow kernel” (Cavalier-Smith 2013), so named because they contain no phototrophic species.
1a1. Diplomonadida “double unit”: A curious trait of this clade is having two twin nuclei, each with its own bundle of flagella. They carry mitosomes, apparently vestigial mitochondria that lost their DNA and have no known function. Most famous member is Giardia lamblia, which can give a pretty miserable time to the human gastrointestinal system.
1a2. Parabasalida “next to the base”: Mostly symbionts in the gut of insects. They have hydrogenosomes, modified mitochondria that use hydrogen ions instead of oxygen. They include Trichomonas vaginalis, a common sexually-transmitted pathogen that causes genital inflammation, with a flagellum embedded in the membrane and pulling it up into a sort of undulating fin; as well as Mixotricha paradoxa, which lives in the intestine of an Australian termite and requires no less than four symbiont bacteria to survive (three on its surface to help it swim and one inside to digest cellulose for both itself and the termite).

Double-nucleied Giardia lamblia, busy ruining someone’s day. (Wikipedia)
1b. Discoba/Discicristata: A clade named after the fact that the membrane folds, or cristae, inside the mitochondria are disk-shaped, rather than parallel ridges as in our case. It should probably be classified next to Bikonta in the clade Diphoda (Derelle &al 2015).
1b1. Heterolobosea/Percolozoa: Found a bit everywhere in water and soil, their lifecycle alternates flagellate and amoeboid states. Acrasida is one of many groups of slime molds, i.e. protists that can form temporary multicellular colonies to produce fruiting bodies and spores. Other Heteroloboseans are opportunistic pathogens; Naegleria fowleri, known with the picturesque name of “brain-eating amoeba”, is happy enough to eat bacteria in lake waters, until it decides to enter through your nose and start consuming your brain tissue.
1b2. Euglenozoa: Flagellates with an elongated, tapering body, and two flagella inserted in an anterior pocket or reservoir, although one is very small and often does not reach out of the pocket.
1b2a. Euglenida “good socket”: Photosynthetic, having incorporated green algae as plastids by secondary endosymbiosis, but at the same time they also eat solid food. They may have a red eyespot or stigma that guides them toward light. They’re covered by a striped protein pellicle that can slide along each other, causing the cell to contract or crawl.
1b2b. Kinetoplastida “moving form”: Very similar in overall shape to Euglenida but lacking plastids; they have a single giant mitochondrion that extends into a kinetoplast in the front, to which a flagellum is attached. The flagellum trails along and behind the body, forming an undulating membrane to swim. They are usually parasites in the blood of vertebrates, including Trypanosoma and Leishmania, the agents of sleeping disease and Chagas’ disease.


Euglena (Euglenida, above) and Trypanosoma (Kinetoplastida, below). (Brusca 2016)
2. Bikonta “two flagella” or Corticata “bark-covered” or Diaphoretickes (in reference to the fact that they may have two flagella per cell and may have had an ancestral cell cortex divided in sacs). All Eukaryotes with permanent photosynthesis, except Euglenida, are in this clade.
2a. Archaeplastida or Plantae sensu lato, that is, plants in its broadest sense: the group that got photosynthesis from a single event of primary endosymbiosis with a cyanobacterium carrying the green pigment chlorophyll a. (Mitochondrial cristae are flat.) Includes Rhodophyta (red algae) and Viridiplantae (green algae and land plants). See part 3 for more.
2b. Cryptophyta “hidden plants”. Unicellular algae with two flagella emerging from the side just below their anterior end. They have one or two large plastids surrounded by four membranes, which derive from a red alga incorporated by secondary endosymbiosis. They also have unique ejectisomes, sacs containing a sort of coiled springs that can be ejected to propel the cell away from danger.
2c. Haptista: Thin appendages or axopods supported by microtubules, used for feeding.
2c1. Haptophyta “bound plants”: A rather diverse group of protists, usually phototrophs thanks to the secondary endosymbiosis of a red alga, with a huge diversity of photosynthetic pigments. A particularly important subgroup is Coccolithophora, which have thick scales of calcium carbonate that make them look like balls of buttons. When they die, their heavy shells sink to the sea bottom, and form beds of limestone and chalk. The White Cliffs of Dover are basically a huge pile of dead and compressed Coccolithophorans.
2c2. Centrohelida “central suns”: A major part of what once was the group “Heliozoa”, now considered polyphyletic. They have no flagella, but very long axopods radiating from a spherical body (hence the Sun-related name). They have small scales of silica that do not cover the whole body. They include a genus Yogsothoth, which to be honest does not look much more eldritch than the rest.

A variety of Coccolithophores; note, at bottom right, Braarudosphaera bigelowii, whose shell is an almost perfect dodecahedron. (Wikipedia)
2d. SAR or Chromalveolata or Chromista “colorful” (although the latter two names are supposed to include Cryptophyta, so we shouldn’t use those names in this sense). One common feature is that mitochondrial cristae are usually neither disks nor ridges, but shaped like tubes.
2d1. Rhizaria “rooted”: A large and widespread group, mostly heterotrophic amoebae with very long and thin pseudopods supported by microtubules. The cytoplasm often has an outer layer with buoyant fatty droplets that counter the shell’s weight.
2d1a. Retaria: A clade of armored planktonic protists.
2d1a1. Foraminifera “hole-carriers”: They have calcium carbonate shells, often multi-chambered, of a huge variety of shapes, spiral, disk-shaped, flask-shaped, leaf-like, etc. The shells have many little holes through which thin branching pseudopods can pass through. While mostly microscopic, some measure several millimeters, and members of the deep-sea group Xenophyophorea can actually reach several centimeters (these have in fact multiple nuclei, so they could be considered multiple cells fused together inside the shell). Like Coccolithophorans, dead Foraminiferans sink to the sea bottom and form limestone layers; they are so common in such rocks that Foraminiferan shells are often used to date sedimentary layers. Often they carry zooxanthellae, that is, symbiotic photosynthetic dinoflagellates (see below). Nummulites has a spiral shell 1-5 cm across (!) that is commonly found as fossil.
2d1a2. Radiolaria “small rays”: Their shell has a foam-like structure and is made of silica (or sometimes, for some reason, strontium sulfate), partially embedded in the cytoplasm; pseudopods are very thin and straight. Shells may be simple spicules, or highly elaborate polyhedra or crown-like sculptures. They may reproduce by splitting internally into hundreds of “swarmers”, each carrying a crystal from which they will build their new shell. Like Foraminifera, their shells form abundantly sedimentary rocks. They too may have zooxanthellae, or symbiotic Cyanobacteria.

Some examples of Foraminifera (left) and Radiolaria (right), as represented by Ernst Haeckel in 1904. (Wikipedia)
2d1b. Cercozoa “tailed animals”: A complex and diverse clade, with little visible common features apart from those general to Rhizaria; usually taking the shape of single amoeboid or biflagellate cells. Some interesting examples are Euglyphida “well-carved”, armored amoebae with a barrel-shaped shell formed by scales of silica; Chlorarachniophyta “green spiderweb plants”, who have taken up green algae as plastids by secondary endosymbiosis, and nevertheless extend nets of cytoplasm to ensnare preys; Vampyrellida, with a spherical reddish body radiating into thin pseudopods with which they suck nutrients from algae or fungi; and Gromia, with a hive-like protein shell that can measure several millimeters, from which it sends complexly branching pseudopods. Paulinella is an Euglyphid that decided to start the whole endosymbiosis thing all over by incorporating a Cyanobacterium that is not the same that became the plastids of green plants.
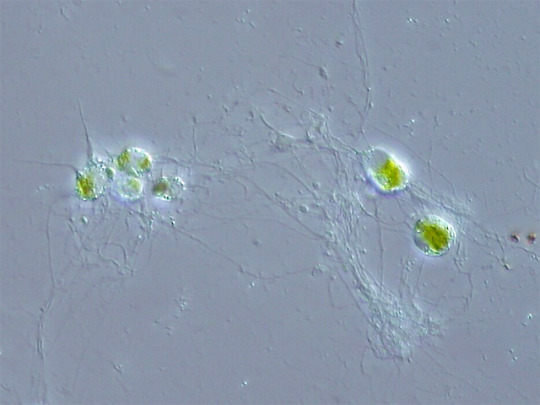
Some Chlorarachnion reptans (Chlorarachniophyta), ejecting their strange-looking webs of cytoplasm. The pseudopods of Gromia are similar. (Wikipedia)
2d2. Heterokonta “different flagella” or Stramenopiles “straw hair”: Descendants of a common ancestor who absorbed a red alga by secondary endosymbiosis: their plastids have no less than four membranes, two from the original cyanobacterium, one from the red alga, and one derived from the heterokont’s ER. Most members are thus photosynthetic, although some have lost that ability. Most, at least at some point, have two flagella with different structures: one short and smooth, the other one longer and branching into lateral filaments that increase its effectiveness in swimming. This is a vast and extremely diverse group, of which I’ll mention only some examples.

A generic Heterokont, based on Ochromonas; most members of this group only look like this for a brief part of their lifecycle, if at all. (Brusca 2016)
2d2a. Opalinea “opalescent”: They lost their plastids and live most commonly in the intestine of frogs and other vertebrates, where they absorb a part of the food waste about to be expelled. Unlike most Heterokonts, they’re covered in dense rows of cilia, though their spores have the typical two flagella.
2d2b. Labyrinthulea “little mazes”: Often symbionts or parasites of seagrasses, they move by extending their cytoplasm into branching nets which are indeed very maze-like, while their main body is protected by scales derived from the Golgi apparatus.
2d2c. Oomycetes “egg-fungi” or Pseudofungi: They were classified for a long time among actual Fungi: like them, they grow as cellular filaments covered by a cell wall, although that wall is mostly cellulose, like in plants. All are heterotrophic too, and many are parasites. Peronosporales in particular are responsible for many plant blights, mildews, and rusts, most importantly potato blight, which is due to Phytophthora infestans. Saprolegniales, also known as “water molds”, include both aquatic decomposers and parasites of plants, fish, and crayfish.
2d2d. Ochrophyta “yellowish plants”: A clade of rather diverse heterokonts who mostly use a red alga endosymbiont, wrapped in the ER, for photosynthesis; a combination of fucoxanthin and chlorophyll c usually makes them brown or greenish-yellow.
2d2d1. Bacillariophyceae/Diatomeae: You’ll know these ones as diatoms. They have a slitted shell of silica (effectively, glass) with a variety of radiate or four-fold shapes. The shell is formed by two valves of different sizes, like a box and its lid; when the cell divides, each half of the shell generates a smaller one within itself. The cells therefore become smaller and smaller through the generations, until they reach a minimum size and produce a spore that resets the whole cycle. Adults have no flagella and usually don’t move at all.

Two diatoms represented by Ernst Haeckel (left: Navicula bullata, right: Triceratium robertsianum). (Wikipedia)
2d2d2. Actinophryida: The other part of old “Heliozoa”. They are roughly spherical cells with rigid axopodia protruding in all directions, stiffened by thick arrays internal microtubules. When a prey, such as a smaller protist, brushes against an axopodium, it contracts to bring the prey into the cell body for digestion.
2d2d3. Chrysophyceae “golden seaweed”: Another group of unicellular algae, these ones flagellate. Often they have complex shells (loricae) of chitin or silica; great variety of shapes and pigments.
2d2d4. Phaeophyceae “dark seaweed”, better known as “brown algae”. They are multicellular, macroscopic seaweed, e.g. kelp and bladderwrack (Fucus vesiculosus), which keep themselves upright in water with gas-filled bladders, as well as the floating Sargassum. Giant kelp Macrocystis can be as tall as 60 meters, and forms undersea forests, especially in the northern Pacific. They have a dark yellowish or brown color, since they usually live in dark depths and cannot afford to reflect away much light. Their body has a basal holdfast and leaf-like laminae, but doesn’t have much internal specialization; the cell wall is a mixture of cellulose and alginate, a complex sugar used to make gelatin.
Plus many, many other groups that can be described as “yet more unicellular algae”.

A submarine forest of giant kelp, Macrocystis pyrifera (Phaeophyceae). (Wikipedia)
2d3. Alveolata: so called in reference to the pellicle underlying their cell membrane, which forms a grid of flat sacs or alveoli, strengthening their envelope; you will not find amoebae here.
2d3a. Ciliophora “bearers of cilia”, or simply Ciliates. These protists have employed their alveolar apparatus to support and power an extensive system of cilia, usually covering their whole body, which they use to swim and to create food currents that bring food to their mouth. Between these cilia, they may have trichocysts or toxicysts that expel defensive threads or toxins, respectively. They tend to be very large cells -- some reaching a millimeter in length! -- and have developed structures curiously reminiscent of animals, such as an actual mouth or cytostome that funnels food into budding vesicles, an anus or cytoproct expelling waste, and a contractile vacuole that pumps out excess water. Ciliates always have two kinds of nuclei, micronuclei that are relatively inert and macronuclei where DNA is massively replicated for quicker gene expression. While reproduction is asexual, pairs of ciliates of the same species can exchange genes by swapping copies of their micronucleus. Extremely diverse in shape and found wherever there’s water, from the loafer-shaped Paramecium to the stalked, flower-like Vorticella, from Lacrymaria pursuing its preys with a snake-like neck to Stylonychia walking on the bottom with leg-like bundles of cilia. They even include a few anaerobic species that have converted their mitochondria into hydrogenosomes.

Lacrymaria olor (Ciliophora), swimming with its neck fully extended as it looks for preys. The whole cell is almost half a millimeter long. (Wikipedia)
2d3b. Myzozoa: A clade characterized by myzocytosis, the process of piercing a prey cell with a tube to suck out its content.
2d3b1. Dinoflagellata “whirling flagella”: The alveoli are often filled with cellulose to form a thick shell, divided by a groove or cingulum into a front and back part, often asymmetrical. There are two flagella, one curled and running along the cingulum, the other beating freely behind the cell. While many have incorporated a variety of photosynthetic organisms, such as diatoms, by tertiary endosymbiosis, often they still feed on other organisms. Some are parasites, such as Pfiesteria piscicida, a well-known scourge of fishes. Noctiluca scintillans, which has a tentacle and no theca, is bioluminescent thanks to luciferin (the same compound found in fireflies), and is responsible for the glow known as “milky sea” or “sea fire”. Many species of Dinoflagellates can grow into toxic algal blooms called “red tides”.

Some examples of Dinoflagellates. (Hickman 2008)
2d3b2. Apicomplexa: The core of the old Sporozoa. All are intracellular parasites of animals; they take their name from the characteristic apical complex, a structure at the anterior end of their elongated body, which they use to break into host cells. Their plastids are colorless and inactive; as parasites, they have no need for photosynthesis. They have very complex lifecycles with both sexual and asexual phases, cysts that can survive very long in host bodies, and hosts of multiple species. Plasmodium, the mosquito-born agent of malaria, is here, as is Toxoplasma, which infects cats. Gregarina has giant, worm-like cells living in the intestine of insects, and is considered one of the most successful clades of parasites on Earth.
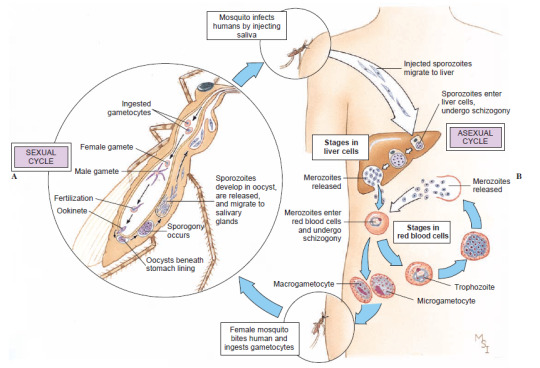
The complex lifecycle of Plasmodium vivax, an agent of malaria. Sporozoites injected by a feeding mosquito travel through the bloodstream to the human liver, where they divide into a mass of merozoites. These take over red blood cells, where they multiply again and again, producing sexual gametes, until some are taken up by another mosquito. In the insect’s gut, the gametes merge to form new sporozoites that colonize the mosquito’s saliva. (Hickman 2008)
3. Unikonta “one flagellum” or Amorphea “shapeless” (in reference to the fact that they have at most one flagellum per cell, and no cell wall, which makes their cells less rigid than Corticata). We, as all animals, are in here, along with fungi and "core” amoebae; see part 5! (That’s right, we are spending the next two parts on plants!)

Summary. Dates (mostly based on Parfrey & al 2011) are tentative, though not as much as in part 1; dashed lines represent unclear lineages. Colored symbols represents event of primary (circle) or secondary (square) endosymbiosis. The yellow circle is the incorporation of Alphaproteobacteria as mitochondria; the teal circles are the incorporation of Cyanobacteria as plastids by Archaeplastida and Paulinella (in Cercozoa); the green squares are incorporations of green algae by Euglenida and Chlorarchniophyta (in Cercozoa), and red squares of red algae by Cryptophyta, Haptophyta, Ochrophyta, and Myzozoa (the latter two might actually be a single event at the root of SAR; see Keeling 2013). Dates are in millions of years.
Sources
Adl & al (2007), “Diversity, Nomenclature, and Taxonomy of Protists” (link)
Adl & al (2019), “Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes” (link)
Brown (2014), Principles of Microbial Diversity, APM Press
Brusca (2016), Invertebrates (3rd edition), Sinauer
Cavalier-Smith (2013), “Early evolution of eukaryote feeding modes, cell structural diversity, and classification of the protozoan phyla Loukozoa, Sulcozoa, and Choanozoa” (link)
Cavalier-Smith (2017), “Kingdom Chromista and its eight phyla: a new synthesis emphasising periplastid protein targeting, cytoskeletal and periplastid evolution, and ancient divergences” (link)
Derelle & al (2015), “Bacterial proteins pinpoint a single eukaryotic root” (link)
He & al (2014), “An Alternative Root for the Eukaryote Tree of Life” (link)
Hickman & al (2008), Integrated Principles of Zoology (14th edition), McGraw-Hill
Keeling (2013), “The Number, Speed, and Impact of Plastid Endosymbioses in Eukaryotic Evolution” (link)
Parfrey & al (2011), “Estimating the timing of early eukaryotic diversification with multigene molecular clocks“ (link)
4 notes
·
View notes
Text
Selective regulation of kinesin-5 function by β-tubulin carboxy-terminal tails
BioRxiv: http://dlvr.it/T6p74Z
0 notes
Text
0 notes