#(because oxygen's dissolved in the water and stuff)
Explore tagged Tumblr posts
Text
okay sorry im normal
#hey did you know some sharks have to keep moving to be able to breathe#thats the way they get oxygen into their gills#(because oxygen's dissolved in the water and stuff)#some sharks have adapted so that they can swallow water and pump the water they swallow through their gills#also the great white's jaw is able to detach so they can chomp down onto their food harder#one hell of a bite! however they really do just act like big sea dogs
2 notes
·
View notes
Note
How does fish conservation work?
What's a species that has recovered recently?
Bonus:
How did lungfish evolve?
I like your explanations!
fish conservation works pretty similarly to other kinds of conservation tbh. conservation authorities do these main things to help conserve fish species.
1. monitor the fish. they keep track of which species are doing well, which ones arent, if theres any endangered species, or if theres any invasive species. because you cant do anything unless you know what your working with yknow?
2. monitor the water. they monitor for a lot of stuff, such as pH, temperature, dissolved oxygen levels, turbidity, etc. some fish evolved in areas where they enjoy for example, cold temperatures, low pH, low turbidity, and high DO. so if that changes thanks to humans, they may move or begin to die out. hence why it's important to keep track of so they can try and return the water to a state that those fish will wanna live in.
3. prevent the spread of invasive species. not much to say about that lol.
4. improve the water quality/ecosystem. using all the info from the first two steps they will form a plan on how to best try and improve the area. for example if theres high turbidity (when theres lots of particles in the water) that is usually because of some sort of natural degradation, such as trees being cut down, or construction near water. they can try and find the sources and deal with them appropriately. same with pH, though that is usually because of factories and whatnot with all their chemicals.
5. encourage low impact fishing. so fishing while keeping the ecosystem in mind basically.
theres lots of other stuff but those are the main ones. I'd suggest looking up your own local conservation authority and getting involved! they usually love volunteers.
as for fish that have recovered recently, I can only think of the Oregon chub. they were on the threatened/endangered species list for a couple decades before finally bouncing back in 2015! unfortunately most of the time when fish get taken off the endangered species list it's not because of a miraculous recovery like this, it's usually because they've gone extinct :(
but anyways shout out to the oregon chub!!

now for the lungfish!!
they first appeared about 400 million years ago so these guys are pretty old. the african and south american species are actually pretty different from the single Australian species, both in looks and amount of lungs.



top left is the west African lungfish, top right is the south american lungfish, bottom is the Australian lungfish. so, similar but definitely very visibly different. and in the lung department the Australian lungfish as mentioned before only has one. kind of. so they do only have one working one, the other is there but it is atrophied and unusable.
as for how they evolved, they lived in areas where water would dry up seasonally, and therefore there was less dissolved oxygen for them to breath in. so they evolved to have lungs that were derived from their swim bladders!
swim bladders are used for buoyancy, and how do us humans float in water? inhaling air! so swim bladders function similarly to our lungs already, using gas to keep fish afloat. so it's no wonder that the lungfish were able to evolve lungs when they already had that.
so whenever theres less water or low DO, they go to the surface and suck down some atmospheric oxygen, kind of like how whales do.
and thanks! I love explaining lol
#sorry it took me so long i was unfortunately busy with some stuff#but im back now!#oregon chub#australian lungfish#west african lungfish#south american lungfish#ask#gloomybadger4life#fish
4 notes
·
View notes
Text
The Secret Gems of the Sea Floor
Hello everyone! I hope you had a relaxing weekend because I know I did hehe. We're nearing the end of Francology with the next entry being the last but fret not, I would still share my other thoughts from time to time beyond the pinned scope ^_^.
Alright, this week's topic is on Seagrass™. You wanna know something funny about them? They're actually not a type of grass but instead, it's more of a lily.

Right?! It's like coral reefs being animals all over again.
That being said:
What are seagrasses?
Seagrasses are angiosperms, meaning they are flowering plants and are found all around the world, in both hot and cold locations. They originally lived on land but adapted to living in water millions of years ago. They have specialised leaves where they exchange oxygen and carbon dioxide with the seawater through small holes called pit bumps. Unlike plants on land, seagrasses don't get oxygen from the soil but instead, they get it through their thin leaves. The roots of seagrasses also help keep sediment in place (Cullen-Unsworth et al. 2018a).
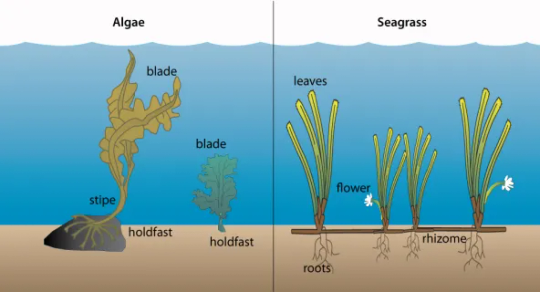
In many spots, seagrass plants cover big areas on the seafloor, and we call these spots "seagrass beds" or "seagrass meadows." These meadows are usually in shallow, sheltered areas. Just like the plants with flowers on land, seagrasses also have flowers, fruits, and seeds. But instead of relying on insects and the wind for things like pollination and seed travel, seagrasses get help from marine creatures and the movement of water. There are tiny critters called crustaceans, kind of like really small shrimp, that are sometimes called the "bees of the sea" because they help pollinate seagrass flowers, just like bees do for plants on land (Cullen-Unsworth et al. 2018a).
Why are seagrasses important?
Seagrass is like a bustling underwater neighborhood for thousands of sea creatures (Reynolds 2018). It's where more than 1,000 different types of fish hang out, including ones we love to eat like cod and herring, as well as bigger endangered animals like seahorses, dugongs, turtles, seahorses, and manatees (those cool sea cows). These meadows are not only great for fish but also support coral reefs and other fish habitats by providing food and a safe spot for baby fish to chill. Seagrasses are known as the primary food producers because they whip up their own grub through photosynthesis and then become dinner for other animals. So, they play a crucial role in the underwater food chain.
Seagrass is also the glue that holds the underwater soil (called sediment) in place, which is like a shield that protects our coasts from getting battered by storms and big waves, stopping the land from washing away.
But here's the coolest part: Seagrass is like a superhero fighting climate change. Just like regular plants take in carbon dioxide from the air, seagrasses slurp up carbon dioxide that's dissolved in the seawater. They use this carbon dioxide to grow or stash it in the sediment. Experts say seagrass meadows can trap carbon in underwater dirt a whopping 40 times faster than tropical forests do in the soil (Fourqurean et al. 2012). This is a big deal because it helps keep the water's pH level stable, which is a measure of how acidic or basic it is. A stable pH is good news for animals with shells or tough outer coverings, like corals and shellfish (think clams and oysters), as it shields them from ocean acidification.
All the ways that seagrass helps out us humans are called "ecosystem services," and these services make seagrass one of the most vital marine ecosystems for our well-being.
Threats to seagrass and what can be done to help
Despite how crucial they are, seagrass meadows worldwide are disappearing pretty fast, at a rate of about 7% every year (Waycott et al. 2009). This loss is often connected to things like building new stuff along the coast, pollution from rivers and streams flowing into the sea, and overfishing.
If we want seagrass to withstand bigger and longer-lasting challenges like climate change, we've got to do some things on the local level. That means improving the quality of the water nearby, making sure we don't mess up seagrass meadows, setting up safe zones for seagrass, cutting back on overfishing, and not letting coastal development stress them out (Cullen-Unsworth and Unsworth 2016b). When we protect seagrass, we're not just looking out for the cool life living there, but we're also doing our part to fight climate change and making sure people have enough to eat from the sea. It might take some big moves to protect and bring back these spots, but if lots of folks take small steps to look out for seagrass, we can give these hidden underwater gardens a brighter future.

References:
Cullen-Unsworth, LC, Jones, BL, Lilley, R and Unsworth, RKF 2018, Secret gardens under the sea: what are seagrass meadows and why are they important?, Frontiers, viewed 17 October 2023, <https://kids.frontiersin.org/articles/10.3389/frym.2018.00002#ref2>.
Cullen-Unsworth, LC and Unsworth, RKF 2016b, 'Strategies to enhance the resilience of the world's seagrass meadows' Journal of Applied Ecology, vol. 53, no. 4, pp. 1 - 6.
Fourqurean, JW, Duarte, CM, Kennedy, H, Marba, N, Holmer, M, Mateo, MA, Apostolaki, ET, Kendrick, GA, Krause-Jensen, D, McGlathery, KJ and Serrano, O 2012, 'Seagrass ecosystems as a globally significant carbon stock', Nature Geoscience, no. 5, pp. 505 - 509.
Reynolds, PL 2018, Seagrass and seagrass beds, Smithsonian, viewed 17 October 2023, <https://ocean.si.edu/ocean-life/plants-algae/seagrass-and-seagrass-beds>.
Waycott, M, Duarte, CM, Carruthers, TJB and Orth, RJ 2009, 'Accelerating loss of seagrass across the globe threatens coastal ecosystems', Proceedings of the National Academy of Sciences, vol. 106, no. 30, pp. 12377 - 12381.
27 notes
·
View notes
Text
Homemade Parodinal Recipe
Makes 150ml developer concentrate for B+W film and paper – enough for many rolls of film, see usage below. Cost: pennies per roll. Typical buy quantities may last a lifetime.
WEAR GLOVES – Caustic Soda burns are nasty! Work outside. It’s easy but be careful.
16 x 500mg tablets paracetamol, crushed (cheapest kind with no added stuff)
20g sodium metabisulphite (ebay, or sold as Camden tablets from brew supply)
19g sodium hydroxide (sold as Caustic soda in hardware stores)
Cooled boiled water (boiling softens and reduces dissolved oxygen), seal while cooling to reduce re-absorption.
1) Dissolve slowly 10g caustic soda in 50ml water. It will get warm. While still warm add the paracetamol, stir and allow to cool (always add soda to water and not the reverse).
2) Dissolve slowly 9g caustic soda in 70ml of water, allow to cool and add metabisulphite.
3) Mix two solutions at room temperature and add water up to 150ml. Decant to a darkened glass bottle that you can exclude air from. I use some well washed and dried discarded small medicine bottles because that’s what was around. Try to have as little air at top as feasible.
4) Allow to 'meld' for at least 72 hours. I gave it a shake now and then, no worries.
There will be crystals that deposit at the bottom of the bottle, those appear to act as oxygen scavengers and should not be removed.
Oh and word of warning - do the mixing in a well ventilated area and nowhere near photo film or paper as sulphur dioxide from camden tablets will fog them. Wear eye and skin protection when mixing, caustic soda burns are nasty. See “Fight Club”... Usage and Dilutions:
You can use like Rodinal developer. The batch you mixed is concentrated. It lasts for decades if well stored. Dilute it with water in various strengths for use. Like Rodinal it gives nice grain with lower ISO films, and pronounced grain, some say ugly even at higher ISO – say 400 and up. Longer development times seem more grainy. You have some control. See: Massive Dev Chart Film Development, Film Developing Database Examples:
1+25, faster, typically ~ 4-5 minutes. eg. 12ml into 300ml water, 150ml batch does 12 rolls of film.
1+50, slightly slower, around ~ 8 minutes but economical. eg. 6ml into 300ml water, gets 25rolls per batch.
1+100, for 60 minute stand development, acts as multi-ISO, grainy. eg. 3ml into 300ml – 50 rolls out of 150ml batch. Yikes.
(pdf printable version available here)
Notes:
This developer is usually made with sodium sulphite but sodium metabisulpite is often more readily available from brew supply shops (and cheaper). From my online reading I believe mixing the latter with sodium hydroxide converts it to the former and that's why it is mixed separately and then combined. In any case I've used the above method and it works fine.
I often fill 5ml syringes with the developer and have on hand for quick and easy measured use, quick mixing and then into film tank. Use old jars for mixing as you don't want this stuff on kitchen food utensils.
The above info was gleened from various online forums and sources. There are youtube videos showing how to make Parodinal as well.
#parodinal#film developer#how to#home made#b+w#bnw#DIY#recipes for the apocalypse#film is not dead#film photography#analog photography
2 notes
·
View notes
Text
With any substrate, things like phosphates, iron, and ammonia come to be gradually and naturally concentrated at the bottom. This is why reddish cyanobacterial blooms happen on the substrate, sometimes, in even marine tanks with perfect water parameters. Its also why the sudden, physical stirring of an established substrate, can rapidly affect the water chemistry as reported by test kits; when 'bad stuff' is immediately sent upwards in the water column, and thus back into solution, because formerly percolated chemicals and particles have been disturbed.
The way in which the saturated substrate has different chemical parameters to the water column up above, means that - for example - the phisphate levels of the aquarium water can suddenly (and negatively) be affected, should the substrate become disturbed, usually by the activities of the aquarist. Needless to say, any such stirring of the substrate must be regular and small scale, for example through the activities of 'live sand' infauna, and those of larger, burrowing ornamental animals, such as shrimp, gobies, echinoderms, and gastropods.
A similar misunderstanding of a substrate involves freshwater tanks, and the fear that 'muck' or detritus in the substrate makes undergravel filtration ineffective. In reality such detritus holds well the beneficial nutrients, that plants require and are able to access with their root systems. Dirty gravel or sand is thus excellent for plant growth, and coarser grains trap more wholesome muck. As with marine tanks, and for the same reason as described above, substrates should not be disturbed large scale if it can be avoided, whether or not an undergravel filter happens to be employed in the tank.
The accumulation of detritus within a gravel or sand substrate, and the processes and products of its decomposition, are (as they say) a rather integral working or feature, and not at all a ‘bug’ to be avoided. Aquaria cannot be spotless, and vacuuming activities should only ever be done lightly, or not at all - and if done at all, then only lightly spruced, and only from the surface of the sand or gravel. It is important that only the surface of the gravel should be disturbed if at all, or its utility for natural biofiltration will be compromised.
Returning to the subject of substrates and filtration; in marine aquariums, but rarely in freshwater tanks, people sometimes advocate what are called 'anoxic plenums' beneath the substrate - the so-called 'Monaco method'. The whole point is that, unlike the workings of undergravel filtration systems, water is not at all pumped through the space, making it a stable volume or 'void space' of water, that is deprived of dissolved oxygen - constituting a plenum. Plenums were once a huge idea in reefkeeping, following the famous aquarium scientist, Jean Jaubert.
However, it seems that the idea of the often imitated Monaco system, is actually based on misunderstood observations of phenomena taking place in aquariums, that have other scientific explanations. Related to the facts and fallacies surrounding the dubious plenum method, is the popular notion that sufficiently deep beds of sand used as a substrate, are in themselves similarly beneficial, thus rendering the creation of a plenum superfluous. Typically associated with marine and especially reef aquaria, these methods have some advocates as regards freshwater tanks.
Where water flow is absent throughout the substrate, there is a risk, however slim, that organic material might end up decomposing deep in the substrate, in a way producing accumulations of bacterial toxins alongside noxious smells - and a possibility that there could be mortalities should this filth be released unto desirable organisms. I have only ever experienced such smells after disturbing a settled substrate, and yet fortunately, there were no mortalities despite a sudden and downward shift in my water quality, that distressed the fish - you should get the picture by now, not to disturb the substrate, wether it is being used as a filter medium or not.
Now returning to the subject of plants in freshwater aquaria. Vascular plants (or tracheophytes) access nutrients via their roots, and natural selection has furnished different plants with different root systems for this purpose. Plants such as Echinodorus and Cryptocoryne are heavy root feeders, equipped with long and deeply penetrating roots, Such plants do better when they are planted in gravel than on sand, because gravel traps detritus better. A reverse flow variant of the undergravel system, would actually help to bring nutrients to the living and feeding plant roots.
#substrate#water chemistry#undergravel filtration#biofiltration#planted tanks#aquarium plants#cyanobacteria#detritus#deep sand beds#plenums#monaco method#gravel vacuums#decomposition
1 note
·
View note
Text
Hey guys, just so you know, the orange stuff called sunstone here may not be a rock. There's an artisanal glass known by so many names it's difficult to keep track of. Goldstone, sunstone, sandstone, aventurine, and likely others. It's made using dissolved copper in a low oxygen atmosphere, and the copper is reduced and precipitated into the glass. Very pretty. Not a stone.
"cat's eye stone" and "moonstone" are both poorly regulated terms which in turn could refer either to a type of glass or an actual stone.
Don't buy brown diamonds called "chocolate diamonds." Not because they're bad or anything, but because they're not nearly as valuable as the company that trademarks that term will tell you they are. If you buy "brown diamonds" you'll get them cheaper.
Tiger's eye is brown to orange. There is a blue variety. If the stone has multiple colours, it's dyed. They'll all have some amount of colour play, but there shouldn't be purple, green, and blue. Not naturally, anyway. Tiger's eye can also be bleached.
Don't go swimming with malachite
Don't wear fluorite as a ring or pretty much anything that can get scratched or dropped or hit.
Mystic topaz, mystic quartz, azotic topaz, etc. are treated stones, having been coated on at least one side with a thin layer of tin or titanium oxide. They're not unnatural per se, but they don't naturally look like that.
I'm not against any of these gemstones by the way, just deceptive marketing practices. Wear what you like!
*except copper bearing minerals like azurite and malachite. Be sure they're sealed, and keep them out of prolonged contact with water. Fluorite is harder than your teeth, but not much else. It will break if you hit it too hard, and sometimes just dropping it is enough. Beware of cleavage planes.
Also, beware of black coral jewelry. It's made using an endangered species of coral, so make sure that any jewelry that uses it is either an antique or recycled.

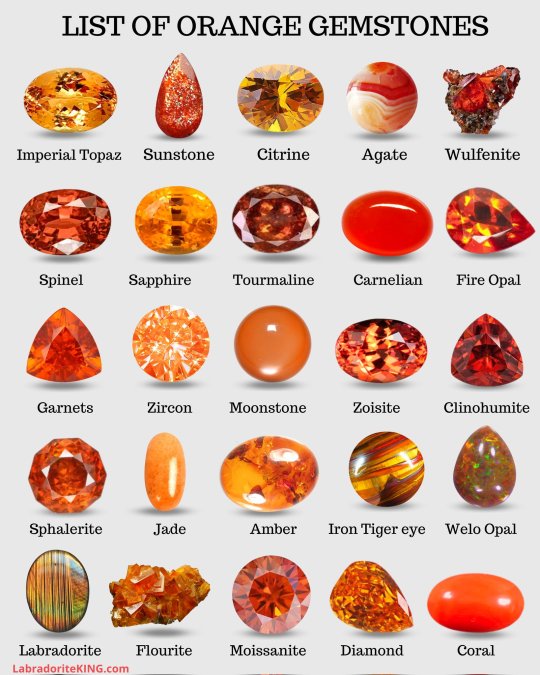


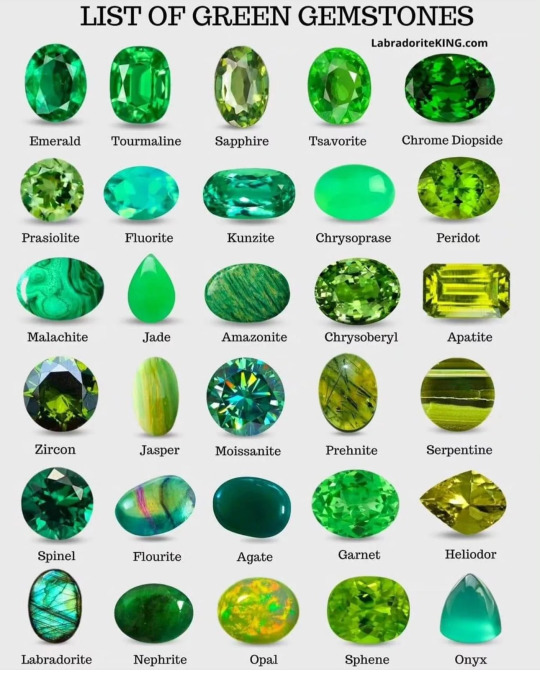

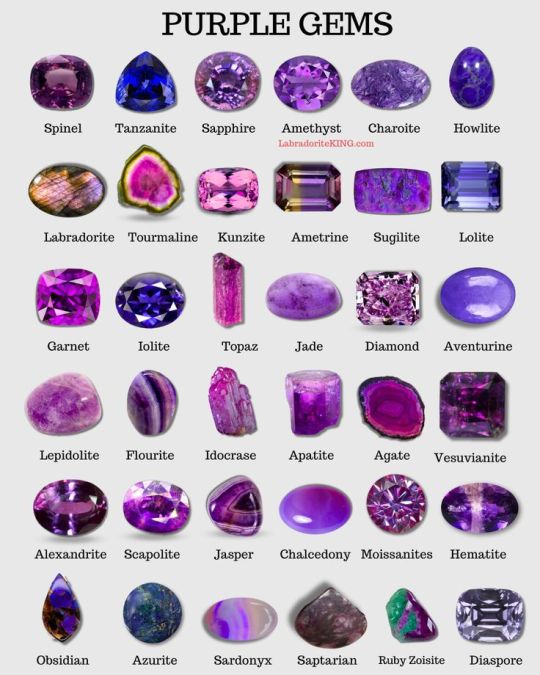





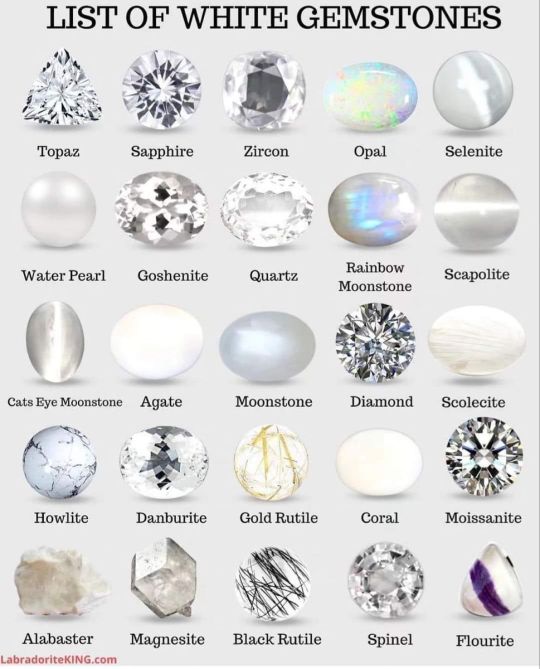



By LabradoriteKing on Pinterest
#jewelry#gemology#conservation#marketing#do your homework#ask questions like#where did this stone come from?#do you know your supplier?#what is the airspeed of an unladen swallow#is this safe for everyday wear?#is this stone treated?#is this a naturally occurring stone?#can this stone be heated?#will you be here in three months if I have any issues?#and stuff like that#.
162K notes
·
View notes
Note
hello i have a question
when the minerals are pokey like for example the kyanite you showed for round 2
do those pokeys be growing out right into the air? or, if they grow into other bits of rock, what is the technique that is used to get rid of the other rock bits to preserve the pokeys?
hope this makes sense and thanks for your cool poll
Oh this is such a fantastic question!!!!
This got away from me so
TL;DR:
Sometimes pokey minerals form into the air (or into water with minerals dissolved in it). These can be minerals like calcite, quartz, or halite.
Minerals also form pokey crystals because their microscopic crystalline structure looks pokey (not really but kinda) and it tells crystals to look pokey on a scale we can see.
When those pokey minerals are formed via recrystallization of material during the metamorphosis of rocks, they will form pokey crystals within the rock itself, no empty space required. This is how it works for kyanite.
That surrounding rock is often worn away to reveal the crystals, if needed.
Many, many more details under the cut.
Sometimes pokey minerals form into the air (or into water with minerals dissolved in it). These can be minerals like calcite, quartz, or halite.
So, yeah, often (but certainly not always) in order to form large crystals of any shape (especially pokey crystals), you need empty space.
So, for example, geodes. Those are rocks with hollow space in them, but they usually have some way for, like, groundwater to get in. And that water has minerals dissolved in it that crystallize out to form the pokey crystals inside a geode.
If you've ever stuck a string in salt water and had salt crystallize on the string, that is the same process of minerals precipitating out of the solution. Stalagmites and stalactites also form from minerals crystallizing out of water (in this case the water drips, like icicles).
If there wasn't a lot of space in the geode, those crystals would (eventually) grow into each other. So they wouldn't be pokey anymore, because they would just be a mass of crystals that grew into each other's space.
Quartz crystals can form this way, as do carbonate minerals like calcite, halide minerals like fluorite or halite, and sulfate minerals like gypsum, barite, celestine, and chalcanthite.
When pointy minerals are formed via recrystallization of material during the metamorphosis of rocks, they will form pointy crystals within rock.
Kyanite is special because it is a metamorphic mineral. Therefore, it grows as rocks are being metamorphosed into other rocks. This has always been something that is difficult for me to wrap my head around, but it's also why I love metamorphic minerals so much (like garnet)!
Metamorphic rocks start as igneous or sedimentary rocks that are put under extreme temperatures and pressures. They can't melt (if the rocks melted from high temperatures, they would be igneous rocks) but they can recrystallize. Which is exactly what they do.
As the rock is being smushed and superheated beneath the ground somewhere, the stuff (as in elements) that makes up the rock, is changing configuration.
In the case of kyanite, it forms when an aluminum-rich rock gets put under high temperatures and pressures. The aluminum (and silicon and oxygen) in the rock re-bond and re-crystallize and form the mineral kyanite. So this is one case where pointy crystals do NOT grow into the air. They grow within the rock as it is getting all messed up from high temperatures and pressures.
Minerals form pointy crystals (even within rocks) because their microscopic crystalline structure looks pointy (kinda) and it tells crystals to look like that on a scale we can see.

Above is a picture of kyanite in a schist. As you can hopefully see, kyanite still has its bladed shape despite being grown within the rock. That's because crystal habits (the shape we see) are directly tied to the crystalline structure of the mineral on the microscopic scale. So the crystal structure of kyanite (triclinic) means that it forms bladed (or tabular, but that's unimportant to this issue) crystals.
For example, halite forms cubes, because the sodium and chlorine ions that make up the mineral bond and form cubes at the microscopic scale. So they (often) form squares that we can see.

That surrounding rock is often worn away to reveal the crystals, if needed.
Now this, of course, leads to your other question about getting rid of the other material to get the pokey crystals. I'm not trained in mineral excavation, but I know a little bit from working at a museum and hearing the museum's curator talk about certain specimens.
From my understanding, a lot of the time you just chip or wear away at the other material because it is softer and easier to wear away at than the mineral itself.
For kyanite specifically, it can be found in schist. Schist is often made up of a lot of mica. Micas (like muscovite and lepidolite in the poll) have a hardness of 2-3, which is super low. If you have hard fingernails, you might be able to scratch certain micas with your finger.
Meanwhile, metamorphic minerals like kyanite have a comparatively higher hardness when compared to the material it might've grown in.
So, as far as I've learned, it could be feasible for some samples, to just chip at the softer material, and the harder material (kyanite crystals) will remain because they're harder to chip away at!
That is how I was told this garnet specimen got to look the way it does. The garnets form those giant dodecahedra, and the schist that they grew in was worn away to reveal them.

I hope all of this makes sense, and if it doesn't, please feel free to ask more questions, and I'll do my absolute best to clarify. The whole "minerals recrystallizing while rocks are being metamorphosed" thing gets kind of weird, and I'm not sure if I explained it as well as I could.
#anon#ask#i was going to write some fanfic these evening but then i saw the notification for this ask and POUNCED#i hope this is clear i know there's a lot of information#i wanted the answer to be thorough but i realized it's also confusing so that's why i did the tldr
27 notes
·
View notes
Text
Extracts from Alan Weisman, The World Without Us, 2007. The book considers the material aspects of human civilization and how long they would last, unattended. If humans were to vanish from Earth, if all maintainance and repairing work ceased, what would happen to what we leave behind?
(The book went on to inspire two speculative documentaries, Life After People by History Channel and Aftermath: Population Zero by National Geographic, emphasizing different aspects of it. They were neat.)
Chapter 2: Unbuilding Our Home
No matter how hermetically you’ve sealed your temperature-tuned interior from the weather, invisible spores penetrate anyway, exploding in sudden outbursts of mold—awful when you see it, worse when you don’t, because it’s hidden behind a painted wall, munching paper sandwiches of gypsum board, rotting studs and floor joists. Or you’ve been colonized by termites, carpenter ants, roaches, hornets, even small mammals.
Most of all, though, you are beset by what in other contexts is the veritable stuff of life: water... moisture enters around the nails. Soon they’re rusting, and their grip begins to loosen... As gravity increases tension on the trusses, the ¼-inch pins securing their now-rusting connector plates pull free from the wet wood, which now sports a fuzzy coating of greenish mold... When the heat went off, pipes burst if you lived where it freezes, and rain is blowing in where windows have cracked from bird collisions and the stress of sagging walls. Even where the glass is still intact, rain and snow mysteriously, inexorably work their way under sills. As the wood continues to rot, trusses start to collapse against each other. Eventually the walls lean to one side, and finally the roof falls in...
While all that disaster was unfolding, squirrels, raccoons, and lizards have been inside, chewing nest holes in the drywall, even as woodpeckers rammed their way through from the other direction... Fallen vinyl siding, whose color began to fade early, is now brittle and cracking as its plasticizers degenerate. The aluminum is in better shape, but salts in water pooling on its surface slowly eat little pits that leave a grainy white coating... Unprotected thin sheet steel disintegrates in a few years. Long before that, the water-soluble gypsum in the sheetrock has washed back into the earth. That leaves the chimney, where all the trouble began. After a century, it’s still standing, but its bricks have begun to drop and break as, little by little, its lime mortar, exposed to temperature swings, crumbles and powders.
If you owned a swimming pool, it’s now a planter box... If the house’s foundation involved a basement, it too is filling with soil and plant life. Brambles and wild grapevines are snaking around steel gas pipes, which will rust away before another century goes by. White plastic PVC plumbing has yellowed and thinned on the side exposed to the light, where its chloride is weathering to hydrochloric acid, dissolving itself and its polyvinyl partners. Only the bathroom tile, the chemical properties of its fired ceramic not unlike those of fossils, is relatively unchanged, although it now lies in a pile mixed with leaf litter.
After 500 years, what is left depends on where in the world you lived. If the climate was temperate, a forest stands in place of a suburb; minus a few hills, it’s begun to resemble what it was before developers, or the farmers they expropriated, first saw it. Amid the trees, half-concealed by a spreading understory, lie aluminum dishwasher parts and stainless steel cookware, their plastic handles splitting but still solid... The chromium alloys that give stainless steel its resilience... will probably continue to do so for millennia, especially if the pots, pans, and carbon-tempered cutlery are buried out of the reach of atmospheric oxygen. One hundred thousand years hence, the intellectual development of whatever creature digs them up might be kicked abruptly to a higher evolutionary plane by the discovery of ready-made tools...
If you were a desert dweller, the plastic components of modern life flake and peel away faster, as polymer chains crack under an ultraviolet barrage of daily sunshine. With less moisture, wood lasts longer there, though any metal in contact with salty desert soils will corrode more quickly. Still, from Roman ruins we can guess that thick cast iron will be around well into the future’s archaeological record, so the odd prospect of fire hydrants sprouting amidst cacti may someday be among the few clues that humanity was here...
In a warmer world... drier, hotter desert climates will be complemented by wetter, stormier mountain weather systems that will send floods roaring downstream, overwhelming dams, spreading over their former alluvial plains, and entombing whatever was built there in annual layers of silt. Within them, fire hydrants, truck tires, shattered plate glass, condominia, and office buildings may remain indefinitely, but as far from sight as the Carboniferous Formation once was.
No memorial will mark their burial, though the roots of cottonwoods, willows, and palms may occasionally make note of their presence. Only eons later, when old mountains have worn away and new ones risen, will young streams cutting fresh canyons through sediments reveal what once, briefly, went on here.
***
Chapter 3: The City Without Us
Under New York, groundwater is always rising… Whenever it rains hard, sewers clog with storm debris… With subway pumps stilled… water would start sluicing away soil under the pavement. Before long, streets start to crater. With no one unclogging sewers, some new watercourses form on the surface… Within 20 years, the water-soaked steel columns that support the street above the East Side’s 4, 5, and 6 trains corrode and buckle. As Lexington Avenue caves in, it becomes a river.
Whenever it is, the repeated freezing and thawing make asphalt and cement split. When snow thaws, water seeps into these fresh cracks. When it freezes, the water expands, and cracks widen… As pavement separates, weeds like mustard, shamrock, and goosegrass blow in from Central Park and work their way down the new cracks, which widen further… The weeds are followed by the city’s most prolific exotic species, the Chinese ailanthus tree… As soil long trapped beneath pavement gets exposed to sun and rain, other species jump in, and soon leaf litter adds to the rising piles of debris clogging the sewer grates.
The early pioneer plants won’t even have to wait for the pavement to fall apart. Starting from the mulch collecting in gutters, a layer of soil will start forming atop New York’s sterile hard shell, and seedlings will sprout…
In the first few years with no heat, pipes burst all over town, the freeze-thaw cycle moves indoors, and things start to seriously deteriorate. Buildings groan as their innards expand and contract; joints between walls and rooflines separate. Where they do, rain leaks in, bolts rust, and facing pops off, exposing insulation. If the city hasn’t burned yet, it will now… with no firemen to answer the call, a dry lightning strike that ignites a decade of dead branches and leaves piling up in Central Park will spread flames through the streets. Within two decades, lightning rods have begun to rust and snap, and roof fires leap among buildings, entering paneled offices filled with paper fuel. Gas lines ignite with a rush of flames that blows out windows. Rain and snow blow in, and soon even poured concrete floors are freezing, thawing, and starting to buckle. Burnt insulation and charred wood add nutrients to Manhattan’s growing soil cap. Native Virginia creeper and poison ivy claw at walls covered with lichens, which thrive in the absence of air pollution. Red-tailed hawks and peregrine falcons nest in increasingly skeletal high-rise structures.
Within two centuries… colonizing trees will have substantially replaced pioneer weeds. Gutters buried under tons of leaf litter provide new, fertile ground for native oaks and maples from city parks. Arriving black locust and autumn olive shrubs fix nitrogen, allowing sunflowers, bluestem, and white snakeroot to move in along with apple trees, their seeds expelled by proliferating birds… as buildings tumble and smash into each other, and lime from crushed concrete raises soil pH, inviting in trees, such as buckthorn and birch, that need less-acidic environments…
In a future that portends stronger and more-frequent hurricanes striking North America’s Atlantic coast, ferocious winds will pummel tall, unsteady structures. Some will topple, knocking down others. Like a gap in the forest when a giant tree falls, new growth will rush in. Gradually, the asphalt jungle will give way to a real one.
***
Chapter 7: What Falls Apart
(context: this chapter describes Varosha, a city in Cyprus evacuated in 1974 after the Turkish invasion, and left abandoned until 2019)
[Two years after abandonment] Asphalt and pavement had cracked… Australian wattles, a fast-growing acacia species used by hotels for landscaping, were popping out midstreet, some nearly three feet high. Creepers from ornamental succulents snaked out of hotel gardens, crossing roads and climbing tree trunks… Concussions from Turkish air force bombs, Cavinder saw, had exploded plate-glass store windows. Boutique mannequins were half-clothed, their imported fabrics flapping in tattered strips…
Pigeon droppings coated everything. Carob rats nested in hotel rooms, living off Yaffa oranges and lemons from former citrus groves… The bell towers of Greek churches were spattered with the blood and feces of hanging bats.
Sheets of sand blew across avenues and covered floors… Now, no bands, just the incessant kneading of the seathat no longer soothed. The wind sighing through open windows became a whine. The cooing of pigeons grew deafening.
Varosha, merely 60 miles from Syria and Lebanon, is too balmy for a freeze-thaw cycle, but its pavement was tossed asunder anyway. The wrecking crews weren’t just trees, Münir marveled, but also flowers. Tiny seeds of wild Cyprus cyclamen had wedged into cracks, germinated, and heaved aside entire slabs of cement…
Two more decades passed… Its encircling fence and barbed wire are now uniformly rusted, but there is nothing left to protect but ghosts. An occasional Coca Cola sign and broadsides posting nightclubs’ cover charges hang on doorways… Fallen limestone facing lies in pieces. Hunks of wall have dropped from buildings to reveal empty rooms… brick-shaped gaps show where mortar has already dissolved. Other than the back-and-forth of pigeons, all that moves is the creaky rotor of one last functioning windmill.
In the meantime, nature continues its reclamation project. Feral geraniums and philodendrons emerge from missing roofs and pour down exterior walls. Flame trees, chinaberries, and thickets of hibiscus, oleander, and passion lilac sprout from nooks where indoors and outdoors now blend. Houses disappear under magenta mounds of bougainvillaea. Lizards and whip snakes skitter through stands of wild asparagus, prickly pear, and six-foot grasses. A spreading ground cover of lemon grass sweetens the air. At night, the darkened beachfront, free of moonlight bathers, crawls with nesting loggerhead and green sea turtles.
***
Chapter 10: The Petro Patch
If, in the immediate aftermath of Homo sapiens petrolerus, the tanks and towers of the Texas petrochemical patch all detonated together in one spectacular roar, after the oily smoke cleared, there would remain melted roads, twisted pipe, crumpled sheathing, and crumbled concrete. White-hot incandescence would have jump-started the corrosion of scrap metals in the salt air, and the polymer chains in hydrocarbon residues would likewise have cracked into smaller, more digestible lengths, hastening biodegradation. Despite the expelled toxins, the soils would also be enriched with burnt carbon, and after a year of rains switchgrass would be growing. A few hardy wildflowers would appear. Gradually, life would resume.
Or, if the faith of Valero Energy’s Fred Newhouse in system safeguards proves warranted—or if the departing oilmen’s last loyal act is to depressurize towers and bank the fires—the disappearance of Texas’s world champion petroleum infrastructure will proceed more slowly. During the first few years, the paint that slows corrosion will go. Over the next two decades, all the storage tanks will exceed their life spans. Soil moisture, rain, salt, and Texas wind will loosen their grip until they leak. Any heavy crude will have hardened by then; weather will crack it, and bugs will eventually eat it.
What liquid fuels that haven’t already evaporated will soak into the ground. When they hit the water table, they’ll float on top because oil is lighter than water. Microbes will find them, realize that they were once only plant life, too, and gradually adapt to eat them. Armadillos will return to burrow in the cleansed soil, among the rotting remains of buried pipe.
Unattended oil drums, pumps, pipes, towers, valves, and bolts will deteriorate at the weakest points, their joints… Until they go, collapsing the metal walls, pigeons that already love to nest atop refinery towers will speed the corruption of carbon steel with their guano, and rattlesnakes will nest in the vacant structures below. As beavers dam the streams that trickle into Galveston Bay, some areas will flood. Houston is generally too warm for a freeze-thaw cycle, but its deltaic clay soils undergo formidable swell-shrink bouts as rains come and go. With no more foundation repairmen to shore up the cracks, in less than a century downtown buildings will start leaning.
… When oil, gas, or groundwater is pumped from beneath the surface, land settles into the space it occupied… Lower the land, raise the seas, add hurricanes far stronger than midsize, Category 3 Alicia, and even before its dams go, the Brazos gets to do again what it did for 80,000 years: like its sister to the east, the Mississippi, it will flood its entire delta… flare towers, catalytic crackers, and fractionating columns, like downtown Houston buildings, will poke out of brackish floodwaters, their foundations rotting while they wait for the waters to recede.
… Below the surface, the oxidizing metal parts of chemical alley will provide a place for Galveston oysters to attach. Silt and oyster shells will slowly bury them, and will then be buried themselves. Within a few million years, enough layers will amass to compress shells into limestone, which will bear an odd, intermittent rusty streak flecked with sparkling traces of nickel, molybdenum, niobium, and chromium. Millions of years after that, someone or something might have the knowledge and tools to recognize the signal of stainless steel. Nothing, however, will remain to suggest that its original form once stood tall over a place called Texas, and breathed fire into the sky.
I cannot really describe the feeling I get from reading these portions in particular, only that it’s the strongest I ever got from any book. It’s certainly not one of joy: I don’t want humans to disappear -- in fact, there are a lot of humans among my family and friends -- and I don’t want human civilization to vanish, after the unspeakable effort it took to put together, with all the promise that, despite everything, it shows. It’s not one of sadness or fear, either. I suppose it’s just one of awe, of terrible grandeur, similar in kind to what I feel when considering the alien horror and beauty of evolved life, its sheer multi-layered complexity, or the unthinkable vastness of geological time.
17 notes
·
View notes
Text
So I think what you're referring to is water's status as a "universal solvent" - that is, it's an extremely common and powerful ionizing solvent (i.e. it dissolves substances by virtue of its highly polar nature, with the oxygen with its electron abundance from the hydrogens being happy to be an electron donor, and the hydrogens, having had their electrons pulled back towards the oxygen, are happy to be an electron acceptor, creating what are known as weak hydrogen bonds).
Notably, this classification as an ionizing solvent is not unique: a bunch of stuff such as ethanol and ammonia work the same way, by serving as electron donors or acceptors stronger than whatever else the solute might want to be attached to. Note also that not all ionizing solvents do both accepting and donating - acetone, for example, is polar, but solely accepts protons due to not having any isolated donor sites, and this specificity can be very useful in some contexts.
This doesn't get into what you can do about dissolving nonpolar substances (short: use a nonpolar solvent that is all about nonpolar bonds where everyone involved is vaguely electronegative), or acids (these act through stronger bonding with donation of H+ or accepting a full covalent bond), but.
So yeah. Water as a solvent is certainly not unique in its mechanisms, but it is highly available, and because of that, there's a lot of "the puddle notes how this pothole was made for them" (Douglas Adams) where mechanisms important for life that pop up with common compounds Just Work because of the specific level of polarity of water. Which is still sick as hell, it's awesome that something this willing to open up and glom onto most things is just. Everywhere.
we should talk about water more often that shit is crazy
134K notes
·
View notes
Note
I'm not sure if you know anything about betta fish but if you do I could really use your help. I got one for college and I'm very scared I'm going to kill him, as I had lots of bad luck as a kid with my fish. I'm very paranoid about anything that might be going wrong with him. I know I don't have all the materials right now to care for him but it'll be a couple of days before I can get to a store. Should I be worried he's spending almost all his time near the top of the tank? In addition, my tank has lots of white algae. Is that an immediate threat to his health?
Is it actually algae (Textured to the touch) or is it a clearish-white slime? If it's slime, it's fine, that can happen in new tanks and it should go away on its own, if it's algae you should remove as much of it as you can mechanically, scrub everything down, and potentially treat it with an anti-algae treatment. HOWEVER if it's algae, that's usually a sign there's something wrong with the tank care; too much light, too much excess food, water parameters are off, etc. So you'll want to correct for those factors as well, as those could definitely be an immediate threat to his health.
Bettas being near the top of their tank is not the same as other fish being near the top of their tank. Bettas have an organ called a labyrinth organ, which allows them to process air-air instead of just oxygen dissolved in the water. Normal fish at the top are gasping for air because their water is not oxygenated well enough. Bettas just actually breathe some air and often hang out at the surface where the air is. This is why, for a very long time, people believed they were "just fine" in vases and other tiny, unheated, still-water "tanks." They are not- they should have a minimum of 5 gallons, with proper filtration and heat. Preferably with substrate, and decor for enrichment.
And this is not going to be what you want to hear, and please know I'm saying this on the behalf of the fishes, but typically when it comes to fish, the tank and other setup should come long before the fish. I don't know the circumstances of why you acquired the fish before the tank, and I'm going to assume in good faith that it couldn't be helped in this case, but in the future if it can be avoided, it should be avoided, as that will go a long way toward protecting your fish. Tanks need a minimum of a week to even start to cycle and grow good bacteria to process ammonia and handle the nitrate/nitrite cycle that maintains the water quality within your tank and prevents your fish from dying from waste issues. In reality, the cycling process can take upwards of a month, sometimes even two, to get going properly and stabilized from a brand new tank. Bettas are pretty resilient little dudes a lot of the time, but they shouldn't have to be. Their tanks should be cycled and stable long before they come home to be the final addition to it.
I hope that you're able to get him all the stuff he needs quickly, and that he makes it through the cycling process when you get his new tank set up. Good luck with him!
43 notes
·
View notes
Text
Interesting things I learned while I was checking Rondo's wiki page.
Their name, Rondo, in japanese is made up of three kanjis (燐舞曲).The first kanji (燐) means phosphorus, the second one (舞) means dance, and the third and final kanji (曲) means song.
I didn't know what 'phosphorus' meant, so I searched it up and found out that it was a chemical element.
I was confused, why a chemical element? So I checked Wikipedia and apparently phosphorus is derived from the Greek word, Φωσφόρος, which means 'light bearer' which refers to the 'Morning star' aka Venus. (In Latin it's translated as Lucifer because of Bible stuff)
There's also this phenomenon called 'phosphorescence' where it absorbs the light and reemits it with a longer wave length. (It's kind of like fluorescence, but it doesn't light up when under a black light.) Even though the actual thing happening with the chemical element it's named after is 'chemiluminescence'. (Which is the emission of light as the result of a chemical reaction.)
Then I remembered that Nagisa's last name is Tsukimiyama. To be specific, the 'Tsuki' part meaning 'moon', the moon is the second brightest thing in the Earth's sky, the first being Venus. It's like how Nagisa already stands out with her guitar and playing alone, but with Rondo, she stands out even more.
Also, there's the fact that in Latin, phosphorus means 'Lucifer' and Satan used be called Lucifer in the Bible until his hubris got the best of him, also there's the fact that Tsubaki used to go to church and the whole everything of prayer and prayer[s].
It already sounds interesting to me, so I read about the white phosphorus' characteristics and this is when everything clicks.
In Hiiro's ten question interview thing she said that 'Aoi has passion, Tsubaki sets it ablaze, and Nagisa stirs up the wind.'
As I read through the Wikipedia, it said that phosphorous, when it comes into contact with air, becomes highly flammable and pyrophoric (self-igniting). Also, when it's exposed to oxygen, it can glow a faint blue or green light and what's Rondo's signature color? Blue.
White phosphorus also can't dissolve in water, but can dissolve in carbon disulfide.
Which unit is connected to water?
Merm4id.
Even in the unit names of Merm4id and Rondo, them being polar opposites is still there! Which is sooooo cool! The thought put into these kinds of things is crazy.
I got a cool science lesson thanks to my favorite unit, thanks Rondo! :D
#crow talks#d4dj groovy mix#d4dj#some people probably already know this and im just stupid but eh whatever#learned a cool science fact and some mythology stuff#which is pretty cool in my book#now the thing i want to know now is how tf did aoi know abt this?#like. while she was making it up did she thought of the name and wondered how to spell it in kanji and went down a science rabbit hole?#like i know that bushiroad is the one who made up the unit names and stuff but from an in-game universe stand point?#how???#instead of me working on my major or my aa au post i just work on this? wow.#u know i have my priorities straight
10 notes
·
View notes
Text
Animal Crossing Fish - Explained #206
Brought to you by a marine biologist and another fresh fellow...
CLICK HERE FOR THE AC FISH EXPLAINED MASTERPOST!
Aquarium fish have a genetic history similar to dogs - most species in the aquarium trade have been bred for fancier body shapes and brighter colors, and sometimes completely different patterns from their wild counterparts. No one knows this better than the humble goldfish or its cousin the koi, but we have covered lots of AC freshwater fish that were *clearly* a domesticated form running feral in the rivers of your island. Today, I present another such friend - the gourami.

The gourami first appeared in Pocket Camp in Fishing Tourney 26 in May 2020, and then actually came back for a full spring season in 2021. Gourami are native to South and Southeast Asia, so makes sense they prefer warmer water (although if I were to be super picky, I’d point out that anywhere that gets snow still has *frigid* water temperatures in the spring thanks to snow melt, but I digress! I’m not actively trying to poke holes in Animal Crossing’s choices when it looks like they thought about it a little in goddamn Pocket Camp!)
“Gourami” is actually the name of an entire family - Osphronemidae. They are nestled with the Order Anabantiformes, and more specifically the SubOrder Anabantoidei, also known as the Labyrinth Fish. We covered fish in this family almost a hundred entries ago when we talked about the Betta, so to review:
Labyrinth fish have what is called the “labyrinth organ” which acts like another version of “lung”, allowing the fish to gulp and use air when their still-water habitats become hypoxic or anoxic, aka, very low in oxygen/has virtually no oxygen dissolved in it. Lots of freshwater fish have to deal with this major issue in their habitats, so there are a plethora of strategies fish have evolved to combat it, including *actual lungs* (which then helped lead the tetrapods further onto land). So, you can think of the labyrinth organ as another version of sack-air-breathing that we could have been using but lungs eventually won out as the equipment of choice for tetrapods. Natural history is fascinating.
The Betta is actually included in Osphronemidae with the other gouramis - they are very closely related. The two fish belong in two separate SubFamilies. The gourami ACPC chose appears to be a domesticated version of the Honey Gourami (Trichogaster chuna).

^So, this is generally what they look like in the wild - males, of course, have bold colors to attract females. In this case, he has a dark bluish-black underside contrasted with orange and yellow on top. Of course, humans saw this and were like “ Ya know what this guy needs? More YELLOW.” And then we made this:

Luckily for the gourami, it seems we’re totally okay with their body shape so far. Species of gourami can get generally large (and I mean in like, the 2 to 4 inch range) and like their cousins the Bettas, some species and individuals can be aggressive to tankmates.
Now, the most noticeable trait of the gourami are its elongated pelvic fins that look like thin tentacles that they kinda use like tentacles. Because they live in murky freshwater habitats, those long fins act like antenna, helping the fish to feel around its environment through touch when it can’t see.
And there you have it. Fascinating stuff, no?
#gourami#honey gourami#fish#freshwater#animal crossing#animal crossing pocket camp#acpc#science in video games#animal crossing fish explained
9 notes
·
View notes
Text
@ifritdiezel replied to your post “By the early 20th century, the field had expanded to include "bogology", "water studies", "water lore", "geobiology", "geology",...”
could you explain water lore? maybe you have a chart with some basics?
Sure! Water is the medium for life. It supports and sustains all living things. It can be divided into two major types: "terrestrial" water, found on the earth's surface, and "marine" water, found in the oceans and rivers. Water in its free state (so-called "brine") has a specific gravity of 1.0, so it's not too dense to hold liquids and gases under pressure. This makes it a good medium for transporting substances and gases through porous objects such as rocks and plants.
Water is a fluid that can flow easily because of its low viscosity, meaning it flows easily through tubes, channels, and around obstacles. (Viscosity describes the resistance of a liquid to flow, like friction -- one way of thinking of it is the "stiffness" of the liquid.) Water also has a small boiling point -- in a vacuum, the temperature where the water will vaporize into steam. This allows water to be the medium through which a great deal of heat energy can be transported, which is especially important in the sun's energy cycle.
It also has large amounts of hydrogen and oxygen and no heavy elements, which means that many substances are soluble (easily-dissolvable) in it. It is very abundant, so it is widely used in industrial processes and is a very common source of water in our world. (The Earth's oceans and land areas have a total mass of about 1000 times that of the atmosphere.)
(Also, in case this is not clear, "water" does not necessarily mean just the stuff that's on our planet. There's also "terrestrial water," which is water on the other planets, and "water lore" (or water religion), but those don't have much to do with the usual ideas of "water.")
6 notes
·
View notes
Note
*asks about job*
Anon you are my favorite.
Okay, so, I'm a laboratory and field technician in a soil and water science lab.
I love my job so goddamn much. I get to work with fancy machines like the gas chromatograph and spectrometer! (The spectrometer is actually really boring but shhh.) I get to go outside and watch birds while I collect water samples! I am getting paid for this! I get to learn data processing and engineering and wilderness safety precautions and how to explain complex science to people whose careers are Not This.
Also, research labs are chaos and I delight in it. Okay maybe not ALL research labs, I'm informed the chemistry department does not look like this. BUT this is an environmental science lab. It is full is bizarre, deeply nerdy, deeply passionate people. Who do things like eat baked potatoes like apples, improvise experimental setups with mason jars and duct tape, and nickname every instrument either a human name or a Pixar reference. I love them so much I have no words.
Crazy and fun things I've done for this job:
Freezer jenga followed by freezer tetris (had to take all the things out of a the freezer, put them in coolers to keep them cold, defrost the freezer, and put them back in except organized this time. I was delighted by this for no logical reason, my boss thought I was nuts).
Okay you know in scifi movies where they have some weird mystery substance and they put it in a box with gloves attatched so they can work with it without actually touching it? I've done that! Not because of hazardous substances, we just needed to put stuff in jars without exposing it to oxygen. But still! It was cool!
Shopping trip to get food for like half a dozen people for three days (I had weird dreams about being overwhelmed with tortilla chips afterwards, this doesn't sound that crazy but I promise you it felt like it).
Taped plastic tubing to 200+ funels until the boxes we were storing them in overflowed and there was no longer floorspace to walk (AFTER cutting the plastic tubing into 200+ equally sized pieces and stuffing it with ion exchange resin, which is like evil microplastic sand. Between all those things, this took WEEKS. It got really boring).
Dissolved like 10kg of KCl (KCl my behated, its very harmless but hell to get off glassware) in water to make 80 LITERS OF KCL SOLUTION (that's over 20 pounds of solid KCl and over 20 gallons of solution! My coworker and I were sort of laughing hysterically over this entire process because come on! 80 liters! For reference most lab protocols need like, a liter or less of whatever solution.) Fun fact about solid KCl, it tends to stick together into a giant brick. We were chiseling at it with scoops, spoons, whatever was on hand (i really wanted to attack it with a screwdriver but it would introduce dirt into the chemicals so i couldn't) and eventually we got so frustrated we went outside and dropped the thing off a second floor balcony. After wrapping it in like 3 layers of plastic bags because we knew at least one bag was gonna break. This did not actually help much but it was very cathartic.
There was a project once where we had to take sealed mason jars and replace all the air in them with nitrogen gas. Repeatedly. For over a hundred jars. My PI (principle invesitgator, means the scientist in charge of a project and usually a lab) is good at building things, so of course he assembled this manifold thing so we could pump nitrogen through a dozen jars at once. Which was great, except it involved two dozen needles, half of them attatched to flexible plastic tubing so they'd kinda bounce around when you pulled them out of the jars. It looked like a very stabby centipede-slash-octopus monster. Impressively, we only stabbed ourselves a couple times each with this thing (and changed the needles of course, we are aware of the risks of transmitting blood diseases).
Actually one of the craziest things about this job in my opinion is how many fucking needles we work with. You see, we study atmospheric gases. And to do that, we need to transfer gases between sealed containers, which means needles and septa (the rubber things they put on vials so you can poke needles through them). So. Many. Needles. Did you know you can only use a needle four times before it gets too dull? It's extremely noticeable as you're using them - not as they get dull, but when you discard an old needle and get out a new one it is a huge difference. I don't know why I find this so fascinating, but working with needles is honestly so fun. I feel like a mad scientist or something. Also, for the first couple months I kept poking myself on accident so I was just walking around with these pinpricks and papercut looking wounds. It felt a bit like a badge of honor, somehow, like a rite of passage for working in the gas lab. Another thing about needles, if you get scratched with one horizontally instead of stabbed, they look like papercuts. It's weird. Also weird is how good you get after a while at not stabbing yourself.
I think I like working with needles because they're something that used to make me nervous. Not horribly, but I have more than typical anxiety and I get nervous about everything. And yet I am now totally chill about needles, because I work with them all the time. It's... freeing I guess. Maybe empowering, even. I am scared of so many things, but I am not scared of this. Ditto large quantities of acid, once you've had to work with dozens of liters of the stuff you stop being scared of it - this was for the same project as the KCl and yes it was equally ridiculous. Dilute acid, thankfully, but to make dilute acid you have to mix the really concentrated stuff with water. It does not come as dilute acid, that would be too easy. So we spent multiple days in a row diluting acid and soaking things in it, there were plastic boxes full of the stuff on every available counter space with handmade warning labels, it was A Thing™️.
Anyways, I'm a person who's scared of everything, except weird stuff like hydrochloric acid, needles, and wasps. I can blame all three of those things on this job, which I love dearly. I love to learn new things, pretend I'm in a scifi movie, be surrounded by crazy people (affectionate), and apparently overcome my numerous fears. You absolutely did not sign up for this big puddle of feelings, anon, but thank you for inspiring it nevertheless.
#something something personal growth and overcoming fears. maybe that's part of why i love this job.#the wasp thing is because i have a field site at a bridge that's basically got wasp nests all over the underside#the wasps like to watch me work#i am genuinely having personal realizations while answering this ask#thank you anon sorry this got out of control i hope my exploits amuse you#some of it i think you just had to be there#also if anyone recognizes these stories shh you didn't see anything#my coworkers had better not see this it would be so embarassing#hylian rambles#hylian does science#anon#asks#thanks anon!#needles tw#i promise proper safety protocols were being followed in all of these stories#the acid involved so. much. ppe.
1 note
·
View note
Text
somehow almost a week has passed since my big surgery. which is insane.
my surgery was scheduled tuesday april 19th for noon and was supposed to last for two - two and a half hours. i arrived at the hospital and started prep at 9:00am. my surgeon was scheduled for a surgery prior to mine and that one went longer than expected. i was wheeled out of the prep area and taken to a room near the OR at 12:30pm where I was prepped me with a nerve block, vital monitors, and something to keep me calm though the iv. and then i waited in that room for an hour and half watching friends. when it became clear my surgery would be delayed, they took me back to the prep area.
they had to keep dosing me with stuff to keep me calm because it kept wearing off. my surgery finally started at 3:30pm. i remember being wheeled back and seeing the big overhead light. i remember the oxygen mask being put over my mouth. i don't remember being told to count down.
my surgery lasted two and a half hours. i spent two hours in recovery afterwards before being brought to my room. my surgeon visited me the following day and told me my surgery was a success and that there were no complications. i didn't have any major blood loss, so there were no transfusions. i only had one fibroid, a severely degenerated one about size of a softball or a small grapefruit. since i had only one fibroid, i only have a 10-15% chance of growing another again. i had no cysts. i had no endometriosis. my ovaries are fine. everything else was fine. i have a four inch incision like a cesarean section about three inches below my naval. i have dissolvable stitches inside of me and glue and steri-strips on the outside.
i spent one night in the hospital. the nurses were super nice. i was given the option to go home on wednesday or spend one more night in the hospital. i debated because the drugs in the hospital are better, but i ultimately decided to leave on wednesday. the nerve block was wearing off and the drive home took three hours. the nerve block helped me through most of that. i had major pain in my shoulders after getting into bed when i got home from gas still trapped in my body after surgery. i put a heating pad on it and took gas x, but i mostly just had to ride that out.
every day since has slowly been getting better. my period started immediately after surgery because of course it did, so i had to get up more than i would have liked to those first few days lest i destroy the bed. i think that's helped me in the long run, though. i did the stairs for the first time on friday. i didn't think i'd be able to do that until well into week 2. i walked standing upright and not bent over for the first time yesterday. i even cooked at the stove a little today. (someone had to get the pot for me and fill it with water, but i did the rest!).
i'm definitely not feeling normal yet, but it's incredible that i had major surgery just a week ago. i don't feel like i did. i've only been using prescription strength tylenol and ibuprofen, which is also incredible to me. like...i did the same thing women do when they have c-sections. it's wild. the hospital gave a binder upon discharge, which helped me significantly. i ordered a better one online and it arrived today. so much support. i feel ready to take on the world now.
no way but up from here.
#my posts#someday i am going to be able to bend over and pick things up from the floor again#i keep dropping things and i need cleaned up after :'D#my big fibroid surgery
4 notes
·
View notes
Text
hello dirt truthers I love you but I do not respect you and here’s why
plants photosynthesise. that’s their whole deal. photosynthesis is when plants convert carbon dioxide and water into glucose and oxygen, in the equation below:
6CO2 + 6H2O -> C6H12O6 + 6O2
that just means they’re taking up 6 carbon dioxide molecules (from the air! famously a gas in the air, you may know her from her recent work in Climate Change) and 6 water molecules (from the soil! but soil is also famously Not made of water and we will get to that later), making 1 glucose molecule and 6 molecules of oxygen (which go back into the air! different kind of air). this is cool because it’s the same process as respiration (how we break down sugars to release energy!) in reverse!
BUT MR PELAGIC — I hear you ask — what about the SUNSHINE?
that’s the fun complicated part for all you contrarian third party motherfuckers :)
the ENERGY for this process comes from light. I said before that this is respiration in reverse: which means that energy needs to go IN, it can’t do this on its own. but! light is VERY good at carrying energy! you may have experienced this with sunburn. you may also know that photosynthesis uses a green pigment called chlorophyll, which is the star of the show here
(I’m lying it’s really rubisco but we’re not going there rn)
yknow when you’re wearing something black when it’s sunny and it gets hot. same thing happens with chlorophyll when it’s absorbing light. but all that energy has to go SOMEWHERE, so instead of just sitting there, the chlorophyll breaks down a little and releases an electron.
stay with me here. we’re getting back to the air. so this electron is carrying a bunch of energy now — this is gonna get passed around and sent to play with the big kids. carbon dioxide is the big kids. as well as her friend ribulose bisphosphate (big carbon based molecule! CO2 has one carbon, this one has 5, and was initially made by joining CO2s together!). the electron here provides the ENERGY for these two to join together — energy was released to break something, needs to be put back in to make something. light -> energy -> bond. which leaves us with glucose! which is MADE of carbon dioxides! a bunch of them!
(you may have noticed the water is missing from this! you remember our friend chlorophyll who’s sad and broken and missing an electron? water (H2O) can ALSO be broken apart by light in the same way, into oxygen, 2 hydrogen ions, and 2 electrons! the oxygen is released as a gas, and the hydrogen ions and some electrons are used as fuel while the rest of the electrons are passed back to patch up ms chlorophyll. everything gets used up!)
SO. altogether, that’s:
carbon dioxide is taken in from the air
water is taken up from the soil (water ≠ soil)
light knocks an electron off the chlorophyll
carbon dioxide joins with ribulose bisphosphate (big 5 carbon molecule) to make glucose, USING THE ENERGY from the electron
water is broken down to replenish electrons and fuel the whole process
NOW! as my dear friend mx splash said above, a big chunk of plants is made of cellulose, which is created by chaining glucose molecules together. most of the things in plants are made of carbon, and all that carbon has to start off in this process -- from glucose molecules, made of carbon dioxide, made of air.
i fear this might be where i lose some of the soil truthers. what about the nutrience?
the main stuff that plants take up from the soil are things like nitrogen, phosphorous, and potassium (absorbed through their roots, dissolved in water). these are what fertilisers are made of! they're needed to make up many things -- all proteins (nitrogen), for example, as well as DNA (phosphorous). however!
MOST OF THOSE MOLECULES ARE MOSTLY CARBON. carbon's super good at making a framework to stick stuff onto. for example, proteins are long chains of amino acids joined together. the simplest amino acid is called glycine:

which has a ratio of 2 carbon atoms for every 1 nitrogen! most amino acids have a couple more carbons (all other amino acids stick something on instead of a hydrogen) -- which means that there is more carbon than nitrogen even in the vast majority of proteins, which make up a whole bunch of the mass of everything currently alive because they can be used for so many things.
(if you want some stats pulled from the first random paper I could find talking about this, under optimal conditions, tomato leaves in this study have a carbon:nitrogen ratio of roughly 6:1 -- there's 6 times more carbon than nitrogen in a given leaf. [1])
water is also fun, because while plants need a bunch of water (they use it like a skeleton! isn't that cool as hell??) their STRUCTURES aren't made of water. water maintains pressure and a consistent environment and keeps everything standing up (vs wilting when they run out of water), but they're not physically made of water for the most part -- we saw earlier that it's mostly used to drive the process of photosynthesis, while stuff is actually built from the carbon dioxide.
soil itself is also made of a lot of carbon: it's dead plants and rocks and shit. mostly dead plants. but this is a one way relationship: we know that plants are mostly carbon, and plants get the vast majority of this carbon as carbon dioxide from the air -- the stuff they need from soil is instead the extra nutrients like nitrogen, which are released as the dead plants and shit decompose. plants become soil, but soil doesn't become plants (soil is made of plants, plants are not made of soil). when everything works right, the nutrients get put back in the soil by things decomposing, and that layer keeps building up (carbon storage!). soil is really good at sittin there. that's also why air plants can exist, and why we can grow most plants in just water -- all they really need from soil is the water, nutrients and support.
IN CONCLUSION:
plants take in carbon dioxide (air) and use it to make glucose (via photosynthesis, powered by energy from light) which is then used to make the majority of the stuff in the plant. soil isn't taken up directly -- plants suck up nitrogen and other nutrients dissolved in water, which are important, but there is so much more carbon than anything else, which is needed for basically everything because it's used as a base to build on.
plants are made of fucking air <3
dirt propaganda: he says ‘the plants eat the dirt with their roots’ and ‘soil has nutrients’
air propaganda: air plants are a thing and also i reckon we would have run out of soil by now otherwise
#if you would like to correct me abt a mistake or a simplification pls assume i already know and i did it on purpose#this is way too long. however. i am only doing this once and there are so many tags abt how it's soil or water or sunlight actually and no.#no it is not <3#plants#bio
3K notes
·
View notes