#Intracellular Events
Text
Pharmacology Unveiled: How Medications Work on a Molecular Level"
Explore the science of pharmacology and delve into the mechanisms of action of commonly prescribed medications, shedding light on how they interact with the body's systems.
In the multifaceted domain of pharmacology, an intricate symphony of molecular interactions orchestrates the therapeutic effects of medications. A profound comprehension of the molecular underpinnings of pharmacological actions is indispensable for healthcare professionals, pharmaceutical scientists, and researchers. In this discourse, we embark on a comprehensive exploration of pharmacodynamics, elucidating the profound intricacies of how medications function at the molecular level.
Pharmacodynamics: A Multilayered Discipline
Pharmacodynamics constitutes the extensive scrutiny of the manner in which drugs interlace with specific molecular targets, often referred to as receptors or enzymes, within the human organism. Medications are meticulously designed to effectuate alterations in biochemical pathways, receptor kinetics, or enzymatic processes, aiming to modulate physiological phenomena to alleviate symptoms or remediate pathological states.
Receptor-Mediated Pharmacological Actions
A pivotal facet of pharmacodynamics lies in the receptor-mediated actions of medications. Receptors are intricate protein entities, frequently situated on the extracellular or intracellular domains of cells, that play a pivotal role in cellular communication and homeostasis. When a medication interfaces with a receptor, it initiates a cascade of molecular events, which, contingent upon the context, may potentiate or impede the cellular response.
Agonists and Antagonists: Puppets of Molecular Dance
In the intricate theater of pharmacodynamics, medications assume roles as either agonists or antagonists. Agonists aptly mimic the endogenous ligands or signaling molecules, seamlessly integrating into the receptor's binding pocket. This engagement sets forth a conformational alteration in the receptor, instigating cellular events replicating or augmenting the physiological response. Conversely, antagonists function as molecular antagonists, obstructing the receptor and forestalling the binding of endogenous signaling molecules. Consequently, the physiological response is negated or attenuated.
Enzymatic Interference: Orchestrating Biochemical Concertos
Certain medications orchestrate their therapeutic influence through the intricate domain of enzyme inhibition. Enzymes are the catalytic workhorses governing biochemical transformations in biological systems. Medications that selectively inhibit or modulate these enzymes effectively regulate the pace or character of these metabolic reactions, rendering them invaluable in conditions characterized by aberrant enzyme function.
Ion Channel Choreography: Modulating Electrophysiological Ballets
A notable mechanism of pharmacological action entails the modulation of ion channels. These proteinaceous conduits, reposing within cellular membranes, govern the flux of ions across these barriers. Medications designed to engage with ion channels effectively influence the electrochemical signaling within cells. The modulation of ion channels is instrumental in conditions such as arrhythmias, epilepsy, and neuropathic pain.
Pharmacogenetics: Personalizing Medication Regimens
The burgeoning realm of pharmacogenetics delves into the impact of an individual's genetic repertoire on their medication response. Genetic polymorphisms can significantly influence drug metabolism, receptor sensitivities, and pharmacological efficacy. Tailoring medication regimens to align with an individual's genetic makeup represents a burgeoning paradigm in personalized medicine.
Pharmacology unfolds as an intricate tapestry of molecular engagements and multifarious mechanisms. Medications, hewn with precision, are intended to engage with specific molecular entities, be it receptors, enzymes, or ion channels, aiming to modulate intricate biochemical processes to achieve therapeutic ends.
References
Rang, H. P., Dale, M. M., Ritter, J. M., & Flower, R. J. (2015). Rang & Dale's Pharmacology. Elsevier.
Katzung, B. G., & Trevor, A. J. (2021). Basic & Clinical Pharmacology. McGraw-Hill Education
Brunton, L. L., Knollmann, B. C., & Hilal-Dandan, R. (2020). Goodman & Gilman's: The Pharmacological Basis of Therapeutics. McGraw-Hill Education.
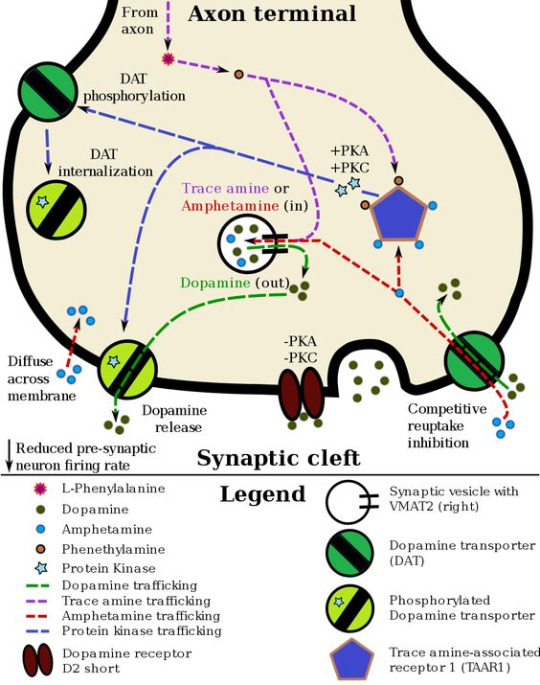
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#pharmacology#pharmacy#medication#med student#med school#medical stuff#drugdiscovery#pharma industry#molecular biology#drugsandresearch#clinicalpharmacology#pharmaceuticalindustry
67 notes
·
View notes
Text
The T Cell Landscape
T cells, a critical component of the adaptive immune system, stand as the body's elite force in combatting infections and diseases. These specialized lymphocytes boast remarkable diversity, each type playing a distinct role in orchestrating a targeted and effective immune response.
T cells, like all blood cells, originate from hematopoietic stem cells residing in the bone marrow. However, their training ground lies within the thymus, a specialized organ located in the chest. Here, they undergo a rigorous selection process known as thymocyte education. During this process, immature T cells, called thymocytes, are presented with self-antigens (molecules unique to the body) by special cells. Thymocytes that bind too strongly to these self-antigens are eliminated, preventing them from attacking healthy tissues later. Only thymocytes that demonstrate the ability to recognize foreign invaders while exhibiting tolerance to self are released into the bloodstream as mature T cells.
Following this rigorous training, mature T cells exit the thymus and embark on their patrol, circulating throughout the bloodstream and lymphatic system. They remain vigilant, constantly scanning for their specific targets – antigens. Antigens are foreign molecules, such as fragments of viruses, bacteria, or even cancerous cells, that trigger the immune response.
The hallmark of a T cell is its T cell receptor (TCR), a highly specialized protein complex embedded on its surface. This receptor acts like a lock, uniquely shaped to fit a specific antigen, the "key." Each T cell develops a unique TCR capable of recognizing only a single antigen, enabling a highly specific immune response.
But how do T cells encounter these hidden antigens lurking within infected or cancerous cells? This critical role is played by antigen-presenting cells (APCs). APCs, such as macrophages and dendritic cells, engulf pathogens or abnormal cells, break them down into smaller fragments (peptides), and present them on their surface complexed with major histocompatibility complex (MHC) molecules. MHC molecules act as identification tags, allowing T cells to distinguish between "self" and "non-self." When a T cell's TCR encounters its specific antigen bound to an MHC molecule on an APC, a dance of activation begins. The T cell becomes stimulated, and a cascade of signaling events is triggered. This leads to the T cell's proliferation, producing an army of clones specifically tailored to combat the recognized threat.
T cells are not a single, monolithic entity. They comprise a diverse population, each type with a specialized function:
Helper T Cells (Th Cells):
Helper T cells, often abbreviated as Th cells, play a central role in coordinating immune responses. They express the CD4 surface marker and can recognize antigens presented by major histocompatibility complex class II (MHC-II) molecules. Subtypes of helper T cells include Th1, Th2, Th17, and regulatory T cells (Tregs), each with distinct functions and cytokine profiles.
Th1 cells mediate cellular immunity by activating macrophages and cytotoxic T cells, crucial for defense against intracellular pathogens.
Th2 cells are involved in humoral immunity, promoting B cell activation and antibody production, thus aiding in defense against extracellular parasites.
Th17 cells contribute to the immune response against extracellular bacteria and fungi, producing pro-inflammatory cytokines. Regulatory T cells (Tregs) maintain immune tolerance and prevent autoimmunity by suppressing excessive immune responses.
Cytotoxic T Cells (Tc Cells):
Cytotoxic T cells, also known as Tc cells or CD8+ T cells, are effector cells responsible for directly killing infected or aberrant cells. They recognize antigens presented by MHC class I molecules on the surface of target cells. Upon activation, cytotoxic T cells release perforin and granzymes, inducing apoptosis in target cells and eliminating the threat.
Memory T Cells:
Memory T cells are a long-lived subset of T cells that persist after the clearance of an infection. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, conferring immunological memory. Memory T cells can be either central memory T cells (TCM), residing in lymphoid organs, or effector memory T cells (TEM), circulating in peripheral tissues.
γδ T Cells:
Unlike conventional αβ T cells, γδ T cells express a distinct T cell receptor (TCR) composed of γ and δ chains. They recognize non-peptide antigens, such as lipids and metabolites, and are involved in immune surveillance at epithelial barriers and responses to stress signals.
Beyond the Battlefield: The Expanding Roles of T Cells: The remarkable capabilities of T cells have opened doors for several groundbreaking applications in medicine:
Vaccines: By presenting weakened or inactivated forms of pathogens, vaccines "train" the immune system to generate memory T cells. This prepares the body to recognize and rapidly eliminate the real pathogen upon future exposure, preventing disease.
Cancer immunotherapy: CAR T-cell therapy, a revolutionary approach, genetically engineers a patient's own T cells to express chimeric antigen receptors (CARs) that recognize and target specific cancer cells. These "supercharged" T cells are then reintroduced into the patient, unleashing a potent attack against the tumor.
Autoimmune disease treatment: Researchers are exploring ways to manipulate T cells to suppress harmful immune responses that underlie autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
The diverse array of T cells underscores the immune system's complexity and adaptability in mounting tailored responses against a myriad of threats. From orchestrating immune reactions to maintaining tolerance and establishing long-term immunity, T cells play multifaceted roles in safeguarding the body's health. Understanding the intricacies of T cell biology not only sheds light on immune-mediated diseases but also paves the way for developing novel therapeutic strategies harnessing the power of the immune system.
T cells represent a fascinating aspect of immunology, with their diversity and specificity driving the complexity of immune responses. As research advances, further insights into T cell biology promise to revolutionize immunotherapy and enhance our ability to combat diseases ranging from infections to cancer. By understanding and harnessing their power, we can unlock new avenues for protecting and improving human health.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#t cells#T helper cells#autoimmune#autoimmunity#helathcare#immunology#immunotherapy#medical care#cancer#human health#research#scientific research#the glass scientists#scientific illustration#research scientist
11 notes
·
View notes
Text
Interesting Papers for Week 31, 2024
Encoding of Predictive Associations in Human Prefrontal and Medial Temporal Neurons During Pavlovian Appetitive Conditioning. Aquino, T. G., Courellis, H., Mamelak, A. N., Rutishauser, U., & O Doherty, J. P. (2024). Journal of Neuroscience, 44(17).
Network-neuron interactions underlying sensory responses of layer 5 pyramidal tract neurons in barrel cortex. Bast, A., Fruengel, R., de Kock, C. P. J., & Oberlaender, M. (2024). PLOS Computational Biology, 20(4), e1011468.
Unifying network model links recency and central tendency biases in working memory. Boboeva, V., Pezzotta, A., Clopath, C., & Akrami, A. (2024). eLife, 12, e86725.3.
Causal phase-dependent control of non-spatial attention in human prefrontal cortex. Brus, J., Heng, J. A., Beliaeva, V., Gonzalez Pinto, F., Cassarà, A. M., Neufeld, E., … Polanía, R. (2024). Nature Human Behaviour, 8(4), 743–757.
Proximity to rewards modulates parameters of effortful control exertion. Devine, S., Roy, M., Beierholm, U., & Otto, A. R. (2024). Journal of Experimental Psychology: General, 153(5), 1257–1267.
Low-dimensional criticality embedded in high-dimensional awake brain dynamics. Fontenele, A. J., Sooter, J. S., Norman, V. K., Gautam, S. H., & Shew, W. L. (2024). Science Advances, 10(17).
Limitations to optimal search in naturalistic active learning. He, L., Richie, R., & Bhatia, S. (2024). Journal of Experimental Psychology: General, 153(5), 1165–1188.
Collective behavior from surprise minimization. Heins, C., Millidge, B., Da Costa, L., Mann, R. P., Friston, K. J., & Couzin, I. D. (2024). Proceedings of the National Academy of Sciences, 121(17), e2320239121.
Human decision making balances reward maximization and policy compression. Lai, L., & Gershman, S. J. (2024). PLOS Computational Biology, 20(4), e1012057.
Distinct engrams control fear and extinction memory. Luft, J. G., Popik, B., Gonçalves, D. A., Cruz, F. C., & de Oliveira Alvares, L. (2024). Hippocampus, 34(5), 230–240.
Bayesianism and wishful thinking are compatible. Melnikoff, D. E., & Strohminger, N. (2024). Nature Human Behaviour, 8(4), 692–701.
Bayesian inference of structured latent spaces from neural population activity with the orthogonal stochastic linear mixing model. Meng, R., & Bouchard, K. E. (2024). PLOS Computational Biology, 20(4), e1011975.
The olivary input to the cerebellum dissociates sensory events from movement plans. Pi, J. S., Fakharian, M. A., Hage, P., Sedaghat-Nejad, E., Muller, S. Z., & Shadmehr, R. (2024). Proceedings of the National Academy of Sciences, 121(17), e2318849121.
Brightness illusions drive a neuronal response in the primary visual cortex under top-down modulation. Saeedi, A., Wang, K., Nikpourian, G., Bartels, A., Logothetis, N. K., Totah, N. K., & Watanabe, M. (2024). Nature Communications, 15, 3141.
Episodic long-term memory formation during slow-wave sleep. Schmidig, F. J., Ruch, S., & Henke, K. (2024). eLife, 12, e89601.3.
Perceptography unveils the causal contribution of inferior temporal cortex to visual perception. Shahbazi, E., Ma, T., Pernuš, M., Scheirer, W., & Afraz, A. (2024). Nature Communications, 15, 3347.
Chaotic neural dynamics facilitate probabilistic computations through sampling. Terada, Y., & Toyoizumi, T. (2024). Proceedings of the National Academy of Sciences, 121(18), e2312992121.
A voltage-based Event-Timing-Dependent Plasticity rule accounts for LTP subthreshold and suprathreshold for dendritic spikes in CA1 pyramidal neurons. Tomko, M., Benuskova, L., & Jedlicka, P. (2024). Journal of Computational Neuroscience, 52(2), 125–131.
Spontaneous eye movements reflect the representational geometries of conceptual spaces. Viganò, S., Bayramova, R., Doeller, C. F., & Bottini, R. (2024). Proceedings of the National Academy of Sciences, 121(17), e2403858121.
Intracellular magnesium optimizes transmission efficiency and plasticity of hippocampal synapses by reconfiguring their connectivity. Zhou, H., Bi, G.-Q., & Liu, G. (2024). Nature Communications, 15, 3406.
#neuroscience#science#research#brain science#scientific publications#cognitive science#neurobiology#cognition#psychophysics#neurons#neural computation#neural networks#computational neuroscience
6 notes
·
View notes
Text
Abstract
Therapeutic applications of synthetic mRNA were proposed more than 30 years ago, and are currently the basis of one of the vaccine platforms used at a massive scale as part of the public health strategy to get COVID-19 under control. To date, there are no published studies on the biodistribution, cellular uptake, endosomal escape, translation rates, functional half-life and inactivation kinetics of synthetic mRNA, rates and duration of vaccine-induced antigen expression in different cell types. Furthermore, despite the assumption that there is no possibility of genomic integration of therapeutic synthetic mRNA, only one recent study has examined interactions between vaccine mRNA and the genome of transfected cells, and reported that an endogenous retrotransposon, LINE-1 is unsilenced following mRNA entry to the cell, leading to reverse transcription of full length vaccine mRNA sequences, and nuclear entry. This finding should be a major safety concern, given the possibility of synthetic mRNA-driven epigenetic and genomic modifications arising. We propose that in susceptible individuals, cytosolic clearance of nucleotide modified synthetic (nms-mRNAs) is impeded. Sustained presence of nms-mRNA in the cytoplasm deregulates and activates endogenous transposable elements (TEs), causing some of the mRNA copies to be reverse transcribed. The cytosolic accumulation of the nms-mRNA and the reverse transcribed cDNA molecules activates RNA and DNA sensory pathways. Their concurrent activation initiates a synchronized innate response against non-self nucleic acids, prompting type-I interferon and pro-inflammatory cytokine production which, if unregulated, leads to autoinflammatory and autoimmune conditions, while activated TEs increase the risk of insertional mutagenesis of the reverse transcribed molecules, which can disrupt coding regions, enhance the risk of mutations in tumour suppressor genes, and lead to sustained DNA damage. Susceptible individuals would then expectedly have an increased risk of DNA damage, chronic autoinflammation, autoimmunity and cancer. In light of the current mass administration of nms-mRNA vaccines, it is essential and urgent to fully understand the intracellular cascades initiated by cellular uptake of synthetic mRNA and the consequences of these molecular events.
4 notes
·
View notes
Text
sharing this really interesting article from Nature; https://www.nature.com/articles/s41586-022-05349-x
the abstract is: "Infectious diseases are among the strongest selective pressures driving human evolution1,2. This includes the single greatest mortality event in recorded history, the first outbreak of the second pandemic of plague, commonly called the Black Death, which was caused by the bacterium Yersinia pestis3. This pandemic devastated Afro-Eurasia, killing up to 30–50% of the population4. To identify loci that may have been under selection during the Black Death, we characterized genetic variation around immune-related genes from 206 ancient DNA extracts, stemming from two different European populations before, during and after the Black Death. Immune loci are strongly enriched for highly differentiated sites relative to a set of non-immune loci, suggesting positive selection. We identify 245 variants that are highly differentiated within the London dataset, four of which were replicated in an independent cohort from Denmark, and represent the strongest candidates for positive selection. The selected allele for one of these variants, rs2549794, is associated with the production of a full-length (versus truncated) ERAP2 transcript, variation in cytokine response to Y. pestis and increased ability to control intracellular Y. pestis in macrophages. Finally, we show that protective variants overlap with alleles that are today associated with increased susceptibility to autoimmune diseases, providing empirical evidence for the role played by past pandemics in shaping present-day susceptibility to disease."
tldr; the genes that allowed our ancestors to survive plagues are also the genes that can cause autoimmune disease in modern populations.
7 notes
·
View notes
Text
Imperial Lates
Upon experiencing my first Imperial late event, here are a few things that I learnt:
- My first lecture focused on precision polymer particles by Professor Rachel O’riley. Although typically such nanoparticles are governed by the ratio of hydrophobic and hydrophilic components for self assembly, here; the focus was on crystallisation driven self assembly (CDSA) which overrides this mechanism. DMA seeds are used as the original seeds on which the polymer begins to form through a process known as living polymerisation growth for greater control that leads to platelet like structures. Studying the complexity of assembly was important on exploring how polymer length, composition and chemistry can be altered to rationally tune nanoparticle shape and in turn it’s properties. Novel applications include directing biological interactions, such as cellular uptake, intracellular trafficking and immune response or the ability to modulate hydrogel mechanical and adhesion properties when used as fillers for antimicrobial and tissue engineering purposes.
- The second talk event was lead by Dr Catherine Kibirige on treating HIV in rural African communities. HIV is particularly difficult to treat and has to be rather suppressed because of it’s elusion from the immune system. Using a continually shifting shield of glycans, it prevent antibodies from detecting and attacking. It also replicates rapidly using reverse transcriptase to develop a pool of diverse HIV strains - called quasi-species - whilst attacking helper T-cells that orchestrate the immune system’s cell signalling. Finally it has a long incubation period and is slow to reveal (typically 5-10 years), where by that point it may have shredded the victims immune system. There are campaigns that have been developed, notably the ongoing UN led campaign of 95-95-95; where 95% people with HIV know their status, then 95% are receiving their treatment and 95% have suppressed their viral load by 2030. Suppression is the best we have at the current moment with no vaccine, with the Undetectable= Untransmittable campaign (U=U), sexual transmissions of HIV can be stopped by lowering the viral load in blood whilst on effective treatment. Dr Kibirige has been a part of the HIVQuant project that is a HIV-1 kit (working on portable solar or battery driven cyclers) that provides a treatment monitoring solution for resource-constrained settings, especially in Africa to minimise monthly hourly trips to district hospitals to meet the 2030 95-95-95 UN target.
- Vera. AI was the final project that grasped my attention as AI is largely underrepresented in the field of healthcare. Being a hyper-personalised digital platform that focuses on gynaecological, hormonal health management and patient education, this AI tools aims to break women’s health taboos in communicating their medical needs. Focusing on improving patient’s ability to understand and learn, Vera.AI improves patient-doctor communication. This is a project still in it’s early stages but with incorporating ChatGPT in the future, it has colossal potential to democratise women’s health medical data.
#medicine#drugs#drug experiments#imperial college london#AI#HIV#chatgpt#nanoparticles#polymers#self help#university#u=u#95-95-95#united nations#immune system#Imperial lates#learning#womens health
6 notes
·
View notes
Text
Spatial transcriptomics unveils the in situ cellular and molecular hallmarks of the lung in fatal COVID-19
Severe Coronavirus disease 2019 (COVID-19) induces heterogeneous and progressive diffuse alveolar damage (DAD) highly disrupting lung tissue architecture and homeostasis, hampering disease management and leading to fatal outcomes. Characterizing DAD pathophysiology across disease progression is of ultimate importance to better understand the molecular and cellular features driving different DAD patterns and to optimize treatment strategies. To contextualize the interplay between cell types and assess their distribution, spatial transcriptomics (ST) techniques have emerged allowing unprecedented resolution to investigate spatial architecture of tissues. To this end, post-mortem lung tissue provides valuable insights into cellular composition and their spatial relationships at the time of death. Here, we have leveraged VisumST technology in post-mortem COVID-19 induced acute and proliferative DAD lungs including control samples with normal morphological appearance to unravel the immunopathological mechanisms underlying DAD providing novel insights into cellular and molecular communication events driving DAD progression in fatal COVID-19. We report a progressive loss of endothelial cell types, pneumocytes type I and natural killer cells coupled with a continuous increase of myeloid and stromal cells, mostly peribronchial fibroblasts, over disease progression. Spatial organization analysis identified variable cellular compartments, ranging from major compartments defined by cell type lineages in control lungs to increased and more specific compartmentalization including immune-specific clusters across DAD spectrum. Importantly, spatially informed ligand-receptor interaction (LRI) analysis revealed an intercellular communication signature defining COVID-19 induced DAD lungs. Transcription factor (TF) activity enrichment analysis identified TGF-B pathway as DAD driver, highlighting SMAD3 and SMAD7 TFs activity role during lung fibrosis. Integration of deregulated LRIs and TFs activity, allowed us to propose a downstream intracellular signaling pathway in peribronchial fibroblasts, suggesting potential novel therapeutic targets. Finally, spatio-temporal trajectories analysis provided insights into the alveolar epithelium regeneration program, characterizing markers of pneumocytes type II differentiation towards pneumocytes type I. In conclusion, we provide a spatial characterization of lung tissue architecture upon COVID-19 induced DAD progression, identifying molecular and cellular hallmarks that may help optimize treatment and patient management. http://dlvr.it/T9C2Pz
0 notes
Text
Fwd: Graduate position: UWarsaw.SymbiosisEvolution
Begin forwarded message:
> From: [email protected]
> Subject: Graduate position: UWarsaw.SymbiosisEvolution
> Date: 15 May 2024 at 06:43:32 BST
> To: [email protected]
>
>
> PhD position in the project: "Unravelling the establishment of
> endosymbiosis: quest for intermediate evolutionary stages among
> microbial eukaryotes (SYMBIOSTART)"
>
>
> Project description:
>
> The phenomenon of endosymbiosis - in which one organism lives within
> another - led to major evolutionary transitions such as the origin of
> mitochondria and plastids. We currently know that there have been
> several independent events of endosymbiosis in early eukaryotic
> evolution, giving rise in some cases to major eukaryotic superclades.
> However, despite its significance, we still do not understand the
> mechanism of endosymbiotic integration. Only by examining more recent
> endosymbioses in various phases of integration can we make progress in
> our understanding of the host-endosymbiont integration process. In
> this regard, the endosymbioses of microbial eukaryotes, due to the
> unicellular nature of the host, provide the most suitable systems for
> studying the establishment of endosymbiosis. However, the number of
> such models is very limited and does not allow the reconstruction of
> the mechanisms of integration. Moreover, recent advances in diversity
> research suggest that intracellular symbioses are common among
> microbial eukaryotes, providing a golden opportunity to identify
> unicellular symbiotic systems in intermediate stages of integration.
>
> To identify the intermediate stages of endosymbiotic integration,
> SYMBIOSTART will combine screening of diverse freshwater environments
> for microbial eukaryotes and their endosymbionts with studies on
> established culturable systems. The goal is to provide a wide range of
> systems in various stages of integration, which will be further
> studied to highlight the genomic and ultrastructural features that
> accompany endosymbiont integration using genomics,transcriptomics and
> microscopy. Overall, SYMBIOSTART will address the most challenging
> questions about endosymbiotic events: how integration occurs and what
> is the relative timing of the steps involved.
>
>
> Specific Tasks:
>
> -Analysis of high-throughput sequencing data: assembly and annotation
> of genomes, metagenomes and transcriptomes, amplicon data analysis
>
> -Isolation and culturing of microbial eukaryotes
>
> -DNA & RNA isolation from environmental samples and cultures, PCR,
> preparation of libraries for total genome, transcriptome & amplicon
> sequencing, Nanopore sequencing
>
> -Publication of the obtained results in conference talks & papers,
> active participation in publishing original research articles
>
> Requirements:
>
> -Master’s degree in biology, biotechnology or related field (obtained
> before September 23rd, 2024)
>
> -Status of a doctoral student at the Doctoral School of Exact and
> Natural Sciences of the University of Warsaw at the beginning of the
> contract
>
> -Being self-motivated, organized, and highly team-oriented
>
> -Proficiency in English (oral and written)
>
> -Background in bioinformatics, especially genomics, transcriptomics
> and metagenomics.
>
> -Familiarity with microscopy, molecular biology techniques, and
> microbiology techniques will be an advantage.
>
>
> The requirements listed above need to be met before the employment
> starts, but not necessarily by the application deadline.
>
>
> We offer a scholarship for up to 48 months. Starting date: 1 October, 2024
>
>
> Application deadline: 3 June, 2024
>
> More details about the application process at the website:
> https://ift.tt/pBfdM8e
>
> Please feel free to reach out with any questions at the email address
> listed below.
>
> Anna Karnkowska
>
> Institute of Evolutionary Biology
>
> Biological and Chemical Research Centre
>
> University of Warsaw, Warsaw, Poland
>
> Web Lab.:
> https://ift.tt/MZDoaKO
>
> Anna Karnkowska
0 notes
Text
Advantages of Flow Cytometry Kits

Advantages of Flow Cytometry Kits
In the dynamic field of life sciences, researchers and scientists are constantly seeking innovative tools and technologies to advance their understanding of cellular processes. Among the revolutionary advancements, flow cytometry has emerged as a powerful technique for analyzing and sorting cells based on their unique characteristics. Denovo Technologies, a leading provider of life science products and services in India, offers a wide range of flow cytometry kits that provide researchers with numerous advantages. In this article, we will delve into the advantages of utilizing flow cytometry kits from Denovo Technologies.
The Advantages Offered by Denovo Technologies
Precise and Accurate Cell Analysis
Denovo Technologies’ flow cytometry kits enable precise and accurate analysis of cells. With the ability to analyze multiple parameters simultaneously, researchers can gain comprehensive insights into cellular populations, including cell size, shape, granularity, and surface markers. This allows for a deeper understanding of cell heterogeneity and facilitates the identification of rare cell populations that may be critical in various research areas, such as immunology, cancer biology, and stem cell research.
Versatile Applications
The flow cytometry kits offered by Denovo Technologies cater to a wide range of applications. Whether it is immunophenotyping, cell cycle analysis, apoptosis detection, or intracellular staining, these kits provide researchers with the flexibility to explore diverse research avenues. By utilizing these kits, scientists can unravel the complexities of cellular processes and gain a comprehensive understanding of biological systems.
Streamlined Workflow
Denovo Technologies’ flow cytometry kits are designed to streamline the experimental workflow, saving researchers valuable time and effort. The kits are optimized for efficient sample preparation, staining protocols, and data acquisition, ensuring reliable and reproducible results. With user-friendly protocols and standardized reagents, researchers can focus more on data interpretation and analysis rather than dealing with technical complexities.
High Sensitivity and Resolution
Flow cytometry kits from Denovo Technologies offer high sensitivity and resolution, enabling the detection of even low-abundance cellular events. This is particularly crucial when studying rare cell populations, such as circulating tumor cells or stem cells. The kits utilize advanced fluorochrome conjugates and optimized antibody panels, ensuring accurate and reliable detection of target molecules.
Customization Options
Recognizing the diverse needs of researchers, Denovo Technologies provides customization options for flow cytometry kits. Researchers can tailor the kits to their specific experimental requirements, selecting the appropriate antibodies and fluorochromes. This flexibility allows for the optimization of experimental conditions and enhances the quality of data obtained, leading to more robust and meaningful scientific discoveries.
Conclusion
Flow cytometry has revolutionized cell analysis and has become an indispensable tool in life science research. Denovo Technologies’ flow cytometry kits offer numerous advantages that empower researchers to delve deeper into the complexities of cellular biology. With their precise analysis capabilities, versatile applications, streamlined workflow, high sensitivity, and customization options, these kits provide researchers with the tools they need to unlock new insights and drive scientific progress. By harnessing the power of flow cytometry kits from Denovo Technologies, researchers in India and beyond can advance their understanding of cellular processes and contribute to breakthrough discoveries in various fields of life sciences.
#luminex instruments#across the spiderverse#luminex assays#best base scope essay#luminexinstruments#bioactivitytestingservices#luminex kits#luminex instrument#luminex custom services#automatedmultiplexelisa
0 notes
Text
Cells, Vol. 13, Pages 183: Autophagy and Apoptosis in Rabies Virus Replication
Rabies virus (RABV) is a single-stranded negative-sense #RNA virus belonging to the Rhabdoviridae family and Lyssavirus genus, which is highly neurotropic and can infect almost all warm-blooded animals, including humans. Autophagy and apoptosis are two #evolutionarily conserved and genetically regulated processes that maintain cellular and organismal homeostasis, respectively. Autophagy recycles unnecessary or dysfunctional intracellular organelles and molecules in a cell, whereas apoptosis eliminates damaged or unwanted cells in an organism. Studies have shown that RABV can induce both autophagy and apoptosis in target cells. To advance our understanding of pathogenesis of rabies, this paper reviews the molecular mechanisms of autophagy and apoptosis induced by RABV and the effects of the two cellular events on RABV replication. https://www.mdpi.com/2073-4409/13/2/183?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Neuromorphic Computing: Mimicking the Human Brain
Neuromorphic computing aims to mimic the way that the brain works. It’s an alternative to von Neumann computer architectures that use fixed-function hardware. Instead, it uses interconnected processors that act more like the brain’s neurons and synapses to perform tasks. It is more flexible and scalable than traditional computers.
Scientists have studied this model for decades, and in the last decade it has become a focus of research with the goal of implementing it into computers. A large variety of designs, implementation methodologies and prototype chips have been developed in this vein. However, they all share one common objective – to achieve human-level artificial intelligence (AGI) within the same energy budget as the human brain does.

This is a significant challenge, especially for complex tasks like pattern recognition which requires a lot of computation. The main problem is that the current generation of computers rely on digital circuitry to do the processing, and it consumes enormous amounts of power.
To lower this power usage, researchers have been experimenting with various neuromorphic designs, including transistors that act like the spiking behavior technology has change over the year of neurons. However, this is only part of the picture. Another crucial factor is capturing the intracellular processes that produce action potentials. To do this, they need to be able to capture the ion channel-based events that occur in neurons when they fire.
The new chip developed by Han and his team is the first to do this. Its artificial neurons are made from a type of vanadium oxide that’s been treated to allow for repeated partial temperature cycling. This enables it to store memory. Han and his team are hoping to eventually apply this technology to autonomous cars, robots and Internet of Things (IoT) sensors.
The artificial neurons are also smaller than those in the human brain, which is one factor that could help them operate at lower power levels. They also use conventional CMOS semiconductor technology, the same kind used in most integrated circuits (or chips) found in today’s devices.
Neuromorphic computers are largely modeled after the neocortex, where higher cognitive functions like sensory perception, motor commands and spatial reasoning occur. They also aim to explore the multitude of neuronal firing patterns that work together to produce consciousness.
Although the chips technology website can only emulate certain aspects of the human brain, scientists have already used them to develop prototype machines that are demonstrating some promising results. For example, the Tianjic chip can recognize objects and follow moving targets. It can also detect odors and answer questions about them. In addition, it can recognize faces and tell the difference between them. The project is currently collaborating with Cornell University to teach it how to identify a range of smells.
1 note
·
View note
Text
Unlocking the Future: The Synergy of Polyclonal and Monoclonal Antibodies in Research
In the ever-evolving landscape of life science research, the synergy between polyclonal antibodies (pAbs) and monoclonal antibodies (mAbs) stands as a cornerstone, propelling scientific inquiry into new realms. As we delve into this fascinating world, the role of advanced technologies such as ELISA kits, secondary antibodies, Cell-Based ELISA, and Sandwich ELISA cannot be overstated. Among the pioneers driving these breakthroughs is Assay Biotechnology, a renowned player in the field, unlocking the future of research.
Antibodies: The Foundation of Discovery
At the heart of every groundbreaking discovery lies the humble antibody. Whether polyclonal or monoclonal, these molecular warriors are indispensable tools for researchers worldwide. Polyclonal antibodies, with their diversity and ability to recognize multiple epitopes, provide a robust and versatile foundation. Monoclonal antibodies, on the other hand, offer precision and specificity, targeting a single epitope with unparalleled accuracy.
Assay Biotechnology, a global contributor since its inception in 2006, has been at the forefront of providing high-quality antibodies that fuel academic and pharmaceutical research. The merger with Immunoway Biotechnology in May 2022 has further strengthened their commitment to advancing life science research.
ELISA Kits: Unraveling Complexity
Enzyme-Linked Immunosorbent Assay (ELISA) kits have revolutionized the way researchers detect and quantify proteins. In the arsenal of Assay Biotechnology, ELISA kits play a pivotal role, facilitating the precise measurement of various biomolecules. From Polyclonal Antibodies to Monoclonal Antibodies, the ELISA platform serves as a versatile and efficient tool for researchers across diverse fields.
Cell-Based ELISA: Probing Cellular Dynamics
The integration of Cell-Based ELISA into research methodologies has opened new avenues for understanding cellular dynamics. This innovative approach allows scientists to study intracellular events in a physiologically relevant context. Assay Biotechnology's commitment to providing cutting-edge solutions is evident in its range of Cell-Based ELISA kits, empowering researchers to explore the intricacies of cellular processes with precision.
Sandwich ELISA: Bridging Gaps in Analysis
The Sandwich ELISA technique, a powerful variant of the traditional ELISA, adds an extra layer of specificity. By using a pair of antibodies to sandwich the target antigen, researchers achieve heightened sensitivity and accuracy in their analyses. Assay Biotechnology's dedication to advancing research is exemplified in its comprehensive range of Sandwich ELISA kits, ensuring researchers have the tools needed to bridge analytical gaps in their studies.
Secondary Antibodies: Enhancing Detection
Secondary antibodies play a crucial role in signal amplification and detection in immunoassays. Assay Biotechnology recognizes the significance of these secondary players and offers a range of high-quality secondary antibodies to enhance the sensitivity and specificity of experiments.
In conclusion, the synergy between Polyclonal and Monoclonal Antibodies, coupled with advanced technologies such as ELISA kits, Secondary Antibodies, Cell-Based ELISA, and Sandwich ELISA, represents a paradigm shift in life science research. Assay Biotechnology's unwavering commitment to providing top-tier products underscores its role as a catalyst in unlocking the future of scientific discovery. As we stand on the precipice of new possibilities, the fusion of these elements promises to unravel the mysteries of biology and medicine, paving the way for groundbreaking advancements in the years to come.
#AntibodyResearch#ELISAKits#PolyclonalMonoclonalSynergy#CellBasedELISA#AssayBiotechnology#LifeScienceInnovation#SecondaryAntibodies
0 notes
Text
Faibles niveaux de glutathion identifiés comme facteur de risque d'AVC (événement cérébrovasculaire).
Low glutathione levels identified as risk factor for STROKE (cerebrovascular event).
Niveles bajos de glutatión identificados como factor de riesgo de ACV (evento cerebrovascular).
Bassi livelli di glutatione identificati come fattore di rischio per l'ictus (evento cerebrovascolare).
Níveis baixos de glutatião identificados como um fator de risco para AVC (evento cerebrovascular).
#niveaux#glutathion#facteurderisque#AVC#accidentcérébrovasculaire#glutathione#levels#riskfactor#stroke#cerebrovascularaccident#niveles#glutatión#factorderiesgo#eventocerebrovascular#livelli#glutatione#fattoredirischio#ictus#eventocerebrovascolare#niveis#glutatião#fatorderisco
0 notes
Text
Cell Autophagy Imaging Analysis
Autophagy is an orderly circulatory system within a cell that can degrade unnecessary cytoplasmic proteins and damaged organelles. It is a dynamic autophagy process that exists in all eukaryotic cells. Autophagy is important for cells to resist intracellular and extracellular stress and maintain metabolic homeostasis. It is involved in many physiological events, including cell renewal, cell growth, immunity and aging. Recent evidence indicates that changes in autophagy often occur in many human diseases, such as neurodegenerative diseases, cancer, and cardiomyopathy. Therefore, finding new drugs that target the autophagy pathway is essential for the treatment of human diseases.
0 notes
Text
What are Growth Factors? By Cheyanne Mallas PA

Growth factors are a group of proteins that play a crucial role in regulating the growth, development, and differentiation of cells. These proteins act as signaling molecules, binding to specific receptors on the surface of cells and initiating a cascade of biochemical reactions that ultimately lead to cellular responses says Cheyanne Mallas.
In an authoritative tone, it is important to note that growth factors are diverse and encompass a wide range of molecules, including cytokines, hormones, and other signaling molecules. They are produced by various cell types and act on specific target cells to stimulate or inhibit cellular processes.
The primary function of growth factors is to promote cell proliferation, which is essential for tissue growth, wound healing, and tissue repair. They stimulate cells to enter the cell cycle and undergo division, leading to an increase in the number of cells. Additionally, growth factors can also regulate cell survival, migration, and differentiation, thereby influencing tissue formation and remodeling.
Growth factors exert their effects through complex signaling pathways, involving the activation of intracellular proteins and the modulation of gene expression. They can act in a paracrine or autocrine manner, where they are produced by one cell and act on neighboring or the same cell, respectively. Furthermore, the binding of growth factors to their receptors can trigger various downstream signaling events, such as the activation of kinases, transcription factors, and other signaling molecules says Cheyanne Mallas.
The dysregulation or malfunction of growth factors can have profound implications for human health. Abnormalities in growth factor signaling have been implicated in various diseases, including cancer, developmental disorders, and immune system disorders. Consequently, growth factors have become promising targets for therapeutic interventions, with the development of growth factor-based therapies and drugs that modulate their activity.
In conclusion, growth factors are a critical component of cellular signaling networks that regulate cell growth, differentiation, and survival says Cheyanne Mallas. Their diverse functions and mechanisms of action make them key players in development, tissue homeostasis, and disease. Understanding the intricacies of growth factor signaling holds great potential for advancements in medicine and the development of targeted therapies.
#Cheyanne Mallas#CheyanneMallas#Cheyanne Mallas PA#CheyanneMallasPA#CheyanneMallasCalifornia#Cheyanne Mallas California#CheyanneMallasLosAngeles
0 notes