#Medical Devices Classification
Explore tagged Tumblr posts
Text
Family: Godzilladae
Genus: Eschatolossus
A species originally thought to only have artificial specimens as the first 5 decades of research had insinuated through the Family's emergence at places of extreme man-made fallout, but it's ecological definition has broadened in recent times.
The first specimen of a mid-life male (Eschatolossus basilieus) washed up deceased, having seeming chocked to death on what was found to be the Eiko-Maru, a half-eaten freighter that had gone missing off the coast of the eastern Tokyo bay.
Following this the Bingo Maru was sent to investigate the area this vessel had registered last. All communications with the crew of the Bingo Maru ceased following an unusually delayed nightfall and a spike on all local measurement devices, no matter what they were meant to read. It was registered as lost within the hour.
Within days, these events were followed by a living specimen of the same species, a female, possibly it's mate, was seen climbing out of the horizon of Tokyo, making landfall and laying waste to the city.
The specimen was finally terminated after multiple hours of rampaging, though a means of medically induced coma, via it's entanglement in a power station's lines and exposure to high amounts of phosgene gas, leading to the creature's asphyxiation and death.
This event would lead to the creation of the EDF, which would later be turned into M̴̠͉̜̂ͅƠ̶̼̪̅͗̐̾N̵̜̥̭͖̞͍̠͌̈́̀Á̷̰͐̋R̴̬̍̾̔̋C̵̢̨̖̳̝̹̃̂̈́͜͠H̵̜̰̔̽̀. Creating a charter, a taxonomical guide to the being that were emerging amongst the Kaijuia Class, in the family of Godzilladae. Very specifically today, we shall be reviewing the files of the Eschatolossus Genus, along with some new, albeit under researched specimens.
New emergences from impossibly natural means have occurred in recent times enough so that, while not fitting what we previously designated as a prerequisite to be considered in this classification-that being evolutionary processes directly marred by man-made environmental hazards to an extent of reaching prehistoric megafauna-esque sizes- but still shows to be possession of all other defining characteristics. So much so in recent times we have take the initiative to remove the stipulation of "Human intervention" henceforth.
So, many previously sub-categorized or completely uncategorized specimens that, due to the aforementioned stipulation, were being monitored due to relevance but could not definitively fit our definitions, and so research of was not properly funded beyond discovery:
A handful of mutant creatures asexually reproducing at such rapid speeds it could literally force it's evolution into the Godzilladae classification (Eschatolossus inactus). - New York, New York; Progenitor specimen, Specimen A, terminated via low yield ordinance due to public safety factor. Multiple incubating offspring were taken into M̴̠͉̜̂ͅƠ̶̼̪̅͗̐̾N̵̜̥̭͖̞͍̠͌̈́̀Á̷̰͐̋R̴̬̍̾̔̋C̵̢̨̖̳̝̹̃̂̈́͜͠H̵̜̰̔̽̀ custody for further research. -Sydney, Australia; Specimen B, No progeny discovered. possible death before maturation due to slight physical difference between itself and Specimen A. Killed by another Godzilladae specimen that's been on file since the earliest years of this organization during its EDF time. Specimen B having been rendered paralyzed, possibly having it's thoracic vertebrae severed from the blunt force when attacked by (Eschatolossus Bellator). It was then killed when it was immolated by (Eschatolossus Bellator)'s "atomic breath", an exhalation of nuclear gasses that most members of the Godzilladea family have in some capacity.
Extra-terrestrial/Extra-planar Specimens: -An alien creature observed via Voyager 1, who fits all new classifications, the largest specimen both within the Godzilladea family, but possibly ever, dwarfing even The Pando Aspen 100-fold. Little can be researched, but it has been given the classification (Eschatolossus mundus rex) -A seperate, much smaller alien creature, having cragy, stone like malformations on it's back seperate from the dorsal plates, seemingly moving from one asteroid to the next. Being imaged on Voyager 2, seemingly following it. No more has been observed, but it has now been given the classification (Eschatolossus Mal Longinquus) - If not for the fact we are mathematically incapable of quantifying it's existence, beyond having footage and eye-witness accounts, this remains our most unknown member of the family (Eschatolossus incomprehensibilis vultus).
It is... scary. Truly. It is the most tangibly intangible, conceivable form that could be given to confoundment. The godly visage at which the stagnation of knowledge for it's barrier exeeds the knowedge neede to go beyond it lay as a foundation to ever begining to understand it.
#shitpost?#godzilla#speculative biology#speculative worldbuilding#speculative evolution#spec evo#speculative taxonomy#creative writing
4 notes
·
View notes
Text
Editor's note: This is the fourth blog in our series that examines how social determinants influence gender biases in public health research, menstrual hygiene product development, and women’s health outcomes.
Period poverty is the circumstance of lacking supplies and facilities to manage menstruation comfortably, safely, and without shame. As a researcher who studies period poverty and its effects on adolescent girls’ school attendance and engagement, I am concerned about the growing body of research documenting the exposure to chemical toxins and heavy metals that accompanies use of commercially produced menstrual hygiene supplies (e.g., tampons and pads). The most recent study documents the presence of 16 heavy metals (including arsenic, mercury, and lead) among 14 tampon brands. Though none of the tampons evaluated contained measurable amounts of all 16 heavy metals, all brands contained some heavy metals. Heavy metal exposure is associated with adverse health outcomes, including impairment of the nervous system, organ damage, and increased cancer risk. A 2019 study found that sanitary pads contain volatile organic compounds (VOCs) and phthalates, both of which are associated with menstrual irregularities and cancer.
The finding of heavy metals in tampons and harmful chemicals in pads, coupled with the fact that the Food and Drug Administration’s (FDA) regulation of these products does not mandate regular testing for toxic chemicals, turned my concern into alarm. Sanitary pads became commercially available in 1921, and tampons followed 10 years later in 1931. This cursory monitoring over the 100-plus years of period products’ commercial existence is consistent with the idea that, as “medical devices” (period products’ classification given by the FDA), the treatment offered is concealment—hiding menstruation from everyone but the person experiencing it. Given this focus on keeping menstruating bodies a secret, it is not surprising that regulation did not extend to studying how products designed to keep that secret affected menstruating bodies.
These findings demand that we expand our efforts beyond concealment—that assessment for health risks to users be a routine aspect of menstrual hygiene product manufacturing. They remind us that medical interventions are enacted upon dynamic organisms operating within complex systems. Neglecting that dynamism and complexity to devise a singular solution can have unintended consequences.
In the current case, the “interventions” are menstrual hygiene products. Menstruation involves not only the vagina (the channel for menstrual blood flow), but the circulatory system, which functions to both nourish organs and absorb native and foreign substances within organ tissue. The unintended consequence is the possibility that these products, in addition to absorbing menstrual blood, introduce an as yet undetermined quantity of heavy metals and potentially harmful chemicals into the bodies of menstruating people. The implications of these findings are that we can no longer afford the simplistic view of menstrual hygiene products as an exercise in concealment. Rather, we must consider product use as a potential entry point for physiologic risk and take appropriate steps to monitor and safeguard the health of the menstruating public.
4 notes
·
View notes
Text
mere haath mein (echo x gn!reader)
》 summary: reader and echo's love story from strangers to friends to lovers throughout the clone wars (a 4+1 type of story)
》 series masterlist: (please read the masterlist before continuing on!)
part 1 | part 2 | part 3 | part 4 | part 4.5.1 📍 (you are here!) | part 4.5.2 | part +1
click here to read on AO3
》 part 4.5.1 word count: ~0.65k
》 part 4.5.1 warnings: none
》 part 4.5.1 spoilers: tcw citadel episode
》 a/n: i'm sorry, it's gonna be a rough one loves :(( also 4.5.1 and 4.5.2 are shorter chapters for more dramatic effect hehe

४.५.१ (4.5.1)
You’re waiting at the ship docking bay when they come back from the Citadel. You have been glued to the reports screen, constantly refreshing every five minutes to see the latest news, but there’s been absolutely nothing. Even with your relatively high rank in the GAR, some reports are just far beyond your reach thanks to the deep classifications.
The shuttle docks and you watch as clones pour out, many wounded. They rush past you, and you move out of their way quickly as the injured men are guided to the medical wing as fast as possible. You try looking at each of their armor, each one of their faces, but you can’t find Fives or Echo. You’re not sure if that’s a positive or not.
And then finally, finally, you see your friend step out along with Rex, Anakin, Ahsoka, and Obi-Wan. Where’s Echo? Everyone knows Echo and Fives are inseparable and probably one of the highest ranking clones on the mission. Why isn’t he with the others? Did he board a different shuttle?
The group is talking amongst themselves and you resist the urge to run up to them, instead digging your heels into the ground and focus on finding Echo, mind bursting with questions. Your eyes scour the troopers pouring out of the shuttles, but you can’t find him anywhere. Your stomach drops to your feet and heavy dread begins to take its place as you frantically search for him.
Fives walks over to you then, seemingly finished with tying up some loose ends of the mission. You turn around and watch him approach, every step of his twisting a knife further into your heart. You hold your breath.
He takes off his helmet and his face. Your mouth drops open in shock at the swirling despair in his eyes. The lines on Fives’ face suddenly seem so deep, so tired. His posture slackens as you just stand there, feet frozen and unable to comprehend what you already know. Fives doesn’t need to say a word.
He drops his helmet, the hollow thud reverberating through your ears, finally prompting you to move. You hold Fives in your arms as he breaks down into your shoulder, whispering I’m sorry, I’m sorry, I’m sorry.
I’m sorry your love is lost.
You clutch the plastoid in your hands, nails digging crescent moons into your skin as you breathe in the carbon-scoring fumes from his armor, tears slipping down your own cheeks because he’s gone.
Echo is gone.
You don’t know how long the both of you stand there, holding on like you’re each other’s last life line. Fives sniffles and pulls away, gazing at you sorrowfully. He answers your burning questions, grief-stricken.
“There… There was this clanker on a turret. Echo, that idiot, grabs a shield from the ground and starts shooting to protect the shuttle. But that kriffin’ clanker… it shot the shuttle and it–it exploded,” Fives whispers the last part and your eyes widen to be as round as moons. An audible gasp slips past your lips as you imagine the horrific scene flash before your eyes.
“I tried to warn him but it was too late. I couldn’t help him. I–I couldn’t save him,” Fives swallows the lump in his throat regretfully. “The last thing I saw was his helmet, smoking.”
“No,” you breathe and the realization hits you in full force.
You could have saved him.
If you had just fixed that stupid bug in your code, if you had just dedicated more time to advancing the technology in the device you gave them, if you had just made it better, you could have saved him.
Fives says nothing.
Your hands curl up in fists as they come up to rest on his plastoid chestplate, leaning into Fives as he holds onto you.
No.
You never got to tell him you love him.
---
part 1 | part 2 | part 3 | part 4 | part 4.5.1 📍 (you are here!) | part 4.5.2 | part +1
please consider reblogging! it really helps me and is super encouraging ^_^
#echo#star wars#tbb#the bad batch#bad batch#echo x reader#arc trooper echo#arc trooper echo x reader#arc trooper echo x you#clone trooper echo#the bad batch x you#the bad batch echo#the bad batch x reader#tbb echo#echo x you#star wars tcw#star wars clone wars#star wars: the clone wars#sw fic#star wars fic
14 notes
·
View notes
Text
The American Diabetes Association developed the term “prediabetes” to bring attention to slightly elevated blood sugar levels in some Americans in 2001. Over the next two decades, the organization expanded the definition of the condition, so that by 2019, as Charles Piller reported for Science magazine, 84 million Americans had prediabetes, “the most common chronic disease after obesity.”
There were no drugs specifically designed for prediabetes, so doctors often relied on off-label treatments, a common medical practice. But because off-label drug interventions coincided with the wholesale expanded classification of millions of people with a novel condition, a new market boomed.
This shift broadened the consumer language for medicalizing weight loss as a preventive strategy to treat not only diabetes, but also supposed — though not always proven — diabetes risk. It armed a wellness machine with the medical terminology of “insulin resistance” and “insulin sensitivity,” without the medical expertise to screen for diabetes risk indicators. People could soon buy an astonishing array of apps and devices to self-diagnose insulin efficiency. Enter Ozempic and Wegovy, perfectly designed for our highly developed consumer palates.
Given all these changes, I wondered what Dr. Richard Kahn, the former chief scientific and medical officer at the American Diabetes Association, who helped establish “prediabetes” as a term, now thought about the phenomenon.
When we talked, Dr. Kahn told me that he regrets his role in developing “prediabetes” and its associated grift, but his giddiness about GLP-1 drugs was palpable. He said that encouraging weight loss through lifestyle changes was an “abject failure.” Now, Ozempic offers patients light and hope.
The problem with these drugs, he said, “is that they cost an enormous amount of money.”
From Tressie McMillan Cottom
Oh, fyi: I weigh about 400lbs. I do not have diabetes or prediabetes.
10 notes
·
View notes
Text
i can be described as nothing less than ruthlessly principled. it's a dangerous quality in a person but i think i may be the one in a million who happened upon some good principles according to reviews so i will share. i was abused as a child by people who suffered psychosis which gave me both an obsessive attention towards harm and an obsessive need for proof before I act, and i love to read. well. it turns out we have an absolute fuckton of data about things that hurt people especially regarding abuse. because people are typically fucking begging you to listen to what hurts them.
and it's like. yeah there's a data threshold. it first has to echo what we know of abuse. all of the -isms and phobias are culturally normalized interpersonal abuses towards a member of a particular group of people. the big abuses of an entire group over another group are built on millions upon millions of the tiny constantly reinforced interpersonal abuses of one human to another human and our collective deafening silence in the face of it.
when a white person does something racist and then gets sad and upset that a person of color was hurt, it's literally fucking classic DARVO. Deny, Attack, Reverse Victim and Offender.
Deny: no i couldn't have been racist i would never be racist im a good person Attack: you're the mean person because you thought i was being racist and failed to provide me with enough benefit of the doubt to know i was always being a good person Reverse Victim and Offender: i can't believe that someone would think so poorly of me i'm so nice and so kind and so good i would never be racist or hurt someone else you hurt me by accusing me of being a bad person and i deserve an apology for being taken in bad faith.
like think about it. for real. think about how your bosses treat you and if you would tolerate it in an acquaintance. think of yourself as a human, a full human with a body and needs and a family and a home life and emotions and an extremely social and intelligent animal who figured out that living in groups makes it easier to survive and take care of our loved ones. then think of every single other organism in the species classification "homo" that exact same way.
you know you're safer in a bigger group that cares for you. if you're american, think about how what you're gonna do if you need to crowdfund a fucking medical bill. think about how much we need each other for every aspect of our lives. how many guys it took to cut and harvest the wood that builds every single one of your buildings and the guys that delivered it and the guys that assembled it into your house and the plumbing and electrical guys and more and more and more who make your house possible. think about the people in the factories who do the dangerous work of making the screws of every size and shape that hold every single one of your devices you couldn't live without together. how many people does your life rely on? and don't they deserve to live well? and doesn't living well necessarily mean freedom from abuse? your boss can scream at you and you probably can't do anything about it. why are we in a human group where it's okay for one member to abuse another.
we are a culture that permits abuse. it's that simple. we say that it's okay to abuse someone in certain ways based on certain characteristics. if you refuse to allow any level of interpersonal abuse in your presence, things get a lot more clear and easy to navigate. verbal denigration is verbal denigration. gaslighting is gaslighting. your culture will tell you that there are types of "good" power imbalances, "necessary" checks to autonomy, verbal denigration that is deserved, or types of gaslighting that are required. it's always a lie. interpersonal abuse of any kind is always violent, always cruel, always controlling. there is no variety that is acceptable. structural power imbalances are obvious and abusive behaviors are consistent if you're brave enough to accurately identify them and recognize them for the harm they are everywhere they are.
#this is not evaluated for ways it can be taken wrong.#people have a right to defend themselves defensive action isnt the same as abuse. raising your voice doesn't mean verbal abuse. idfk.#this post feels cursed. it got rbs too fast.
7 notes
·
View notes
Text
The 2024 Regulation on Medical Laboratories in Turkey

The 2024 Regulation on Medical Laboratories in Turkey was newly declared in the Official Gazette, as of June 4, 2024.
The 2024 Regulation on Medical Laboratories abolishes the previous one, dated 9.10.2023. That change is very critical to navigate dynamics for medical professionals and healthcare lawyers.
It is beyond doubt that medical laboratories play a crucial role in better promotion of healthcare services. This article will give a detailed outline about the new regulation applicable to Turkey healthcare system after June 2024.
What are core legal healthcare instruments in Turkey?
It is significant to underline at the outset that health services are performed under the framework of the Law Numbered 1219 coming from 1928. Additionally, a new legislation on Health Services Law Numbered 3359 entered into force in 1987. Besides, particular services including genetic testing activities and medical device quality management systems are mainly governed by Regulations. Especially the Regulation on Genetic Diseases Evaluation Center and Regulation on Medical Laboratories are of utmost importance in constituting workable standards and principles for the subject matter.
For a comprehensive article on medical device quality management system, take a look at our article on Medical Device Quality Management System in Turkey
What is the aim of the 2024 Regulation on Medical Laboratories?
The purpose of this Regulation is to regulate the procedures and principles regarding the planning, licensing, opening, regulation of activities, classification, monitoring, inspection and termination of activities of medical laboratories and to ensure that they provide quality and efficient service.
What is the news from the 2024 Regulation on Medical Laboratories?
The first thing capturing our attention is the 2024 Regulation on Medical Laboratories begins with a long list of terminologies including biological material, operating license, medical laboratory license, national reference laboratory. The new Regulation is designed for fulfilling all previous gaps of 2013 Regulation from these aspects.
Besides, Article 7 clarifies the four main categories of medical laboratories that may be licensed for operating:
Medical biochemistry laboratory,
Medical microbiology laboratory,
Medical pathology laboratory,
Tissue typing laboratory.
In addition to that medical laboratories are classified into five categories in terms of service content and activities:
Supervised medical laboratory,
Comprehensive medical laboratory
Regional laboratory,
Reference authorized laboratory on a test basis.
National reference laboratory.
Additionally, Article 9 stipulates the procedures in a detailed manner for application about license and activity permit [ruhsat in Turkish]. It is clearly underscored that a medical laboratory is only opened with a license and activity permit issued by the Ministry.
Additionally, the medical laboratory unit established within the health facility is recorded in the facility’s license/activity permit as well.
Last but not least, physical conditions and working conditions of medical laboratories are thoroughly addressed in Article 12 and 13.
Medical Laboratories Planning under the 2024 Regulation on Medical Laboratories?
It is very important to note that according to the new Regulation, medical laboratories that need to operate are planned by the Ministry with the intention of ensuring quality and efficiency.
Conclusion
Having regard to all foregoing, this article already displayed that the 2024 Regulation on Medical Laboratories is intended to cope with previous handicaps arising from shortcomings of 2013 Regulation. All practitioners including medical lawyers are required to consider core potential solutions by the 2024 Regulation on Medical Laboratories. It is worth reiterating that working conditions and license requirements are formulated much more comprehensively in the the 2024 Regulation on Medical Laboratories.
Pi Legal Consultancy is a legal & business consulting and international law firm in Turkey and turkish law firm.
We have three offices based in İstanbul, Ankara and Batman. We also enjoy solution partners and lawyers located in most European Countries, Africa, Canada and United States of America. We offer legal consultancy and advocacy in key legal areas, among others, international trade, investment, citizenship, healthcare, real estate, corporate governance, banking and finance.
2 notes
·
View notes
Text
Made this one in a hurry, cuz I'm excited for something... also working on the site
TW: Disgusting anomaly

ANM-683: "Sanitary Worms"
Classification: Sikur 🟢
Containment: ANM-683 must be kept in a reinforced containment cell resembling a standard bathroom, located in Department-17. Access to ANM-683 is restricted to Level 3 personnel and above. All interactions with ANM-683 must be pre-approved by the Department Director and supervised by at least two security personnel. Bi-monthly maintenance and sanitation protocols must be strictly followed. Any deviation from established procedures must be reported immediately.
All personnel interacting with ANM-683 must undergo mandatory quarantine and medical evaluation, followed by weekly monitoring for a minimum of six months. Any report of abdominal pain, abnormal bowel changes, or unexplained weight loss must be treated as a high-priority incident.
Description: ANM-683 is a standard porcelain toilet, approximately 400 mm in height and width, mounted on a concrete base. Despite its ordinary appearance, ANM-683 exhibits anomalous properties when used.
When an individual attempts to defecate using ANM-683, they will begin to feel severe discomfort and pain in the rectal area within 30 seconds. This sensation rapidly intensifies, resulting in severe cramps and bleeding. Within 24-48 hours, the affected individual will develop multiple rectal pathologies, including but not limited to hemorrhoids, anal fissures, and proctitis.
After approximately 7-10 days, the individual’s digestive system will become infested with thousands of vermiform parasites (Ascaris lumbricoides). These worms will grow and reproduce at an accelerated rate, causing severe abdominal pain, nausea, vomiting, diarrhea, and significant weight loss. In advanced stages, the worms may attempt to exit the host's body through the mouth, nostrils, or even rupture the abdominal wall.
If ANM-683 is damaged or tampered with, ANM-683-B will manifest. 683-B is a humanoid entity approximately 60 cm tall, resembling a malformed infant with distorted facial features, bulging eyes without eyelids, and rows of needle-like teeth. Its skin is completely covered in blood and feces, with visible internal structures, including a pulsating digestive tract filled with worms.
ANM-683-B is highly aggressive and will attack any human within its proximity. It demonstrates extraordinary agility and strength disproportionate to its size, making it difficult to contain or neutralize. It typically moves by writhing on the ground while screaming. ANM-683-B feeds on organic material, with a particular preference for fecal matter. If deprived of sustenance, it will become increasingly violent and unpredictable, targeting live subjects to extract biological material.
After feeding, it will retreat into the toilet, disappearing into the bowl as if passing through solid matter. Attempts to track or follow ANM-683-B into ANM-683 have failed, as exploration devices become nonfunctional upon entry.
Addendum 683-1: Following Incident ANM-683-1, it was discovered that the anomaly’s properties extend beyond its immediate environment. Anyone who has used the toilet will experience intense anal and rectal pain similar to the effects of ANM-683 when exposed to any conventional toilet. This reaction is accompanied by spontaneous worm infestations, regardless of prior medical treatment or intervention.
This effect persists indefinitely unless treated with a regimen of high-dose anthelmintics, antibiotics, and a rigorous intestinal management protocol. Personnel exhibiting symptoms after interaction must be placed under indefinite medical supervision.
Incident Reports:
ANM-683-1: On ██/██/20██, Dr. ████████ accessed ANM-683 without authorization, resulting in severe rectal trauma and worm infestation. Despite emergency intervention, Dr. ████████ exhibited advanced parasitic growth, necessitating experimental treatments. He subsequently developed a compulsive desire to repeatedly use ANM-683, despite the extreme pain and damage, and was placed on indefinite medical leave and transferred to a specialized care unit.
ANM-683-2: On ██/██/20██, during a containment breach, multiple personnel were exposed, and ANM-683-B emerged, attacking the Department staff, causing multiple casualties and severe injuries. Post-incident analysis revealed that ANM-683-B had grown to 80 cm in height and demonstrated increased intelligence and coordination in its attacks. The entity was eventually subdued and re-contained after extensive efforts. Psychological evaluations of surviving personnel indicated elevated levels of paranoia and fear of sanitary facilities.
Approved by Counselor O3-██, ██/██/20██.
Warning: This document contains Class-A restricted information. Unauthorized access, reproduction, or distribution is strictly prohibited and will result in immediate disciplinary action, including dismissal and/or legal proceedings.
1 note
·
View note
Text
«────── « HEADCANON » ──────»
As late-night boredom often has it, I decided to look at ETH.3N (Ethan) and try to determine his approximate composition and overall weight… Because unlike other robots on this blog, there really isn't a frame of reference to make a quick guess (e.g., Legion being more directly comparable to a living, organic species).
Or maybe there is, but I decided to take the complicated route!
So, like I have done for both Therapont and Alduin, I have decided to sit down and calculate these things manually. Despite me being bad at both math and chemistry! Both subjects I have almost failed at least once.
… This should go over well.
Put below the cut because I took explained everything like I was writing a damn thesis.
To start off, I want to clarify where I'm starting from. There are two key assumptions being made here:
Ethan is proportionally modeled after the average athletic human man. This is because the C6 classification of robots are designed to be worker drones and, specifically in Ethan's case, to be stand-ins for human soldiers.
Ethan is primarily composed of a 2-phase alpha-beta type Titanium alloy known as Ti-6AI-4V Titanium (6AI-4V), which is lightweight, corrosion-resistant, and incredibly strong. This type of metal is used in various modern military pieces of hardware, spacecraft components, medical/surgical devices, jet engine compressor blades, and other recreational equipment uses.
To explain a bit more on why 6AI-4V Titanium is one of the stronger (if not, strongest) metallic alloys, I want to briefly explain what a "2-phase alpha-beta type" really is. And to do that, I'm going to quote Yoshiki Oshida MS, PhD, in Bioscience and Bioengineering of Titanium Materials (Second Edition), 2013… or rather, the excerpt reposted onto Science Direct's website [LINK]:
Basically, titanium and titanium-based alloys can be classified into α type (HCP: hexagonal-closed packed crystalline structure), near α type, (α+β) type, and β type (BCC: body-centered cubic crystalline structure) alloy groups. Alloying elements added to titanium are divided into two groups: alpha (α) stabilizers and beta (β) stabilizers. Elements, such as Al, Sn, Ga, Zr, and interstitial elements (either singly of C, O, and N or in combination), dissolve into the titanium matrix and are strong solid solution strengtheners which produce little change at the transformation temperature (β-transus: 885°C for pure Ti) from the HCP (α) to the BCC (β) structure of pure titanium when heating and from BCC to HCP when cooling. Hence, they are known as α-stabilizers and exhibit good high-temperature performance. Alloying elements that decrease this phase transformation temperature are referred to as β-stabilizers. Generally, β-stabilizing elements are the transition metals, such as V, Mo, Nb, Ta, and Cr, providing much friability. Besides these alloying elements, Fe, Cu, Ni, Si, and B are frequently added to Ti-based alloys for improving mechanical strength, chemical stability, castability, and/or grain refining. By increasing the α-phase portion, it is generally recognized that (1) β-transus temperature increases, (2) creep strength as well as high-temperature strengths enhance, (3) flow stress increases, and (4) weldability improves. By increasing the β-phase portion, it is known that (1) room temperature strength increases, (2) heat-treatment and forming capabilities enhance, and (3) strain-rate sensitivity increases so that superplastic forming (SPF) is more favorably applicable.
What this translates to is that through mixing certain elements into the titanium, you get the properties of these elements reflected into the newly developed alloy. And titanium is already… fucking incredible.
So you add more shit to it, and you get something very, very versatile.
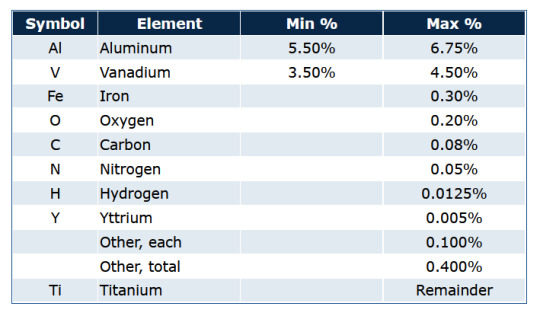
Particularly, with 6AI-4V Titanium, the primary elements being added to create the alloy are Al (Aluminum) and V (Vanadium), with both making up approximately 5.50%-6.75% and 3.50%-4.50% of the alloy respectively. Of course, there are smaller traces of other elements, such as iron, carbon, yttrium, etc., but these are trace compositions with little to no overall impact on the actual alloy. For those curious about the breakdown, here is a screenshot of the average chemical composition of 6AI-4V Titanium, courtesy of the Service Steel Aerospace Corporation:
With that background, we can get into the mathematical portion… almost. There is still the matter of Ethan's proportions. I couldn't get through to the original source material, but I did find the below image in "Modeling and Simulation of a Passive Lower-Body Mechanism for Rehabilitation" by Singh et al. (2016) [LINK]
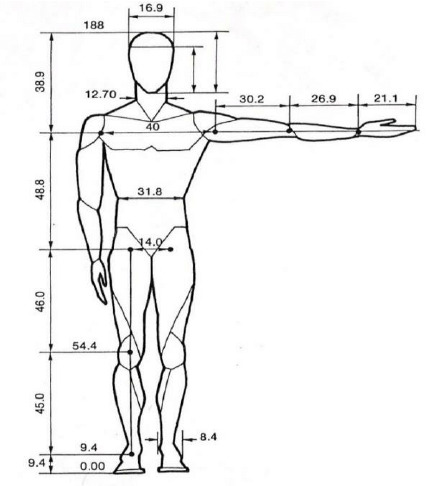
With these proportions in mind, we can assume Ethan is about 188 cm, or about 6 feet and 2 inches.
And then we get approximate breakdowns for each limb section (e.g., upper arm, lower arm).
Of course, these are not entirely accurate, as Ethan's build is not 1:1 with the human body. However, looking at a full body concept of Ethan (pictured below), we can forego really making any major adjustments as he's close… enough.

A bit on the stockier side, so I will account for this by simply adding 1.5x the baseline calculations. Why 1.5? It's an easy number. It adds up quick, though, so I would say it's a good guess.
And finally—par a brief disclaimer—we can get into the math.
DISCLAIMER: I am using TW Metals' calculator [LINK] to determine the approximate weights of each section. They do not have the specific alloy of titanium listed on their site, as they only list what they sell (I'm assuming). So, for the closest weight, I utilized the calculator's input for pure titanium. The weight will be off, but it's the closest I can calculate given the information I have on hand.
So! Let's begin.
First, to utilize the calculator, I have to break Ethan down into parts. Sad.
But it's fairly simple, given the visual references on hand.
Given the nature of Ethan's design, most of the metal is going to be plated (thicker than sheets), so I can really just break down the human body diagram and get a baseline for the amount of plates he has (minus the intricate joints and more defined features, such as his chest chassis and the various abdominal-like grooves).
I then make calculations using the calculator, assuming a varying number (between 6 and 8) of sheets are used per limb section (front, back, and sides; not a super thought out measurement, just an estimate), assume the sheets themselves are (at the bare minimum) 6mm thick, and that's how I got my final estimated range.
With these assumptions noted and input, we get the following approximations for each body section:
HEAD - 8.29 kg // 18.28 lbs TORSO - 35.34 kg // 77.92 lbs ARMS - 15.7 kg // 34.62 lbs HANDS - 2.88 kg // 6.35 lbs HIPS - 12.6 kg // 27.78 lbs LEGS - 47.3 kg // 104.28 lbs FEET - 2.66 kg // 5.86 lbs
And for that bulk adjustment mentioned earlier:
HEAD - 12.44 kg // 27.43 lbs TORSO - 53.01 kg // 116.87 lbs ARMS - 23.55 kg // 51.92 lbs HANDS - 4.32 kg // 9.52 lbs HIPS - 18.9 kg // 41.67 lbs LEGS - 70.95 kg // 156.42 lbs FEET - 3.99 kg // 8.80 lbs
Yeah, not a lot of math to really showcase, so I'll skip right to the good shit:
Ethan would, given my assumptions are fairly accurate, weigh between 124.77 kg // 275.07 lbs and 187.16 kg // 412.62 lbs.
Give or take. Not accounting for the weight of his circuitry and motors.
Which is a pretty sizeable margin, but again, that simple little 1.5x adjustment for bulk makes the difference. So, in my classic fashion, I will simply take the average of these variables, and use that as my canonical weight.
AKA: Ethan weighs 155.97 kg // 343.85 lbs.
Yay.
#(headcanon)#(m: ETH.3N)#(long post)#( why is me doing math at ungodly hours becoming a normal occurrence on this blog? someone put me down- )
2 notes
·
View notes
Text

Dmitry/researcher Makarov anomaly classification
Dmitry not only is a researcher working for the medical department of the site as head psychiatrist but it's also helped by an anomaly that he naturally has since he was born. He's not contained in any way but I'd love to give him a classification and some definitions:
CONTAINMENT CLASS:
-Pending;
-Neutralized after his death.
SECONDARY CLASS :
-Ticonderoga, does not need to be contained.
DISRUPTION CLASS:
-Vlam, affecting few people.
RISK CLASS:
-Caution or Warning.
Other possible classifications:



Mind-control device lightning strike weapon excluded
#scp mtf#scp researcher oc#scp foundation#scp oc#scp oc art#scp original character#original characters#original character
5 notes
·
View notes
Text
Personal Electronic Dosimeter Market Size, Share, Growth, Trends, Demand and Opportunity Analysis
Executive Summary Personal Electronic Dosimeter Market :
Data Bridge Market Research analyses that the global personal electronic dosimeter market which was USD 3,414.45 million in 2022, would rocket up to USD 4,813.61 million by 2030, and is expected to undergo a CAGR of 7.11% during the forecast period.
Personal Electronic Dosimeter Market business report truly acts as a backbone to the business. Moreover, global market report encompasses all the company profiles of the major players and brands. With this market research report it becomes easy to develop a successful Market strategy for the business. A right utilization of recognized statistical tools and coherent models for analysis and forecasting of market data makes this report outshining. The comprehensive Personal Electronic Dosimeter Market research report takes into account key product developments and tracks recent acquisitions, mergers and research in the industry by the top market players.
The large scale Personal Electronic Dosimeter Market report is a thorough and professional report that focuses on primary and secondary drivers, market share, leading segments and geographical analysis. These calculations will provide estimations about how the Personal Electronic Dosimeter Market is going to perform in the forecast years by informing what the market definition, classifications, applications, and engagements are. 2022 is the base year while 2021 is the historic year for calculation in the report. Being an excellent in quality, this market research report gains customer confidence and trust. Personal Electronic Dosimeter Market report comprises of a chapter on the global market and allied companies with their profiles, which delivers essential data pertaining to their insights in terms of finances, product portfolios, investment plans, and Market and business strategies.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Personal Electronic Dosimeter Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-personal-electronic-dosimeter-market
Personal Electronic Dosimeter Market Overview
**Segments**
- By Product Type: Passive Dosimeters, Active Dosimeters - By Modularity: Built-in Dosimeters, External Dosimeters - By Application: Medical (Hospitals, Radiology Centers), Industrial (Nuclear Power Plants, Oil & Gas), Defense & Homeland Security
The global personal electronic dosimeter market is segmented based on product type, modularity, and application. In terms of product type, the market is categorized into passive dosimeters and active dosimeters. Passive dosimeters are widely used due to their simplicity and reliability in measuring radiation exposure over time. Active dosimeters, on the other hand, provide real-time monitoring of radiation levels, making them ideal for high-risk environments. Based on modularity, personal electronic dosimeters are classified as built-in dosimeters or external dosimeters. Built-in dosimeters are integrated into personal protective equipment, while external dosimeters are standalone devices that can be attached to clothing or accessories. The market is further segmented by application, including medical, industrial, and defense & homeland security sectors. In the medical field, personal electronic dosimeters are essential for monitoring radiation exposure among healthcare professionals working in hospitals and radiology centers. Industrial applications encompass nuclear power plants, oil & gas facilities, and other workplaces where radiation hazards are present. The defense & homeland security sector utilizes personal electronic dosimeters to safeguard personnel against radiological threats during military operations and emergency response situations.
**Market Players**
- Mirion Technologies, Inc. - Thermo Fisher Scientific Inc. - Landauer, Inc. - Panasonic Corporation - Unfors RaySafe (a subsidiary of Fluke Biomedical) - Ludlum Measurements, Inc. - ATOMTEX - Fuji Electric Co., Ltd. - Polimaster - Bertin Instruments
Key players in the global personal electronic dosimeter market include Mirion Technologies, Inc., Thermo Fisher Scientific Inc., Landauer, Inc., Panasonic Corporation, Unfors RaySafe, Ludlum Measurements, Inc., ATOMTEX, Fuji Electric Co., Ltd., Polimaster, and Bertin Instruments. These companies are actively involved in product development, strategic partnerships, and market expansion initiatives to enhance their market presence and cater to the growing demand for radiation monitoring solutions. The competitive landscape is characterized by technological advancements, regulatory compliance, and a focus on user-friendly features to improve the safety and efficiency of personal electronic dosimeters across various end-user industries.
The global personal electronic dosimeter market is poised for significant growth in the coming years as the demand for radiation monitoring solutions continues to rise across various industries. One key trend that is likely to impact the market is the increasing focus on enhancing the precision and accuracy of dosimeters to ensure optimal radiation exposure monitoring for individuals working in high-risk environments. Market players are investing in research and development activities to introduce advanced technologies such as real-time monitoring capabilities, data analytics, and connectivity features to meet the evolving needs of end-users.
Moreover, the market is witnessing a surge in strategic collaborations and partnerships among key players to expand their product portfolios and gain a competitive edge in the industry. Collaborations between technology companies and healthcare institutions, industrial organizations, and defense agencies are becoming increasingly common to develop customized solutions for specific applications. These partnerships not only drive innovation in the personal electronic dosimeter market but also help in addressing complex challenges related to radiation safety in diverse sectors.
Furthermore, the growing emphasis on regulatory compliance and certification standards is shaping the competitive landscape of the personal electronic dosimeter market. Market players are required to adhere to stringent regulations governing radiation monitoring devices to ensure the safety and reliability of their products. Compliance with international standards and certifications is crucial for building trust among end-users and gaining market acceptance for personal electronic dosimeters in critical industries such as healthcare, nuclear power, and defense.
Another notable development in the market is the increasing adoption of wearable technology in personal electronic dosimeters. Wearable dosimeters offer convenience, comfort, and mobility to users, allowing them to monitor their radiation exposure levels in real-time and take necessary precautions promptly. With the integration of sensors, communication capabilities, and ergonomic design, wearable dosimeters are becoming popular among professionals working in dynamic environments where radiation exposure monitoring is essential for safety protocols.
In conclusion, the global personal electronic dosimeter market is witnessing dynamic growth driven by technological advancements, strategic partnerships, regulatory compliance, and the adoption of wearable technology. As market players continue to innovate and collaborate to address the evolving needs of end-users across medical, industrial, and defense sectors, the personal electronic dosimeter market is expected to experience steady expansion and offer lucrative opportunities for growth and development in the coming years.The global personal electronic dosimeter market is poised for substantial growth driven by increasing demand for radiation monitoring solutions across diverse industries. One key trend shaping the market is the emphasis on enhancing dosimeter precision and accuracy to ensure optimal radiation exposure monitoring in high-risk environments. Market players are focusing on research and development to introduce advanced technologies like real-time monitoring, data analytics, and connectivity features to meet evolving end-user needs. Strategic collaborations and partnerships are on the rise among key market players to broaden product portfolios and gain a competitive advantage. These partnerships aim to develop customized solutions for specific applications, fostering innovation in the personal electronic dosimeter market and addressing complex challenges related to radiation safety across sectors like healthcare, industrial, and defense.
Moreover, regulatory compliance and certification standards play a crucial role in shaping the competitive landscape of the personal electronic dosimeter market. Adherence to strict regulations governing radiation monitoring devices is essential to ensure product safety and reliability. Compliance with international standards is vital for building trust among end-users, especially in critical industries such as healthcare and nuclear power. The market is witnessing a shift towards the adoption of wearable technology in personal electronic dosimeters. Wearable dosimeters offer users convenience, comfort, and mobility, enabling real-time monitoring of radiation exposure levels and prompt precautionary measures. By integrating sensors, communication capabilities, and ergonomic design, wearable dosimeters are gaining popularity among professionals working in dynamic environments where radiation exposure monitoring is essential for safety protocols.
In conclusion, the personal electronic dosimeter market is experiencing significant growth due to technological advancements, strategic partnerships, regulatory compliance, and the increasing adoption of wearable technology. Continuous innovation and collaboration among market players to meet the evolving needs of end-users across various sectors will drive steady market expansion and present lucrative growth opportunities in the foreseeable future. The market's trajectory indicates a promising outlook for stakeholders looking to capitalize on the demand for advanced radiation monitoring solutions in today's increasingly safety-conscious industries.
The Personal Electronic Dosimeter Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-personal-electronic-dosimeter-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Radical conclusions of the report:
Industry overview with a futuristic perspective
Analysis of production costs and analysis of the industrial chain
Full regional analysis
Benchmarking the competitive landscape
Personal Electronic Dosimeter Market Growth Trends: Current and emerging
Technological developments and products
Comprehensive coverage of market factors, restraints, opportunities, threats, limitations, and outlook for the Market
SWOT Analysis, Porter's Five Forces Analysis, Feasibility Analysis, and ROI Analysis
Browse More Reports:
Global Flavour Systems Market Global Automotive Coolant AfterMarket France Fruit Concentrate Market Global Fiber-Reinforced Composites Market North America Extreme Lateral Interbody Fusion (XLIF) Surgery Market Europe Fluoroscopy - C Arms Market Global Service Integration and Management Market Global Erythema Nodosum Market North America Quicklime Market Global Cast Saw Devices Market Global Methanol-to-olefins Market Global Medical Clothing Market Global Bubble Wrap Packaging Market Europe Cyclodextrins in Pharma Market Global Home Fragrances Market Southeast Asia Third Party Logistics Market Global Fiber Optics Components Market Global Herbs and Spices Kombucha Market Global Memory Packaging Market Global On-Site Oil Condition Monitoring Market Asia-Pacific Network Packet Broker Market Global Household Robots Market Global Cereals & Grains Crop Oil Concentrates Market Global Oxygenated Solvents Market Global Periodic Paralyzes Treatment Market Global Rice Transplanter Market Global Respiratory Syncytial Virus Infection Market Global Ethyl Tertiary-butyl Ether Market Asia-Pacific Medical Clothing Market Global Phenol Market Global Anti-Anxiety Drug Market Global Immunofluorescence Market Global Magnesium Alloys Market Europe Chinese Hamster Ovary (CHO) Cells Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag:- Personal Electronic Dosimeter Market, Personal Electronic Dosimeter Market Trends, Personal Electronic Dosimeter Market Growth, Personal Electronic Dosimeter Market Demand, Personal Electronic Dosimeter Market Size, Personal Electronic Dosimeter Market Scope, Personal Electronic Dosimeter Market Insights, Personal Electronic Dosimeter Market Analysis
0 notes
Text
How to Register a Medical Device in Saudi Arabia?

To register a medical device in Saudi Arabia, manufacturers must comply with SFDA (Saudi Food and Drug Authority) regulations. Here’s a simplified step-by-step process:
Classify the Device: Follow the IMDRF risk-based classification (Class A to D for both general and IVD devices).
Appoint a Local Authorized Representative (AR): A Saudi-based AR is mandatory to manage communications with SFDA.
Obtain Medical Device Marketing Authorization (MDMA): Submit a Technical File including design, safety, clinical data, and regulatory approvals (CE, FDA, etc.).
Register on the GHAD Portal: Submit applications and documentation electronically via SFDA’s online platform.
Comply with ISO 13485:2016 QMS Standards: Your manufacturing process must meet international quality system requirements.
Labeling and Post-Market Surveillance: Devices must include Arabic and English labeling, and you must have PMS systems in place.
Click Now:
https://operonstrategist.com/en-sa/register-a-medical-device-in-saudi-arabia/?utm_source=Google&utm_campaign=Offpage7
Get Expert Guidance from Operon Strategist Call: 9028043428 | 9370283428 | 9325283428
0 notes
Text
Delhi Police Syllabus 2025 – Latest Exam Pattern & Subject-Wise Topics
ChatGPT said: The Delhi Police Syllabus 2025 has been designed to assess a candidate’s intellectual ability, reasoning skills, and awareness of current events. The recruitment process typically includes a Computer-Based Examination (CBE), followed by Physical Endurance and Measurement Tests (PE&MT), and a Medical Examination. The CBE is the most crucial stage, featuring a total of 100 questions for 100 marks with a duration of 90 minutes.

The syllabus is divided into four main sections: General Knowledge/Current Affairs, Reasoning, Numerical Ability, and Computer Fundamentals. The General Knowledge section evaluates candidates on topics such as Indian History, Constitution, Geography, Economics, Science, Static GK, and Current Affairs with emphasis on national and international developments, awards, sports, books, and government schemes. The Reasoning section includes both verbal and non-verbal reasoning such as coding-decoding, analogies, classification, series, syllogism, mirror images, and blood relations. This section aims to test the logical and analytical thinking of candidates. In the Numerical Ability section, questions are framed from topics like Number Systems, Percentages, Profit and Loss, Simple and Compound Interest, Time and Work, Time and Distance, Mensuration, Data Interpretation, Ratio and Proportion, and Average. This part examines mathematical aptitude and problem-solving skills. The Computer Fundamentals section checks basic computer knowledge, including topics such as hardware and software, internet usage, MS Office (Word, Excel, PowerPoint), input and output devices, and basic troubleshooting. The exam follows a negative marking system, where 0.25 marks are deducted for every wrong answer, so accuracy is vital. The questions are set in both Hindi and English, ensuring bilingual accessibility. Candidates who clear the written test are shortlisted for the Physical Endurance and Measurement Test (PE&MT), which varies for male and female candidates based on age and includes tasks like running, long jump, and high jump, along with specific height and chest measurements. The final selection is made after the Medical Examination to ensure candidates are physically and medically fit for police service. Preparing strategically for each section with the right resources and mock tests can help aspirants significantly improve their chances. Candidates are advised to focus especially on current affairs, reasoning tricks, and fast calculation techniques. With a structured study plan and consistent practice, cracking the Delhi Police 2025 exam becomes highly achievable. The syllabus not only aims at testing theoretical knowledge but also practical understanding, decision-making skills, and overall aptitude suitable for law enforcement roles. Updated notifications, syllabus changes, and pattern modifications should be regularly tracked via the official Delhi Police or SSC websites. In summary, the Delhi Police Syllabus 2025 offers a comprehensive test of mental, physical, and practical competencies, ensuring that selected candidates are well-rounded and capable of upholding public safety and law. It is essential for aspirants to stay disciplined, revise frequently, and attempt previous year papers to gauge their preparation level. Thorough understanding of the syllabus topics, along with time management and accuracy, remains the key to success in this competitive recruitment exam.
0 notes
Text
The Role of Regulatory Compliance Consulting Services in Successful Product Approvals

Bringing a new drug, device, or therapy to market is a journey filled with promise—and potential pitfalls. The difference between timely success and costly delay often comes down to one critical factor: the strength of your regulatory submission strategy. At Innovate Research, they believe that expert regulatory compliance consulting is not just a service, but a catalyst for innovation and global impact.
What Are Regulatory Compliance Consulting Services—And Who Needs Them?
Regulatory compliance consulting services are specialized solutions designed to guide pharmaceutical, biotechnology, medical device, and even food and beverage companies through the maze of national and international regulations. Whether you’re a startup seeking your first approval or an established manufacturer entering new markets, the expertise of medical regulatory consultants is essential to avoid missteps and accelerate your product’s path to patients.
Navigating Complex, Evolving Global Regulations
The regulatory landscape is in constant flux. From the FDA and EMA to CDSCO and other global agencies, requirements for regulatory submission are frequently updated and increasingly stringent. Innovate Research’s team of experts stays ahead of these changes, ensuring your product always meets the latest standards—no matter the region.
Key Steps in Preparing for Regulatory Submissions
A successful regulatory submission is built on meticulous preparation. Innovate Research offers end-to-end support, including:
Dossier Preparation: Comprehensive CMC, clinical, and non-clinical documentation, tailored for both paper and eCTD formats.
Gap Analysis & Audit Readiness: Identifying and addressing compliance gaps before they become obstacles.
Regulatory Strategy & Intelligence: Mapping the optimal route from molecule to market, with foresight into potential challenges.
Their medical device regulatory services are especially robust, supporting everything from initial classification and risk assessment to clinical evaluation reports and labeling.
How Regulatory Consultants Help You Avoid Delays
Regulatory hurdles can derail even the most promising innovations. Innovate Research’s regulatory compliance consulting services are designed to:
Prevent common pitfalls in documentation and submission
Streamline communication with regulatory agencies
Anticipate and address queries or deficiencies before they become barriers
With their guidance, clients have consistently achieved faster approvals and smoother market entries, saving both time and resources.
Staying Ahead: The Importance of Regulatory Intelligence
In a world where guidelines evolve rapidly, staying informed is non-negotiable. IR’s consultants monitor updates from FDA, EMA, CDSCO, and other authorities, ensuring your strategy is always current. This proactive approach not only reduces risk but also positions your product for global success.
Value-Added Services for Every Stage of Development
Innovate Research goes beyond basic consulting. Our suite of value-added services includes:
Training for your teams on the latest regulatory requirements
Process optimization for greater efficiency and compliance
Lifecycle management to support your product from development through post-marketing
They also offer regulatory agency liaising and business outsourcing consultancy, making them a true partner in your growth.
Future-Proofing Your Submissions
The future of regulatory submission is digital and interconnected. Innovate Research is at the forefront of trends like eCTD submissions, real-time data sharing, and the harmonization of global standards. Their digital-first mindset ensures your submissions are not just compliant today, but ready for tomorrow’s demands.
Why Choose Innovate Research?
Their team combines deep regulatory expertise with hands-on experience across pharmaceuticals, medical devices, and more. Whether you need targeted medical device regulatory services or comprehensive support from medical regulatory consultants, Innovate Research delivers seamless, transparent, and timely solutions that set you apart in a crowded market.
Ready to accelerate your next regulatory submission?
Partner with Innovate Research and experience the difference that expert regulatory compliance consulting services can make. Contact them today to start your journey to successful product approval.
#Medical Device Regulatory Services#Regulatory Compliance Consulting Services#Regulatory Submission
1 note
·
View note
Text

Book Your CDSCO Registration Consultation Now
Navigating CDSCO approvals for medical devices, cosmetics, or drug licenses? Let our experts simplify the process for you — fast, compliant, and stress-free.
✅ End-to-end documentation support ✅ Guidance on product classification ✅ Filing through SUGAM Portal ✅ Expert assistance for imports & manufacturing Limited slots available this week! Book your FREE 15-min consultation now and get clarity on your CDSCO compliance journey.
0 notes
Text
Global Postpartum Hemorrhage Treatment Devices Market Trends | Report [2025-2033]
Global Postpartum Hemorrhage Treatment Devices Market research report provides a complete overview of the market by examining it both qualitatively and statistically, including particular data and in-depth insights from several market segments. While the qualitative analysis of market dynamics, which includes growth drivers, challenges, constraints, and so on, offers in-depth insight into the market's current and potential, the quantitative analysis includes historical and forecast statistics of major market segments.Get Free Request Sample : https://www.globalgrowthinsights.com/enquiry/request-sample-pdf/postpartum-hemorrhage-treatment-devices-market-100116Who is the Top largest companies (Marketing heads, regional heads) of Global Postpartum Hemorrhage Treatment Devices Market?3rd Stone Design, Program for Appropriate Technology In Health (Path), R. Bard, Bactiguard, Davol, Utah Medical Products, Teleflex Incorporated, Becton Dickinson, Ge Healthcare, Cook MedicalMarket Segmentations:On the thought of the product, this report displays the assembly, revenue, price, Classifications market share and rate of growth of each type, primarily split intoUterine Balloon Tamponade, Uniject Prefilled Injection System, OtherOn the thought of the highest users/applications, this report focuses on the status and outlook for major applications/end users, consumption (sales), market share and rate of growth for each application, includingHospitals, Clinics, OtherKey Drivers of the Global Postpartum Hemorrhage Treatment Devices Market MarketTechnological Innovation: The pulse of the Global Postpartum Hemorrhage Treatment Devices Market market is its ongoing technological evolution, enhancing product and service efficiency. Innovations span materials, manufacturing, and digital technologies.Surging Demand: Factors like population growth, urbanization, and shifts in consumer preferences are fueling a rising demand for Global Postpartum Hemorrhage Treatment Devices Market products and services, propelling market expansion.Regulatory Encouragement: Supportive government measures, including incentives and regulations favoring Global Postpartum Hemorrhage Treatment Devices Market adoptions, such as renewable energy subsidies and carbon pricing, are catalyzing market growth.Environmental Consciousness: The growing awareness of environmental issues and carbon footprint reduction is accelerating the uptake of eco-friendly and renewable Global Postpartum Hemorrhage Treatment Devices Market solutions.Cost Efficiency: The decreasing costs associated with producing and deploying Global Postpartum Hemorrhage Treatment Devices Market solutions, thanks to technological progress, competitive markets, and scale economies, are making these options increasingly attainable. View Full Report @: https://www.globalgrowthinsights.com/market-reports/postpartum-hemorrhage-treatment-devices-market-100116 About Us: Global Growth Insights is the credible source for gaining the market reports that will provide you with the lead your business needs. At GlobalGrowthInsights.com, our objective is providing a platform for many top-notch market research firms worldwide to publish their research reports, as well as helping the decision makers in finding most suitable market research solutions under one roof. Our aim is to provide the best solution that matches the exact customer requirements. This drives us to provide you with custom or syndicated research reports.
#Marketsize#Markettrends#growth#Researchreport#trendingreport#Business#Businessgrowth#businessTrends#GGI#Globalgrowthinsights
0 notes
Text
Laminar Flow Clean Benches: The Ultimate Guide for Lab Professionals

In industries where sterility and contamination control are critical, a laminar flow clean bench is not just useful—it’s essential. Whether you work in pharmaceuticals, biotechnology, research, or electronics, maintaining a controlled clean environment is non-negotiable. In this article, we’ll explore what a laminar flow clean bench is, how it works, its benefits, common applications, and what to look for when purchasing or maintaining one.
🔍 What Is a Laminar Flow Clean Bench?
A laminar flow clean bench is a specialized workstation that provides a contaminant-free environment by directing air through high-efficiency filters and over the workspace in a uniform direction. It is primarily used in laboratories and manufacturing environments where product protection is essential.
The term “laminar flow” refers to air that moves in parallel layers, with minimal disruption between the layers. This smooth airflow helps sweep away airborne contaminants, ensuring a clean and sterile workspace.
There are two main types:
Horizontal Laminar Flow Benches: Air flows from the back of the bench toward the user.
Vertical Laminar Flow Benches: Air flows from the top of the bench downward onto the work surface.
Both types use HEPA (High-Efficiency Particulate Air) filters to remove 99.99% of airborne particles as small as 0.3 microns.
⚙️ How Does a Laminar Flow Clean Bench Work?
The operation of a laminar flow clean bench can be broken down into a few simple steps:
Air Intake: Room air is drawn into the unit.
Pre-Filtration: Large particles are removed via a pre-filter.
HEPA Filtration: Air passes through a HEPA filter, removing fine particulates, bacteria, and other contaminants.
Laminar Airflow: The cleaned air is then directed over the work surface in a smooth, continuous stream.
The result is a workspace that meets stringent cleanliness standards, ideal for handling sensitive materials.
✅ Key Benefits of Using a Laminar Flow Clean Bench
1. Contamination Control
The primary advantage is its ability to create a particle-free environment. This is vital for experiments, sample preparations, and assembly tasks that require extreme cleanliness.
2. Cost-Efficient Clean Area
Rather than converting an entire room into a cleanroom, a laminar flow clean bench provides a localized clean space, saving on infrastructure and maintenance costs.
3. HEPA Filtration
These benches remove nearly all airborne contaminants using high-efficiency filters, helping ensure product integrity and experiment accuracy.
4. Versatile Applications
Used in multiple industries including microbiology, semiconductor manufacturing, medical device production, and more.
🧫 Common Applications
Pharmaceutical Research: For compounding sterile products and testing formulations.
Microbiology: To culture and examine samples without risk of cross-contamination.
Electronics Assembly: Protects sensitive components from dust and static.
IV Preparation in Hospitals: Ensures sterile conditions for mixing medications.
Tissue Culture: Used widely in biotech labs for growing cells in a contaminant-free environment.
🛠️ Choosing the Right Laminar Flow Clean Bench
When selecting a clean bench, consider the following factors:
✅ Size & Workspace
Choose a bench that fits your available lab space and accommodates the equipment you’ll be using.
✅ Airflow Direction
Horizontal benches offer better user comfort and are easier to clean.
Vertical benches offer better product protection and are ideal for working with hazardous substances (when paired with proper containment).
✅ Filter Efficiency
Always opt for certified HEPA or ULPA (Ultra Low Particulate Air) filters for optimal performance.
✅ Compliance Standards
Make sure the unit complies with ISO 14644-1 cleanroom classifications and industry-specific guidelines.
✅ Ergonomics & Accessibility
Look for features like adjustable height, glare-free lighting, and silent operation to improve user comfort and reduce fatigue during extended use.
🔧 Maintenance Tips for Long-Term Use
Maintaining your laminar flow clean bench is crucial for ensuring long-term performance and contamination control.
Regular Filter Checks: HEPA filters should be tested and replaced based on manufacturer recommendations (typically every 3–5 years).
Routine Cleaning: Clean interior surfaces with lint-free cloths and appropriate disinfectants.
Airflow Testing: Annual airflow and particle testing ensure the unit continues to meet performance standards.
Avoid Overloading: Keep the workspace clutter-free to maintain unobstructed airflow.
⚠️ Common Mistakes to Avoid
Using the bench to store non-sterile items like cardboard boxes or personal items.
Turning off the unit between short intervals—this disrupts air balance.
Not allowing sufficient warm-up time after startup (usually 10–15 minutes).
🧾 Conclusion
A laminar flow clean bench is more than just a piece of lab furniture—it’s a critical tool for ensuring product integrity, preventing contamination, and supporting precision in high-stakes environments. From biotech research to electronics manufacturing, clean benches are a cornerstone of modern sterile techniques.
When chosen, used, and maintained correctly, a laminar flow clean bench can provide years of reliable, high-performance service. Whether you’re equipping a new lab or upgrading existing facilities, understanding the role and function of these clean benches is the first step toward superior contamination control.
0 notes