#calcium-rods
Note
i am SO silly
(haiii could i get a cubfan135 with the aro flag, hold the vex, extra sculk? thanx)

creature :)
198 notes
·
View notes
Note
Your art style is really cool + I like your ocs' vibes :D
TYYY
:DDDD
2 notes
·
View notes
Note
your art style is good i would like to hold it
teehee thank you :3 im hoping to make an actually active art blog on here sometime soon so i can compile my stuff into a bit of a portfolio(?) to start doing coms online but my main problem is i forget to post any of my art like a sillybilly (anime girl blehh pose)
1 note
·
View note
Text
do you ever want to summon the moon to cover the land in eternal darkness forever
0 notes
Text
Cybertronian Drinks and Food
So wanted to try and make a little interactive thing for y'all to send in as request. I'm sure you guys have seen this piece I've made for some of the Energon and Minerals the Transformers consume. Send in to my ask box

So I'm giving you guys the power to send in different things from this list to be made into energon drink art. Or edibles of a sort.
Energon and Fuels
Dark Energon
Synthetic Energon
Tox-En
Red Energon
engex
High grade
Energon wine
Energon Z
Natural Energon
Pure energon
Biofuel
super energon
Energon rod
EnerGULP
Diesel
unleaded petrol
95-octane
E10
E85 - flex-fuel
Jet A and Jet A1 Jet B
JET-A, JP5, JP8
LH2/LOX
RP-1/LOX
kerosene
LSFO
Oil
Minerals, Crystal's and Chemicals
Petroleum
Hydrogen
Copper
Aluminium
Titanium
Lead
Tin
Nickel
Gold
Zinc
Magnesium
Cobalt
Tungsten
Platinum
Chromium
Silver
Manganese
Sodium
Beryllium
Vanadium
Molybdenum
Palladium
Uranium
Zirconium
Bismuth
Cadmium
Mercury
Hafnium
Lanthanum
Niobium
Rhodium
Scandium
Tantalum
Yttrium
Cerium
Plutonium
Lithium
Neptunium
Meitnerium
Seaborgium
Dubnium
Francium
Gallium
Indium
Potassium
Rubidium
Strontium
Thallium
Barium
Calcium
Cesium
Calcite
Pyrite
Copper
Quartz
Benitoite
Diamond
Fluorite
Galena
Garnet
Gold
Oxide
Sulfides
Gypsum
Halite
Phosphates
Sulfates
Carbonates
Iron
__________
Let me know if you would like to be added to tag list (tagged for every fic)
Taglist
@angelxcvxc
@saturnhas82moons
@kgonbeiden
@murkyponds
@autobot79
@buddee
@bubblyjoonjoon
@chaihena
@pyreemo
@lovenotcomputed
@mskenway97
@delectableworm
@cheesecaketyrant
@ladyofnegativity
@desertrosesmetaldune
@stellasfallow
@coffee-or-hot-cocoa
@shinseiokami
@tea-loving-frog
@aquaioart
@daniel-meyer-03
@pupap123
@dannyaleksis
@averysillylittlefellow
@wosemoose1
#transformers#transformers idw#mtmte#transformers lost light#transformers prime#world building#transformers worldbuilding
152 notes
·
View notes
Note
post your tangotek ramblings
you asked for it
Tango (mostly general blazeborne) anatomical ramblings and notes and head canons and stuff . I’m using tango as an example apart from the other head canons which are unique to him
The little like. Legs? Blaze rods remind me of arms, so henceforth my tango design has for arms and to compensate for the other he has an oddly shaped spine similar to a diamond kind of? Blazes obviously don’t have spines but I think they’d be distantly related to withers which Ultimately bleeds into the human/blaze cross aspect of tango. Blazes have white eyes, but tango’s eyes are red due to redstone poisoning (confirmed by tango himself), I think his claw or fingertips would be red as well like Taki dust core mf. tango has an overbite in my design (mid ramble snack and holy shit this chicken is so salty) and he’s definitely a messy eater, like izustumi from dungeon meshi he holds his spoons weird ok
I thinkthe idea of the fire hair is definitely one of my favourite design choices and I can 100% imagine his friends cooking over it. They prolly got booked for open flames a few times which is why the back of tango’s vest in has a fire-proof hood, to hide that feature.
he’s talented at nether exploration mostly bc He’s from the nether.. His natural mapping ability helped him to develop decked out with such a big area.
Prolly has a sparkbox- (For reference, a ‘spark box’ is what dragons have to ignite fire in their throats, their ‘saliva’ acts like a gasoline of sorts, the anatomy of a dragon breathing fire is just spitting Venom/saliva and it igniting. it’s pretty cool. I read it in a book when I was like. Eight. Dragononology with my own twists) anyway, he has the ability to use it whenever he wants but if he uses it too much his throat hurts.. so he just doesn’t really tend to use it unless super important anyway. immunity to heat fire and stuff, this wouldn’t apply in hermitcraft or life to make it fair, but in general if we’re not going on minecraft logic, blah blah blah)
also dungeon master tango was a result of a prank; it was dry ice mixed with soul soil into a shampoo the hermits made for him on a whim bc they thought it’d be a funny prank.. which tango ended up just liking anyway so the prank was a bit of a failure ☠️☠️ but hooray for tango it made him blue for his cool dungeon master bit
Anyways imo Blazes typically have extremely light bodies which Is why they can spin, held together by idk. Wistful magic and shit and they float, because of the wind uproar from below them. Tango cannot do that because of his human bones, which are, unfortunately, slightly more fragile due to the mix of dna In the density and structure and calcium and stuff
I’m really autistic over him And his species don’t hmu


#tangotek#hermitcraft tango#tango fanart#life series#hermitcraft#blaze#minecraft blaze#blazeborn tango#minecraft#anatomy#ramblings#rambles#autism
23 notes
·
View notes
Text

Who's the man who can conquer death?
I had the delight and pleasure of working on a piece for the Mini Hermit Fan-Zine organized by @calcium-rods!
This was the premier run of the Mini Hermit Fan-Zine, a Hermitcraft zine dedicated to showcasing smaller creators in the community! The theme was "Alternate Hermits", and due to the reason for the season there are many spooky entries!
Please check out all the amazing pieces in this zine and the talented artists involved!
237 notes
·
View notes
Text









wizbean - charlie - zoe - kin - kane - milo - sunny - valentín - ashiaga
tumblrs: @duck-that-does-stuff @calcium-rods
i have officially surpassed my bloom total of 100 attacks! i have no goal now so im just going until AF ends
4 notes
·
View notes
Text
Quick Health Update.
Chest CT Scan w/ Contrast & Calcium Score. Calcium score is 0. Can’t get better than that so yay? They didn’t find anything unusual but I had a bad reaction to the contrast. Blacked out a bit from the pain (not usually painful for most people so idk what that was about. Felt like a red hot metal rod being jammed in my arm) and missed the breathing cue so had to do it again with even more contrast which flooded the image and made it really hard to see the right side of the heart. Cardiologists conclusion is that I’m fine. I don’t want another CT scan ever again. That was really awful.
Upper Endoscopy. Anesthesiologist was really mad bc of my complex medical history she said I should have had it scheduled at a hospital and not their clinic. She was mad on my behalf that the cardiologist isn’t doing anything to treat my crazy heart stuff and she almost didn’t let me do the endoscopy but she went through all of my test reports over this past year (ct. echo. ekg. lung function. mri) and finally agreed. All those reports saying “you’re tests are normal” were finally good for something 😒 She warned me of all the side effects of the endoscopy & biopsy and said they would transfer me to an emergency room if anything went wrong but tbh it was the most pain relief I’ve had in months and this is what has finally convinced me I need to give methotrexate a try bc I can’t keep living in this much pain.
Oh and the results from the endoscopy is that I don’t have any ulcers but I do have inflammation. The crazy thing is that the inflammation looks more related to my autoimmune issues than like stomach acid problems or whatever. Gastroenterologist is putting me on Omeprazole to protect my stomach from the NSAIDs and I’m going to follow up with the Rheumatologist on the Methotrexate. (I’m also in the process of getting Tirosint approved by my insurance bc I know I’ve been reacting to the fillers in my levothyroxine)
12 notes
·
View notes
Note
your 2AL au has been rotating in my head consistently now, and fun fact (that is probably universal but whatever) is turtles bask so much bc they can't absorb calcium without the Vitamin D3, meaning their bones and shells get dangerously soft, so if Leo's prosthetic is connected skeletally by that titanium rod Don mentioned, i can see him and probably f!leo too having Absolutely Mandatory Bask times to make sure the port stays healthy :) just a little mental image i thought i'd share!
As a massive fan of biology over here that is so cool..?? Oh my gosh thank you for sharing!!! But yeah, the rod and the port as a whole is definitely connected skeletally for the sake of what it has to support! Honestly I should probably try drawing up some diagram of how I believe it would be connected,, And oh I could so see him just sun bathing on days when the port is sore...
46 notes
·
View notes
Text
i think I was supposed to post something so here
@calcium-rods

6 notes
·
View notes
Note
how would placeholder mcdoctorate react to having the four foot mareep from The Meme given to him? idk just wanted to make you draw your babygirl

sometimes i feel bad for lillian cause he just cant stop talking about pataphysics
#asks#calcium-rods#scp#lillian lillihammer#placeholder mcdoctorate#phmd#thank you for letting me draw this love u for that <3#though he js NOT a babygirl. more of a mipy imo#kiku_reading#kiku_scp_art
34 notes
·
View notes
Text
Minecraft youtubers who should get a duo name together. If you want more, ask. If you got ideas, comment. If you have questions, just ask.
Part 9!!
Doc and Fwhip-hog duo
Oli and Wilbur-theater duo
Grian and Dream-life duo
Oli and sausage-orb duo
Pix and Gem-lore duo
Cub and iskall-ice cube duo
Pearl and False-double duo
False and Xornoth-introvert duo
Bdubs and Gem-dawn duo
So. Official now 100 duo names!! Anyway, I like to credit @apollo-the-story-teller for lore duo, and double duo name ideas. @calcium-rods made the amazing Ice cube duo name. Again, this is for Empires x Hermitcraft crossover, but there will be other youtubers present. :). I'm in many fandoms of mcyt.
#docm77#fwhip#orionsound#wilbur soot#grian#dreamwastaken#mythicalsausage#pixlriffs#geminitay#cubfan135#iskall85#pearlescentmoon#falsesymmetry#xornoth#bdubbleo100#mcyt#empires x hermitcraft#duo names
28 notes
·
View notes
Note
oh there are lab grown pearls but i thought it meant the pearls are grown without a mollusk :^(
There are pearls you can get without an animal involved, fortunately! And some are still good quality! But they're still not lab grown, and I'm being pedantic because the reason we can't make fully synthetic pearls is pretty cool actually!
Fake pearls are not *grown* at all, and not made in labs the way some gemstones are. As a shorthand, some companies call them lab grown, but they are always "manufactured pearls" or "imitation pearls." They're mass produced in factories out of other materials.
Lab grown gemstones are really really cool, but this isn't that process, and no lab is involved. In theory, you could take the materials a pearl is made out of and coax it into growing...something. But that mineral would end up being crystalline (trying to grow it slowly into its natural form) or flat (trying to grow it in deposited layers).
The cool thing about pearls is that they get their shape, lustre, and physical properties from the way the calcium carbonate is layered up in a sphere from around the initial nucleation site! Even their unique colors have to do with the microstructures and what's incorporated into the material as it grows. It's so far completely impossible to make them synthetically. You *have* to use a natural material (mollusk shell) or fake it (glass, plastic).
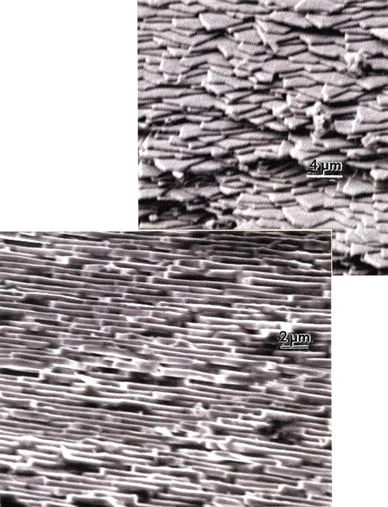
SEM images at different angles of observation on a cultured pearl curved surface region showing aragonite mesolayers and polygonal tile edges. Magnification markers are 4 and 2 microns, respectively. Murr, L.E., Ramirez, D.A. The Microstructure of the Cultured Freshwater Pearl. JOM 64, 469–474 (2012).
We haven't spent as much time trying to come up with a way to lab-grow (genuinely grow) pearls. They don't have as many uses as, say, sapphire (which we lab grow a lot, perfectly!). BUT.
A LOT of research has been done on another cool thing about bivalves...
Muscle foot glue! There are a ton of applications and inspiration we can use muscle foot protein for, like using this glue instead of stitches or rods for shoulder surgeries, or for underwater adhesive applications for structures, boats, pipes, etc.
Bivalves have a lot of their coastal territories in danger because of climate change and development. They clean water and actively sequester carbon and they're super super high in protein so they are all around super cool creatures that don't get enough love!
#pearls#gemstones#pearl is my birth stone and i am a materials scientist so i have read so much about pearls#mimicking natural materials is a whole field it's super cool!!#we're not there yet tho#the muscle article is about just one group's work but hundreds of groups are working on it?#its so cool???!#anyway now my weird bivalve interest is public
6 notes
·
View notes
Text
Will Vinegar Damage a Hot Water Heater? Exploring the Truth and Solutions
Introduction
Hot water heaters are essential appliances in our homes, providing us with the comfort of warm showers, clean dishes, and sanitized laundry. Regular maintenance is crucial to ensure these systems function efficiently and have a long lifespan. One popular method for cleaning and maintaining hot water heaters involves using vinegar. However, there is a common concern: will vinegar damage a hot water heater? In this article, we will explore this question in-depth, separating fact from fiction and providing solutions to safely maintain your hot water heater.
The Role of Vinegar in Hot Water Heater Maintenance
Vinegar is a versatile household item known for its acidic properties, making it effective for cleaning various appliances, including hot water heaters. It is primarily used to address two common issues:
Sediment Buildup: Over time, sediment, such as calcium and magnesium deposits, can accumulate in the bottom of your hot water heater tank. This buildup can reduce efficiency, decrease heating capacity, and even lead to corrosion if not addressed.
Anode Rod Maintenance: Hot water heaters are equipped with an anode rod designed to sacrifice itself to protect the tank from corrosion. Vinegar can be used to clean and extend the life of this rod.
Vinegar's Acidity and Its Impact on Hot Water Heaters
Vinegar is acidic, typically with a pH level around 2 to 3. While this acidity is effective in breaking down mineral deposits and rust, it can raise concerns about potential damage to the hot water heater's components. Let's examine the impact of vinegar on different parts of the hot water heater:
Tank Lining: Most hot water heater tanks are lined with glass or porcelain to prevent corrosion. Vinegar is generally safe for these linings and won't cause significant damage.
Anode Rod: As mentioned earlier, vinegar can be used to clean the anode rod, which is a sacrificial component designed to protect the tank from corrosion. Vinegar's acidity is mild enough not to harm the rod, but it is essential to follow proper procedures for cleaning to avoid damage.
Heating Elements: Electric hot water heaters contain heating elements that may be susceptible to corrosion if exposed to acidic solutions for extended periods. It is advisable to avoid prolonged contact between vinegar and heating elements.
Drain Valve and Pipes: Vinegar can corrode metal components over time, so it's essential to flush the vinegar out of the system thoroughly after use.
Solutions for Safe Hot Water Heater Maintenance with Vinegar
To ensure that vinegar does not damage your hot water heater while effectively addressing sediment buildup and anode rod maintenance, follow these safe procedures:
Drain the Tank: Turn off the power or gas supply to your hot water heater and allow it to cool. Connect a hose to the drain valve at the bottom of the tank and open the valve to drain any sediment. This step alone can help improve the heater's efficiency.
Clean the Anode Rod: If your hot water heater has a removable anode rod, carefully remove it and inspect its condition. If it is covered in sediment or corroded, you can clean it by soaking it in a vinegar solution (one part vinegar to three parts water) for a few hours. After cleaning, thoroughly rinse the rod with clean water and reinstall it.
Prevent Corrosion: To prevent corrosion of metal components, including heating elements and pipes, ensure you thoroughly flush the system with clean water after using vinegar. Run hot water from a faucet to remove any residual vinegar from the tank.
Regular Maintenance: Incorporate hot water heater maintenance into your regular home care routine. Consider flushing the tank and cleaning the anode rod annually or as recommended by the manufacturer.
Conclusion
In conclusion, vinegar can be a useful tool for maintaining your hot water heater, especially for addressing sediment buildup and cleaning the anode rod. However, it is essential to use vinegar carefully and follow recommended procedures to prevent potential damage to certain components. By practicing safe hot water heater maintenance with vinegar, you can extend the lifespan of your appliance, improve energy efficiency, and continue enjoying the benefits of reliable hot water in your home.
2 notes
·
View notes
Text
A brief history of colors and some popular pigments
Black pigment
Black pigment has a long history, starting with charcoal paintings in Stone Age caves. Through centuries of research, people have known how to change the burning conditions and choose the type of wood to customize the shade of this black. Coal can be pressed into a dry bar, or it can be ground into a powder and mixed with water or other liquids to produce the black dye that is now known as carbon black, with the pigment code PBk 7.
During the renaissance, artists often worked with black obtained from the soot of oil lamps, known as lamp black pigment – PBk 6. This pigment has a matte black color with a slightly cool tint. The lamp black is also used in Egyptian tombs and murals, replacing charcoal, which is denser but less pure.
Ivory black or bone black was originally created by boiling the crumbs obtained during the ivory making process to remove fat and gelatine, then ground and concentrated into a harder and coarser form to produce black. The production of ivory pigments was stopped in the 1930s, and today the pigment is mainly made from animal bones, with the color index name PBk 9. This pigment is semi-transparent, has a tinting strength is lower than that of carbon-based black pigments, but has a unique feature of deep yellow or brown undertones.
PBk 11 is an inorganic iron oxide pigment, which is different from all the black pigments mentioned above because there is no carbon in the composition. PBk 11 has a very high color fastness and is almost indestructible. In a mixture of colors, it can easily overwhelm all other colors. PBK 11 is also known as Mars black, named after Mars, the god of war in Greek mythology.
white pigment
The first white substance in history is thought to be natural calcium carbonate chalk, which is an exceptionally soft limestone, formed from the shells and bones of microscopic organisms deposited and compacted over millions of years. The calcium carbonate (CaCO3) that gives the white color can also be obtained from eggshells, oyster shells, ... In European literature, the term "shell white" is often used to refer to these ancient whites. Natural white pigment with calcium carbonate composition is still used to this day, has the pigment code PW 18.
The first synthetic white pigment produced on a commercial scale was lead white (PW 1), which dates back to around 300 BC. Despite its proven toxicity, white lead remained in widespread use until the late 19th century, when the superior zinc white and titanium white appeared and replaced it. There are many documents detailing how to make white lead. In the oldest process, lead rods were exposed to vinegar fumes in sealed clay pots. These pots were buried in manure or tree bark to maintain the temperature for several months so that the lead converted to white lead.
Zinc white (PW 4) with zinc oxide composition (ZnO) is produced by burning zinc in an oxidizing medium or zinc ore in a reducing medium. This pigment has the special property of being able to emit yellow fluorescence under long-wave ultraviolet light. Zinc whites are not toxic, nor are they as clearly affected by hydrogen sulfide as lead whites, but their whiteness is clearly inferior.
In 1908 in New York, a metallurgist named Auguste Rossi invented a brilliant white pigment, titanium dioxide (Titanium White - PW 6). This pigment is extremely stable, it is not affected by heat, dilute acids or alkalis, light or hydrogen sulfide. Most importantly, titanium white reflects about 97% of light, making it the best white ever known. Because of that, it quickly became popular in many fields. Titanium dioxide also provides UV absorption, which greatly improves weather resistance and durability for outdoor applications.
The chemical classification of titanium dioxide, which for many years was considered no problem, is even widely used in the pharmaceutical and cosmetic industries. However, they have become the subject of heated discussion over the past few years, when a European Union authority has changed the way they are classified and evaluated. Titanium dioxide (TiO2) particles with an aerodynamic diameter ≤ 10 μm are considered hazardous when inhaled.
Before titanium white appeared, the dominance in the segment belonged to Lithopone white (PW 5). They once accounted for 60% of the white pigment market, outperforming both lead white and zinc white combined. Lithopone is moderately strong in blends, not as strong as Titanium white, but not as gentle as Zinc white either. For those who are concerned about the durability of zinc white, but don't like the opacity of titanium white, PW 5 can be a good alternative.
Red pigment
Red is a color associated with love, excitement, and danger. This color also symbolizes good luck in many Eastern cultures. There are many red pigments that have been found and present in the pigment database.
The earliest red pigment discovered by mankind was red ocher, which is clay that has been colored by rusted (oxidized) iron. The red iron oxide pigment consists of the mineral hematite with some minor minerals such as clay, chalk and quartz. Red ocher differs from yellow ocher and brown ocher in that they do not contain H2O in their chemical structure. Today, synthetic iron oxide red pigments have the pigment code PR 101. They are chemically very similar to natural red iron oxide (PR 102), but transparent and more vibrant in color.
Lead red (minium) is an ancient pigment that is considered to be one of the first synthetic man-made pigments. Red lead is made by heating white lead to oxidize it at high temperatures. They are still used to this day, in anti-rust paints for steel structures, especially used a lot on ships.
The famous Chinese red color dating back to the fourth century BC is “Chusha” (Cinnabar), which is used to make paints, lacquers, ceramic glazes and calligraphy ink. Many people mistakenly believe that cinnabar is a plant because it is also a medicine in traditional medicine, but in fact, cinnabar is a mineral with the main component mercury sulfide (HgS). Artificial cinnabar, used by the Romans since the Middle Ages. This pigment is named Vermillion, has a color index name PR 106. Vermillion is much more vibrant than natural cinnabar, but both are quite toxic.
Besides the red pigments obtained from minerals, history also records some organic red pigments obtained from plants such as kermes tree, brazilwood or some palm species in Asia. The Incas also had their own red pigment for dyeing their robes, obtained from the Cochineal - a beetle that feeds on cactus.
The age-old reds made from lead and mercury, though toxic, have been commonly used throughout human history. The real alternatives have only appeared for more than a century, with the development of modern chemistry.
Red Cadmium (PR 108) is a dual product of zinc ore. PR 108 can include many different shades of red, for example Cadmium Red Light leans more towards orange, while Cadmium Red Deep is slightly maroon.
Naphthol red pigments PR 5, PR 9, PR 112, PR 170 and PR 188 are a large group of synthetic organic red pigments. While PR 5 can serve as the primary, medium-tone red in the palette, PR 9 is more of an orange hue. PR112 has a soft bright orange color, PR 170 includes Naphthol Red Light with an orange-red color and Naphthol Red Deep leaning towards purple.
Alizarin Crimson (PR 83) has a deep, cold red color and has high tinting intensity. Mixing PR 83 with Viridian Blue (PG 18) or Phthalo Blue (PG 7) creates a very deep black. PR 122 – Quinacridone Magenta is a vibrant red with a blue tint that makes them almost purple. The same Quinacridone family also has a red color PR 202 which is a bit greener than PR 122, PR 206 - Quinacridone Maroon has a red color that turns brown, while PR 207 - Quinacridone Scarlet is bright coral red.
Yellow pigment
Of all the pigment groups, yellow is the largest and most diverse because there are many substances in nature that can produce this color.
The oldest yellow pigment in prehistoric cave paintings, is yellow ocher, also crumbly clay colored by iron oxides. They are still in use today, with the pigment code PY 43 for natural yellow iron oxide and PY 42 for synthetic yellow ocher. Both natural ocher yellow and synthetics are both great colors in art, as they will produce very natural looking greens when mixed with blue pigments.
In early civilizations in Asia, Egypt and Greece, human used a yellow substance called Orpiment, which was synthesized by subliming a mixture of sulfur and a small amount of arsenic oxide. The Babylonians used Napoli yellow, which was prepared by heating a mixture of oxides of lead and antimony. Napoli yellow currently has a pigment code of PY 41.
Another well-known yellow is Indian yellow, which is said to be made from the bladder gravel of cows eating mango leaves. However, the original Indian yellow color dating from the 15th century no longer exists. Today's Indian yellow is Diarylide yellow (PY 83).
The 19th century saw the introduction of more modern inorganic pigments such as chromium yellow (lead chromate), cadmium gold (PY 35 and PY 37), nickel gold (PY 53 and PY 150), …
Some other yellow pigments include: Hansa yellow group (PY 3) with bright greenish yellow, PY 65 with deep yellow and PY 97 medium yellow, Barium Chromat lemon yellow (PY 31), Strontium chromate (PY32), cobalt yellow (PY 40), arylide yellow (PY73 and PY74), Isoindolinone yellow (PY 110), Diazo yellow (PY 128), Quinophthalone yellow (PY 138), Benzimidazolone yellow (PY 151, PY154 and PY 154). PY175), …
Blue pigment
Blue includes sky blue and navy blue which are very rare colors in nature. Less than a tenth of plants have this color, and in animals it's even rarer. Even if they're blue, it's not because they actually have a blue pigment, but they've actually done light tricks to achieve.
In plants, blue color is achieved by mixing or altering natural pigments, most commonly by altering the acidity on red anthocyanin pigments such as in canaries, bellflowers, and hydrangeas.
Instead of mixing or changing pigment, the blue color in many animals is caused by structures on their bodies that are able to change the wavelength of light. For example, the Morpho butterfly is blue because the scales of its wings are ridged, causing light to bend, making the only wavelength of light it reflects is blue. The only exception in nature is the Obrina Olivewing butterfly, the only animal known to have a real blue pigment.
The raw material of mankind's first green pigment was "lapis lazuli", a precious stone originating from mines in Afghanistan. "Lapis" means "stone" in Latin, "lazuli" comes from the Persian word "lazuward", meaning "blue". The blue created from this stone is called Ultramarine Blue, is a most perfect pigment, with its qualities said to be unique and unsurpassable. The color index name PB 29 is assigned to both natural and synthetic Ultramarine blue pigments, but today's natural Ultramarine Blue is actually exhausted. Lapis lazuli contains sulfur anions held in an ordered lattice. These sulfur anions have charged particles that move from molecule to molecule, traveling along the surface, helping to create a spatial effect and a deep blue color.
The synthetic blue pigment Ultramarine was discovered in 1826 by the French chemist Jean-Baptiste Guimet. He made a pigment chemically identical to lapis lazuli, by heating kaolinite, sodium carbonate, and sulfur. Synthetic ultramarine usually has a warm red-blue color, which is even more vivid than natural ultramarine blue but is not as pure and has the same depth.
Lapis lazuli is very precious and expensive. Faced with the need for an available and affordable blue pigment, the Egyptians invented the world's first synthetic pigment: Egyptian blue. Calcium copper silicate is calcined at extremely high temperatures, producing a blue-green compound resembling glass. When ground into a powder and mixed with a binder, they create a pigment that persists over time.
Across the ocean, the ancient Maya also found their own color of blue: Maya blue. Scientists are all confused about the origin of this brilliant blue color. It was not until the 1960s that scientists were able to determine the origin of this pigment. It is made by mixing a rare clay (attapulgite or palygorskite) with a dye from a plant in the indigo family.
In 1704 in Berlin, the first modern pigment was accidentally discovered by a dye maker named Diesbach: Prussian Blue (PB 27), also known as Berlin blue. Pigments made from iron ferrocyanide quickly became popular, simply because they were much cheaper than the earlier blues.
Phthalocyanine dark blue was first sold commercially in 1935 under the trade name Monastral Blue or Phthalocynanine Blue. This is a transparent, highly pigmented and reliable blue pigment. PB 15 is divided into 2 types: PB 15:1 – Phthalocyanine Blue Red Shade is more red and warmer, while PB 15:3 – Phthalocyanine Green Shade has a shade closer to green.
Mangan blue (PB 33) is produced by heating a mixture of sodium sulfate, potassium permanganate and barium nitrate at high temperature. This pigment is inert, unaffected by light, heat, acids or alkalis. Manganese blue pigment has now been discontinued due to concerns about the environmental impact of the manufacturing process.
The most common blue color is cobalt blue (cobalt aluminum oxide), which includes a range of pigments such as PB 28 which has a slightly greenish blue tint, PB 29 which is a bit darker, PB 35 which is sky blue, PB 36 is blue with a green tint, while PB 74 contains a little bit of red. Various cobalt minerals have been used since ancient times to color glass and ceramics, but the first synthetic cobalt blue pigment was discovered in 1802 by Louis Jacques Thénard. He discovered that the combination of cobalt oxide and aluminum oxide produced a highly stable blue pigment. Of all the blue pigments, cobalt blue is the only one with opacity.
It was not until 2009, more than two centuries after the last blue was discovered (cobalt blue), that humans found a new blue pigment, and also the last blue pigment up to the present time. It was YinMn Blue (PB 86), which was discovered by chance by a graduate student at Oregon State University. This pigment gets its name from the elements within it including Yttrium (Y), Indium (In) and Manganese (Mn).
3 notes
·
View notes