#cell immunology
Text
Adaptive Immune Response Phases - Diagram

Patreon
#studyblr#notes#my notes#medblr#medical notes#med notes#immunology#immunology notes#immune system#immune system notes#immune cells#immunity#pathology#pathophysiology#immune system cells#leukocytes#white blood cells#cell biology#cytology#cytology notes#cell biology notes#cellular immunology#cell immunology#diseases#diseases and disorders#science#scienceblr#life science#health science#note cards
4 notes
·
View notes
Text
For roughly one in every hundred people, food containing even the smallest amounts of gluten can deliver a gutful of hurt.
While a domino effect of immunological reactions can be traced back to their genetic roots, a number of contributing factors are also involved, making it difficult to map the precise chain of events that causes an allergy to gluten to emerge.
Using transgenic mice, an international team led by scientists from McMaster University in Canada has identified a crucial role played by the very cells making up the gut's lining, describing a major stepping stone that could lead to new therapies.
Continue Reading.
354 notes
·
View notes
Text
this is my other oc, neutro the neutrophil! a neutrophil is s type of white blood cell
ik the names r basic ugghhh
its one of the granulocyte brothers


#artists on tumblr#anime#art#moe art#moeart#my art#anime art#biology#cells#cells at work#neutrophil#white blood cell#immunology#immune system#cute#diagram#science#medicine#study motivation#premed#cat art#artists of tumblr#cellular biology#furry#oc#original charater art#original character#neutro
42 notes
·
View notes
Text

#shitpost#immunology#immunology shitposting#meme#mhc#b cell receptor#bcr crosslinking#this meme brought to you by my mom and I making fun of a question on a quiz I had for literature class#where one of the questions was ‘which of these is NOT a feature of the Greek underworld’#and I guess the professor just picked a random Greek sounding word but the specific word they picked was leukocyte#so we were pretending that leukocytes were in different literary traditions#and that morphed into talking about ‘immuno-christian values’ and I got inspired to throw this nonsense together
85 notes
·
View notes
Text
Interior Redesigners
Triggered to attack, T cells of the immune system bind their target molecule via a specific receptor on their surface – named? you guessed it, the T-cell receptor. This interaction sparks activity reorganising the T cell's inner protein scaffold (cytoskeleton) enabling optimal contact and function. Here are revealed the roles of cellular enzymes (LMK1 & SSH1) in orchestrating the structural remodelling and T-cell activation
Read the published research article here
Video from work by Álvaro Gómez-Morón and Sergio Alegre-Gómez, and colleagues
Department of Immunology, Ophthalmology and ENT, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain
Video originally published with a Creative Commons Attribution – NonCommercial – NoDerivs (CC BY-NC-ND 4.0)
Published in Communications Biology, July 2024
You can also follow BPoD on Instagram, Twitter and Facebook
13 notes
·
View notes
Text
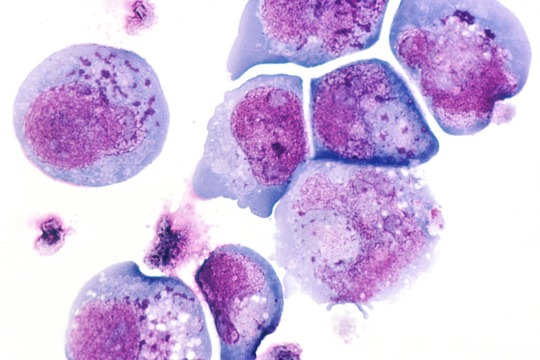
Human herpesvirus 6
“Light micrograph of infected T-lymphocytes with typical so-called inclusion bodies (HE staining). Inclusions in HHV-6 infection (dark blue dots).” - via Wikimedia Commons (original description translated from German using Google Translate)
#human betaherpesvirus 6a#herpesvirus#hhv#hhv-6#human herpesvirus 6#histopathology#micrograph#t lymphocytes#t cells#infectious diseases#immunology#fertility#infertility#wikipedia#wikipedia pictures#nature#wikimedia commons#medicine#medical#herpesviridae#medical microbiology#virology#virus#viruses#diseases#herpesvirales#roseolovirus#betaherpesvirus#orthoherpesviridae#he staining
19 notes
·
View notes
Text

A professional-looking professional antigen-presenting cell!
What are dendritic cells?
Dendritic cells are immune cells which display antigens on their surface to present these antigens to T cells. They do this through engulfing something such as a bacterium and breaking it down into lots of small parts. Then peptides (short proteins) are presented in specialised molecules on the surface of the dendritic cell.
Why are dendritic cells ‘professional’ antigen-presenting cells?
Almost all of our cells are able to present antigens, through displaying peptides from the proteins the cell is producing on the surface of the cell in specialised molecules. This allows the immune system to ‘look inside’ the cell to see whether it is producing any abnormal proteins - these may be due to mutated DNA or a viral infection. Professional antigen-presenting cells use slightly different molecules to present antigens and also provide an additional activating signal to T cells.
#science side of tumblr#immunology#immune system#immunology meme#science#science memes#science communication#dendritic cell
8 notes
·
View notes
Text
Toll-Like Receptor 2 and 4 as novel receptors for SARS-CoV-2
#covid-19#covid-19 vaccine#SARS-CoV-2#John Paul#natural killer cells#immunology#print this off later
7 notes
·
View notes
Text
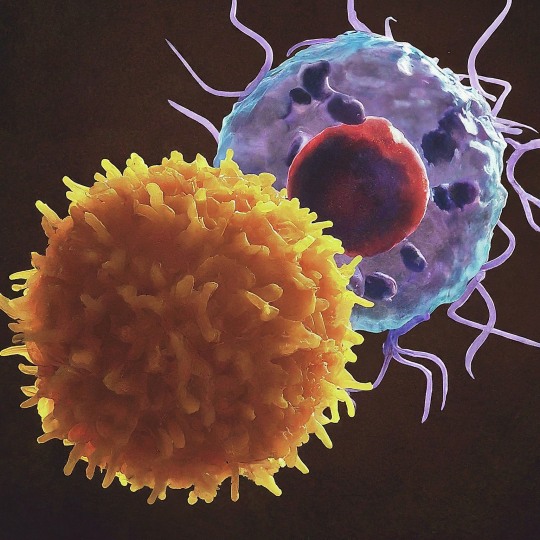
The T Cell Landscape
T cells, a critical component of the adaptive immune system, stand as the body's elite force in combatting infections and diseases. These specialized lymphocytes boast remarkable diversity, each type playing a distinct role in orchestrating a targeted and effective immune response.
T cells, like all blood cells, originate from hematopoietic stem cells residing in the bone marrow. However, their training ground lies within the thymus, a specialized organ located in the chest. Here, they undergo a rigorous selection process known as thymocyte education. During this process, immature T cells, called thymocytes, are presented with self-antigens (molecules unique to the body) by special cells. Thymocytes that bind too strongly to these self-antigens are eliminated, preventing them from attacking healthy tissues later. Only thymocytes that demonstrate the ability to recognize foreign invaders while exhibiting tolerance to self are released into the bloodstream as mature T cells.
Following this rigorous training, mature T cells exit the thymus and embark on their patrol, circulating throughout the bloodstream and lymphatic system. They remain vigilant, constantly scanning for their specific targets – antigens. Antigens are foreign molecules, such as fragments of viruses, bacteria, or even cancerous cells, that trigger the immune response.
The hallmark of a T cell is its T cell receptor (TCR), a highly specialized protein complex embedded on its surface. This receptor acts like a lock, uniquely shaped to fit a specific antigen, the "key." Each T cell develops a unique TCR capable of recognizing only a single antigen, enabling a highly specific immune response.
But how do T cells encounter these hidden antigens lurking within infected or cancerous cells? This critical role is played by antigen-presenting cells (APCs). APCs, such as macrophages and dendritic cells, engulf pathogens or abnormal cells, break them down into smaller fragments (peptides), and present them on their surface complexed with major histocompatibility complex (MHC) molecules. MHC molecules act as identification tags, allowing T cells to distinguish between "self" and "non-self." When a T cell's TCR encounters its specific antigen bound to an MHC molecule on an APC, a dance of activation begins. The T cell becomes stimulated, and a cascade of signaling events is triggered. This leads to the T cell's proliferation, producing an army of clones specifically tailored to combat the recognized threat.
T cells are not a single, monolithic entity. They comprise a diverse population, each type with a specialized function:
Helper T Cells (Th Cells):
Helper T cells, often abbreviated as Th cells, play a central role in coordinating immune responses. They express the CD4 surface marker and can recognize antigens presented by major histocompatibility complex class II (MHC-II) molecules. Subtypes of helper T cells include Th1, Th2, Th17, and regulatory T cells (Tregs), each with distinct functions and cytokine profiles.
Th1 cells mediate cellular immunity by activating macrophages and cytotoxic T cells, crucial for defense against intracellular pathogens.
Th2 cells are involved in humoral immunity, promoting B cell activation and antibody production, thus aiding in defense against extracellular parasites.
Th17 cells contribute to the immune response against extracellular bacteria and fungi, producing pro-inflammatory cytokines. Regulatory T cells (Tregs) maintain immune tolerance and prevent autoimmunity by suppressing excessive immune responses.
Cytotoxic T Cells (Tc Cells):
Cytotoxic T cells, also known as Tc cells or CD8+ T cells, are effector cells responsible for directly killing infected or aberrant cells. They recognize antigens presented by MHC class I molecules on the surface of target cells. Upon activation, cytotoxic T cells release perforin and granzymes, inducing apoptosis in target cells and eliminating the threat.
Memory T Cells:
Memory T cells are a long-lived subset of T cells that persist after the clearance of an infection. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, conferring immunological memory. Memory T cells can be either central memory T cells (TCM), residing in lymphoid organs, or effector memory T cells (TEM), circulating in peripheral tissues.
γδ T Cells:
Unlike conventional αβ T cells, γδ T cells express a distinct T cell receptor (TCR) composed of γ and δ chains. They recognize non-peptide antigens, such as lipids and metabolites, and are involved in immune surveillance at epithelial barriers and responses to stress signals.
Beyond the Battlefield: The Expanding Roles of T Cells: The remarkable capabilities of T cells have opened doors for several groundbreaking applications in medicine:
Vaccines: By presenting weakened or inactivated forms of pathogens, vaccines "train" the immune system to generate memory T cells. This prepares the body to recognize and rapidly eliminate the real pathogen upon future exposure, preventing disease.
Cancer immunotherapy: CAR T-cell therapy, a revolutionary approach, genetically engineers a patient's own T cells to express chimeric antigen receptors (CARs) that recognize and target specific cancer cells. These "supercharged" T cells are then reintroduced into the patient, unleashing a potent attack against the tumor.
Autoimmune disease treatment: Researchers are exploring ways to manipulate T cells to suppress harmful immune responses that underlie autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
The diverse array of T cells underscores the immune system's complexity and adaptability in mounting tailored responses against a myriad of threats. From orchestrating immune reactions to maintaining tolerance and establishing long-term immunity, T cells play multifaceted roles in safeguarding the body's health. Understanding the intricacies of T cell biology not only sheds light on immune-mediated diseases but also paves the way for developing novel therapeutic strategies harnessing the power of the immune system.
T cells represent a fascinating aspect of immunology, with their diversity and specificity driving the complexity of immune responses. As research advances, further insights into T cell biology promise to revolutionize immunotherapy and enhance our ability to combat diseases ranging from infections to cancer. By understanding and harnessing their power, we can unlock new avenues for protecting and improving human health.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#t cells#T helper cells#autoimmune#autoimmunity#helathcare#immunology#immunotherapy#medical care#cancer#human health#research#scientific research#the glass scientists#scientific illustration#research scientist
11 notes
·
View notes
Text
WE HAVE SOLVED THE MYSTERY OF WHY CELLS HATE NEUTROPHILS SO MUCH IN CAW
So, last night, my boyfriend and I got into some random discussion about programmed cell death and some new breakthroughs in medicine, you know, the usual things you talk about with your boyfriend at 1 AM. It is well known that leftover bodies of dead cells are phagocytosed (literally consumed) by macrophages. And that’s why I always wondered why aren’t cells scared of macrophages as much as they are of neutrophils, since neutrophils don’t consume the dead cells. With my limited understanding of immunity (which we technically don’t learn a lot about in biology) I thought that neutrophils only consumed invader bacteria and fungi.
And OH BOY was I wrong about that.
Because (and yes I have spent whole night researching this, I’ll provide the links to papers in the end lol) neutrophils are little freaks and not only do they phagocytose leftovers of cells they actually cause them to die in the first place. This happens during infections, especially with viruses that cause the excess release of cytokines (like Coronaviridae). Cytokines activate neutrophils who basically just follow the signal towards the infection site and there all hell breaks loose. Neutrophils phagocytose bacteria and virions (those are viruses that haven’t infected a cell yet) which is fine, but they also degranulate and NETose. I’ll explain this in simple terms to my best ability.
Degranulation is when granulocytes (neutrophils, eosinophils, basophils and mastocytes are all different granulocytes) release their granules which are kind of like little sacks inside their cytoplasm which contain various chemicals. Releasing these chemicals happens when the cell receives appropriate stimulus, the little granules expel their contents out of the cell’s interior. In the case of neutrophils, granules contain very toxic compounds that cause the formation of free radicals which damage DNA and proteins of the surrounding cells, as well as granules filled with digestive enzymes which, well, digest the surrounding tissues.
NETosis is a special type of cell death specific to neutrophils in which they literally degranulate pieces of their own, or their mitochondrial DNA together with more toxic compounds. This creates a net of DNA strands called chromatin which entangles invading bacteria and severely damages them and also marks them for phagocytosis by macrophages. But this process is not well controlled and some of that chromatin and toxic compounds can land onto neighboring cells which is, as you can conclude, very bad for them.
With these two abilities at hand, neutrophils are very well equipped to kill cells and destroy tissue. Which is good in cases when the cells are infected and the tissue is damaged, but their quite aggressive methods can damage healthy cells in the area as well, some of them will die and neutrophils will phagocytose their dead particles.
Basically, to neutrophils every infection is a huge kill and eat all you can buffet. They literally phagocytose until they physically cannot anymore and then go to the spleen or bone marrow to die. They also allow macrophages to consume them and thus pass on the antigens for antigen presentation which influences further immune response. But they can also cause a lot of damage, especially if cytokine storm happens and they completely lose control. This is what causes SARS and it can kill you if it’s severe enough.
Biologically speaking, neutrophils are very important because they are the first ones to come to the sight of infection and their crazy methods usually finish the things before they get too severe. They themselves produce cytokines that mobilize macrophages and dendritic cells so that more immune cells can join and help them. They also have a role in repairing the tissues they damaged.
However, other immune cells, including macrophages and killer T cells, simply don’t cause as much damage. Neutrophils just go all out, which is why they live for such a short period of time compared to their colleagues (they live for only few days, compared to macrophages who can live up to a month and lymphocytes who can live for months, even years).
So, yeah, my boyfriend and I have concluded (at 4AM this morning) that neutrophils are so feared because they damage tissue, go crazy and violently kill healthy cells by accident, then consume them and that’s not by accident, it’s a mechanism to repair tissues.
I can’t believe I wasted whole night just for this. My boyfriend is also disappointed. But I hope that we finally have an explanation for this mystery. Tell me what you think lol.
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8589350/
https://www.nature.com/articles/nri.2017.105
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5820392/#:~:text=Neutrophils%20contribute%20to%20tissue%20injury,detail%20here%20(Kruger%20et%20al.
#cells at work#hataraku saibou#neutrophils#immunology#medicine#science#biology#i actually need help#it’s 4am
66 notes
·
View notes
Text

Long time no see!
It’s been a while since I’ve posted and that’s because school doesn’t want to give me a break >:( On top of that, I’m currently writing a thesis AND a research paper so I’m constantly reading something (rip my free time)
I’m excited though because this is supposed to be my final semester! I’m so close to the finish line, I can taste it. Just a few more months of mental torture… in the mean time, enjoy these pathways of immune system activation as I’m studying for my immunology exam!
#studyblr#100 days of productivity#100dop#studying#chemistry#student life#studyblr community#study motivation#cell biology#microbiology#immune system#immunology#biology#stem academia
2 notes
·
View notes
Text
Uses of Orasone
Acute graft rejection
Autoimmune disease
Lymphoid tumors
.
Patreon
#studyblr#notes#my notes#medblr#medical notes#med notes#immunology#immunology notes#immune system#immune system notes#immune cells#immunity#pathology#pathophysiology#immune system cells#leukocytes#white blood cells#cell biology#cytology#cytology notes#cell biology notes#cellular immunology#cell immunology#diseases#diseases and disorders#science#scienceblr#life science#health science#note cards
1 note
·
View note
Text
Researchers have revealed the regulatory mechanism of a specific protein that plays a key role in balancing the immune response triggered by viral infections in mammal cells. These findings could help drive the development of antiviral therapies and nucleic acid medicines to treat genetic disorders. The research is published in the journal Nucleic Acids Research.
For cells to protect themselves from viral infections, a series of immune responses typically occur, including programmed cell death called apoptosis and interferon signaling. While apoptosis is a normal process, that occurs with or without the presence of viral molecules, following a cascade of steps to end with the death of a cell—which might not sound advantageous to the host—it can help prevent the reproduction of abnormal cells, including those infected by viruses, and eliminate them from the body.
Continue Reading.
61 notes
·
View notes
Text
platelet
one of my ideas for its design

#anime#artists on tumblr#art#moeart#my art#moe art#anime art#biology#cells at work#cells#biology art#medicine#medicine art#cellular#cell#platelet#neuro and hemo#blood cells#immunology#immunotherapy#immune system#clotting#healing#scabs#cute#cats#oc#original character#oc art#original character art
10 notes
·
View notes
Text
Quanxi as an NK cell.
#nk cell#quanxi#chainsaw man#csm fanart#leukocytes#immunology#immunology fanart#chainsaw man immunology#I'm not an artist I tried my best
16 notes
·
View notes
Text

Anti-cancer Courier
A virus-like particle engineered to deliver cancer-specific antigens [molecule, complex or fragment with potential trigger an immune response] to dendritic cells of the immune system which will in turn activate an attack by T cells on the tumour
Read the published research article here
Image from work by Ruijing Tang and colleagues
The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in eLife (reviewed preprint), June 2024
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#biology#cancer#oncology#immunology#immune system#t cells#dendritic cells#immunofluorescence#virus
12 notes
·
View notes