#esterification
Explore tagged Tumblr posts
Text
As an example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#fischer esterification#esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#solvent#water#ethyl ethanoate#chemical reactions
2 notes
·
View notes
Text
Hey girl, are you the alcohol to my carboxylic acid? because together we'd be... kind of fruity...
#shitpost#hilarious only to me#esterification#chemistry#in most contexts i do not like being called 'fruit'. however. i think this justifies it#pickup lines
2 notes
·
View notes
Text
Synthesis and Characterization of Methyl Myristate: A Chemical Perspective

Synthesizing Methyl Myristate involves a series of chemical reactions that transform starting materials into this valuable ester. The process typically begins with the esterification of myristic acid with methanol, resulting in the formation of Methyl Myristate. This reaction, catalyzed by an acid or base, proceeds under controlled conditions to yield the desired product. Methyl Myristate, once synthesized, undergoes thorough characterization to confirm its purity, identity, and chemical properties.
Characterization of Methyl Myristate involves a comprehensive analysis of its physical and chemical attributes. Techniques such as chromatography, spectroscopy, and mass spectrometry are commonly employed to elucidate its molecular structure, determine its purity, and identify any impurities present. Gas chromatography, in particular, is widely used to quantify the percentage of Methyl Myristate in a sample and assess the efficiency of the synthesis process.
The molecular structure of Methyl Myristate is elucidated through spectroscopic techniques such as infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. These methods provide valuable insights into the functional groups present in the molecule and confirm its identity based on characteristic spectral peaks. By comparing experimental spectra with reference data, researchers can verify the successful synthesis of Methyl Myristate and ensure its quality for further applications.
In addition to structural analysis, characterization of Methyl Myristate includes the determination of its physical properties, such as melting point, boiling point, and density. These parameters provide valuable information about the compound's behavior under different conditions and its suitability for various applications. For example, the melting point of Methyl Myristate influences its solidification temperature in cosmetic formulations, while its density affects its solubility and compatibility with other ingredients. Get More Insights On This Topic: Methyl Myristate
#Methyl Myristate#Chemical Synthesis#Esterification#Spectroscopic Analysis#Physical Properties#Chemical Characterization#Industrial Applications#Cosmetic Ingredients
0 notes
Text
Caustic Soda
MCT Caustic Soda Prills (Sodium Hydroxide), weighing 17 kg, is readily available for shipping at a discounted price of AED 93.45 per piece, inclusive of VAT, with the option for easy installment payments. The product, composed of solid small fused white pearls that are color- and odorless, serves various purposes such as unblocking drains and soap making. Free of anti-caking and flow agents, it outshines conventional granules or flakes and finds significant applications in the paper and pulp industry. Moreover, it is a crucial component in soap, cleaning products, drain treatments, degreasing agents, oven cleaners, and is employed as an esterification and transesterification reagent in addition to its role in food preparation. The stock is located in the United Arab Emirates, with an estimated lead time of 4 business days, and international delivery options are available, along with associated costs and shipping times displayed during checkout.
Caustic Soda prills, also referred to as Sodium Hydroxide, is characterized by the chemical formula NaOH.
This inorganic compound serves a variety of purposes, including unblocking drains and soap production.
One of its key functions involves converting fats and grease that tend to clog pipes into soap through a chemical process.

#alramiz#are#MCT#CausticSodaPrills#SodiumHydroxide#ChemicalCompound#CleaningProducts#DrainTreatment#SoapMaking#DegreasingAgents#OvenCleaners#Esterification#Transesterification#PaperIndustry#PulpIndustry#ChemicalFormula#UnblockingDrains#SolidPrills#WhitePearls#VATInclusive#DiscountedPrice#InstallmentPayments#FreeOfAntiCakingAgents#InternationalDelivery#LeadTime#chemicalprocesses
1 note
·
View note
Text
Esterification Tower
An esterification tower is a piece of equipment used in chemical processes, specifically in the field of organic chemistry and petrochemical refining. It plays a crucial role in the esterification reaction, which is a type of chemical reaction between an alcohol (or a compound with a hydroxyl group) and a carboxylic acid to form an ester and water. The esterification tower is designed to facilitate the esterification reaction by providing an environment where the reactants can interact, and the reaction can take place efficiently.
Indonesia Project in 2013-T-201B Esterification Tower
Designing Pressure
0.1Mpa
Test pressure
0.41Mpa
Working Temperature
130℃
Material
stainless steel
Net weight
13564kg
Application
For chemical plants to produce ethyl acetate.
Esterification Tower Working Principle
In the process of polyester production, the role of the Esterification tower is to separate the gas phase water and ethan glycol mixture generated during the esterization reaction process, separate the low-boiling water from the top of the tower, and separate the high-boiling ethylene glycol. Currency is condensed at the bottom of the tower, and ethylene glycol, as the main raw material for polyester production, can be re-recovered to reactors for reproduction.
Esterization reactions are a type of organic chemical reactions, which are divided into three types: carboxylic acid reacts with alcohol, or inorganic oxygenic acid reacts with alcohol, or inorganic strong acid reacts with alcohol. The esterization reaction of carboxylic acid and alcohol is reversible, and the general response is extremely slow, so concentrated sulfuric acid is often used as a catalyst. Multi-carboxylic acid and alcohol reaction can generate multiple esters. The reaction of inorganic acid and mellow is generally faster. The typical esterization reactions include the reaction of ethanol and acetic acid, and the generic acetate with aromatic smell is a raw material for manufacturing and medicine. The esterization tower are widely used in areas such as organic synthesis.

0 notes
Text
An example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:
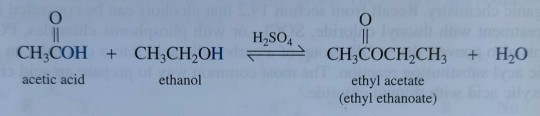

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#fischer esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#ethyl ethanoate#water#chemical reactions#glue#nail polish remover
0 notes
Text
proven chemistry | lee juyeon

𝒑𝒂𝒊𝒓𝒊𝒏𝒈 lee juyeon x fem!reader
𝒘𝒐𝒓𝒅 𝒄𝒐𝒖𝒏𝒕 1.3 k
𝒈𝒆𝒏𝒓𝒆 fluff | college au
𝒘𝒂𝒓𝒏𝒊𝒏𝒈𝒔 none
you see juyeon laughing with someone else and, driven by jealousy, you finally admit how you feel about him.
**•̩̩͙✩•̩̩͙*˚ ˚*•̩̩͙✩•̩̩͙*˚* **•̩̩͙✩•̩̩͙*˚ ˚*•̩̩͙✩•̩̩͙*˚* **•̩̩͙✩•̩̩͙*˚ ˚
You have always known that Juyeon wears his heart on his sleeve. He couldn’t hide his true reactions even if he tried, and that’s something you loved about him. Sometimes it could be hard to get explanations out of him, but you can usually guess what’s going on by how his eyes light up, stare in deep thought, or just avoid your gaze.
“Why is he so giggly?”
Your best friend looks at you with a tiny smirk, and you know what comes next.
“You’re jealous” she giggles, nodding her head.
“I’m not!” you cease to look at the scene displaying just a couple of meters from you and face Jihye, who is waiting for you to elaborate “It’s annoying how he just flirts with her in the middle of the library.”
“Relax, dear Jesus. He’s just laughing at what she said! They are partners in Mr. Choi’s project, they have to interact with one another. Remember?”
Your Chemistry professor had randomly assigned your partners for your final project. You got lucky enough to have been partnered with Jihye, not only because she was your best friend, but also because with her, choosing the topic was fairly simple: it was either related to environmental or green issues. However, as you sneakily look at the pair again, you wished you were in that girl’s place.
“What’s so funny about molecules?”
Juyeon had told you everything about their project and how well their first trials on the software had gone. They had decided to simulate molecules and predict their properties, but instead of experimenting physically, they did it virtually. Now, they just had to write down the first draft to hand in.
“I don’t know, but we still have to narrow down the conclusions on this thing, or else Mr. Choi will dump this paper right in front of our eyes!”
Although your chosen topic was way too interesting for you, esterification (making esters for flavors and scents) and how to replace the acid catalyst with an enzyme/greener catalyst to eliminate hazardous byproducts was proving to be nowhere as entertaining as looking at Juyeon from across the room.
“I think I need to get out of here”
The rest of the evening was spent back at your dorm, surrounded by lab trials on paper, coffee cups, various sweets, and the typing sound in two different laptops going on for the longest time you’ve ever heard.
“Last one?” Jihye asked with hope, looking at you with reddened eyes. The topic was no longer of you guys’ interest. You just needed some sleep.
“Think so! ‘For the last approach…’” You took the lead, ready to get over with it “‘Lipases (enzymes) were used to catalyze the reaction under mild conditions.’
After typing for some minutes, your best friend added “And we conclude that… ‘Every method proved to be efficient’?
“Well, they all were so…”
“Are we done?”
“Hell yeah”
Your celebratory dance was interrupted by a knock on your door. You checked the time — 9:48 PM. Had your roommate forgotten her key?
You stretch out on your way to the door, finally feeling your butt after hours of sitting down in the only chair you could bear. You were in the middle of a yawn when you opened it, and of course, he would be there, laughing at you in that state.
“Still working for Mr. Choi?” Juyeon asked, giggling at the sight of you.
“How did you guess?” you hid your face behind your hands, trying to make your burning cheeks less obvious. “What are you doing here?”
Completely ignoring your question, he walked past you and greeted your friend, who was already packing all her ‘study pack’, as she liked to call it, to go back to her dorm.
“You guys have a good night! I can’t handle myself any longer”
Juyeon got himself comfortable on your small couch and eyed you as you started putting your stuff away. “How did it go?” he asked in a low tone, well aware of how strict quiet hours were in your campus buildings.
“We’ll hand it in on Monday. The topic was fun at the beginning but after writing so much about it, it just fell out of my interest. What about you?”
“We finished it in the library a couple of hours ago. I thought you guys would be there as well.” When you finally sat down next to him, he pressed his back on the armrest so he could better see you.
“Hye and I decided to leave-”
“Because…” he inquired.
“It was too loud”
“The library?” he smiled, not tearing his eyes away from your increasingly reddened cheeks. “I know you were looking at me. At us” he corrected, his smile turning into a bigger grin.
“I don’t know what you’re talking about” You looked away, busying yourself with the hem of your top.
“Your cheeks seem to know”
“You were being too loud” you murmured, unable to return the gaze.
“What? I didn’t hear you” Juyeon replies, clearly messing with you. By this point, it was obvious that he knew more than you’d like to. He showed it, proved it, and said it.
But how could you just tell him the truth? How could you admit that your heart beats faster when he’s near you? How could you preserve the amazing friendship that had blossomed over college years if you had to tell him that you like him more than that?
“Do you promise not to panic after I tell you?”
“Y/N…” he sighed, making the small distance between your bodies disappear. He took your hands in his, and kissed each one with a care you could’ve never seen coming “I like you too.” And if your cheeks had been red before, you now probably looked like an extra spicy hot sauce bottle. How…? When…?
“I-I… what?”
“I was testing you out before I said anything. It may have slipped from Jihye at some point…” he giggled at the face you made. That little bastard…“But I wanted to make sure that it was not just another of her ideas. Well… this afternoon, it was pretty clear to me”
“I couldn’t disguise it, huh?”
“You were shooting daggers at her.” Juyeon chuckled, playing with your fingers. His touch felt so intimate and gentle that you couldn’t stop looking at the way he caressed you “I mean, don’t get me wrong, I felt relieved in so many ways. I had been in the dark about your feelings for way too long and that was a very evident sign.”
“Are you sure you like me?” you whispered, too shy to look at him.
It had been hard enough to picture what being romantically with Juyeon could look like, that you never wanted to have to do that again. So many different scenarios, so many ways in which he showed his love for you, and so many caring stares that you didn’t want to imagine anymore. Cuddling with him? Kissing until your lips hurt? Meeting his family? Sharing a home? It had to be real, or else…
Your heart wouldn’t be able to handle seeing him in any other way than being yours.
“Do you want me to prove it?”
You snapped out of your thoughts, ready to finally have a taste of what you have longed for so many years. “Please”
Your whisper was rapidly cut short by his soft lips on your own, his big and rough hands cupping the sides of your face to angle you better. You couldn't help but smile in the middle of the kiss, too entranced in his touch to care to hide your true feelings. He took his mouth away from yours with the cutest smile you have ever seen.
“I might need a little bit more convincing, sir. Just to make sure… ” Your grin was now sly, daring. It was ridiculous how down-bad you were for him.
“Lee Juyeon at your service, madam”
His kiss was confident and eager. His lips moved at your pace, making sure he never crossed your boundaries.
“I’m officially addicted to your kisses” He whispered in between pecks, leaving your lips a little to continue pressing them all over your face and neck.
“How could we miss this for so long?”
“Better later than never, darling.”
**•̩̩͙✩•̩̩͙*˚ ˚*•̩̩͙✩•̩̩͙*˚* **•̩̩͙✩•̩̩͙*˚ ˚*•̩̩͙✩•̩̩͙*˚* **•̩̩͙✩•̩̩͙*˚ ˚
back at it! missed writing for my boyz so much 💙
#kpop#kpop imagines#kpop x reader#kpop angst#kpop fluff#kpop smut#the boyz#the boyz imagines#the boyz x reader#the boyz fluff#the boyz angst#the boyz smut#lee juyeon#lee juyeon x reader#juyeon imagines#juyeon x reader#juyeon smut#juyeon fluff#juyeon angst
101 notes
·
View notes
Text


4 March 2025
The first chemical technology lab was super fun! I'm surprised I enjoyed it as much as I did, given it's kind of... ochem-heavy and I absolutely detested ochem labs. But I had a good time! Fingers crossed that won't change.
Lab partner and I made biofuel via esterification and transesterification reactions. The former was a reaction between oleic acid and methanol in acidic solution. The resulting ester - methyl oleate - has a greater density than methanol which is why it falls to the bottom of the cylinder, as you can see in the gif. It was so cool to see these "bubbles" in the entire volume just floating downwards as the reaction progressed. I love chemistry! 💖
#the other pairs made such cool things#soaps and creams and foams#so yeah i think im going to like these labs a lot#mine#op#studyblr#chemblr#chemistry#studyspo#study motivation
52 notes
·
View notes
Text
06/11/24; 🌪️
so basically, i am exhausted. tomorrow i have a physics simulation, probably will end up staying up all night.
ʾ ໑ ˖ ࣪ ◌ today's notes:
01. Chemistry: organic reactions summary, like esterification, neutralization and about three types of oxidation, did some questions too.
02. Chemistry: ionic equilibrium summary, with review of acids, bases and hydrolysis of salts, more questions done. A lot of pH too.
03. Chemistry: stoichiometry review, probably my favorite topic from the subject 💕.
ʾ ໑ ˖ ࣪ ◌ net hours of study: 4 hours and 51 minutes. (i'll study more)


#studystudystudy#study with me#study space#study notes#study inspiration#study aesthetic#study blog#study#studyspo
14 notes
·
View notes
Text
i have a running list of random things my chemistry prof says in lecture (aka when he lets the impulsive thoughts win) and today's was when right after explaining Fischer esterification he went on a tangent about the amount of microplastics in the average adult brain, followed by "don't you love living in an advanced society 😃," followed by him explaining saponification. and i just want to know what the sequence of thoughts were in this man's mind that got us there today.
#*insert the society comment*#'anyway do y'all know what triacylglycerides are?'#bro please not the whiplash#personal#stem major shenanigans
2 notes
·
View notes
Text
WHOEVER CAME UP WITH FISCHER ESTERIFICATION SHOULD EAT SHIT
2 notes
·
View notes
Text
methyl salicylate synthesis via fischer esterification. don't know if im doing this right but it seems like its working
1 note
·
View note
Text
Global Dabigatran Manufacturer
Understanding Dabigatran Manufacturing
In the ever-evolving landscape of pharmaceuticals, anticoagulants have emerged as crucial tools in preventing life-threatening conditions such as strokes and venous thromboembolism. Among these, dabigatran stands out as a widely used oral direct thrombin inhibitor. Though commonly prescribed, the manufacturing of dabigatran is a complex, tightly regulated process that blends high-end chemistry with rigorous safety standards. This blog explores the process of dabigatran manufacturing, highlighting key aspects like synthesis, formulation, quality control, and regulatory compliance.
What is Dabigatran?
Dabigatran is an oral anticoagulant that functions by inhibiting thrombin, a key enzyme in the blood-clotting process. It is typically used to prevent stroke and systemic embolism in patients with non-valvular atrial fibrillation, and also for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE).
It is usually administered in its prodrug form, dabigatran etexilate, which becomes active after metabolism in the liver.
Manufacturing Dabigatran: From Lab to Tablet
Manufacturing dabigatran involves a sophisticated process that includes chemical synthesis, formulation, and packaging, all under stringent quality assurance protocols.
1. Active Pharmaceutical Ingredient (API) Synthesis
The core of any pharmaceutical product is the Active Pharmaceutical Ingredient (API). Dabigatran’s API, dabigatran etexilate mesylate, is synthesized through a series of multi-step organic reactions. These reactions involve precise control of chemical conditions such as pH, temperature, and solvent choice.
The synthesis pathway is complex and typically starts with hydroxybenzamidine derivatives, which undergo reactions including esterification, amidation, and salt formation to yield the final mesylate salt. Each intermediate stage must be meticulously purified and analyzed to ensure the desired stereochemistry and chemical stability are maintained.
Key considerations during API synthesis include:
Purity: Impurities must be controlled to meet international pharmacopeia standards.
Yield: Efficient chemical processes are essential to keep production economically viable.
Environmental and Worker Safety: Proper handling of solvents, reagents, and byproducts is critical to minimize health and environmental risks.
2. Formulation of the Final Dosage Form
Once the API is synthesized and verified, the next step is the development of the oral solid dosage form, typically a capsule. Dabigatran etexilate has low bioavailability and is sensitive to moisture and acidity, which makes its formulation particularly challenging.
Key elements in the formulation phase include:
Pellet Technology: Dabigatran is often formulated into small pellets coated with functional layers that protect the drug and control its release.
Enteric Coating: This prevents degradation in the stomach and ensures the drug is absorbed in the intestine.
Excipients: These inactive ingredients aid in stability, bioavailability, and capsule integrity.
The formulation is carried out in Good Manufacturing Practice (GMP) certified facilities using high-precision equipment like fluid bed coaters and granulators to ensure consistency.
3. Quality Assurance and Testing
Every batch of dabigatran undergoes rigorous quality testing to ensure compliance with regulatory standards such as those set by the U.S. FDA, EMA, and other global authorities.
Testing includes:
Identity and Purity Tests: Confirm the chemical structure and absence of harmful impurities.
Dissolution Testing: Ensures the drug releases properly in the gastrointestinal tract.
Stability Studies: Long-term and accelerated testing to ensure the product remains effective over time.
Microbiological Testing: Confirms the absence of microbial contamination.
Each step in the manufacturing process is documented in detail, and full traceability is maintained from raw materials to the final packaged product.
4. Packaging and Serialization
Due to dabigatran’s sensitivity to moisture, its packaging is done in blister packs with desiccant features to ensure shelf-life stability. Additionally, in compliance with anti-counterfeit regulations, manufacturers must implement serialization—unique barcodes or digital identifiers on each unit of sale.
Packaging processes are automated and validated to ensure consistency. Labels must include all regulatory information, usage guidelines, and safety warnings.
Challenges in Dabigatran Manufacturing
Manufacturing dabigatran is far from straightforward. The primary challenges include:
Solubility and Bioavailability: Dabigatran etexilate is poorly soluble in water, making formulation a technological hurdle.
Cost of Production: Multi-step synthesis and specialized formulation techniques increase production costs.
Regulatory Scrutiny: As a high-risk medication, it falls under strict post-marketing surveillance and pharmacovigilance.
Patent and Market Competition: While originator patents have expired in many countries, producing a bioequivalent generic still requires significant investment and regulatory approval.
Global Landscape and Future Prospects
The manufacture of dabigatran has expanded globally with the rise of generic drug manufacturers. The expiration of key patents has opened up opportunities for more affordable versions, increasing global accessibility. However, ensuring consistent quality across different manufacturers remains a top priority for health regulators.
In the future, innovation may focus on improving formulations (e.g., extended-release versions), enhancing patient compliance, or integrating AI and automation into production lines for better quality control.
Conclusion
Dabigatran represents a critical advancement in anticoagulant therapy, but its availability and efficacy are rooted in a highly intricate manufacturing process. From chemical synthesis to final packaging, each stage is governed by science, precision, and stringent quality controls. As the pharmaceutical industry evolves, so too does the technology and oversight behind medications like dabigatran—ensuring patients receive safe, effective treatment every time.
URL: For more information, visit Bhasya International: Dabigatran manufacturer
0 notes
Text
What is the catalyst used in conversion
In chemical engineering and industrial processes, the term "conversion catalyst" refers to a substance that accelerates a chemical reaction without being consumed in the process. These catalysts are pivotal in optimizing reaction rates, improving selectivity, and reducing energy consumption. This article explores the role, types, and applications of conversion catalysts across various industries.
The Role of Conversion Catalysts Conversion catalysts lower the activation energy required for a reaction, enabling it to proceed at milder conditions (e.g., lower temperatures or pressures). For instance, in petroleum refining, catalysts facilitate the cracking of heavy hydrocarbons into lighter, more valuable products like gasoline and diesel. Without catalysts, these reactions would require extreme conditions, leading to higher costs and environmental impacts.
Catalysts also enhance selectivity, ensuring that the desired product is formed preferentially over by-products. In the synthesis of ammonia (Haber-Bosch process), an iron-based conversion catalyst directs the reaction toward NH₃ formation while minimizing side reactions. This efficiency reduces waste and improves process economics.
Types of Conversion Catalysts A. Heterogeneous Catalysts Heterogeneous catalysts exist in a different phase (solid, liquid, or gas) than the reactants. Common examples include:
Zeolites: Used in fluid catalytic cracking (FCC) units, zeolites convert heavy oil fractions into gasoline. Their porous structure provides high surface area, enhancing reactivity. Metal Catalysts (e.g., Platinum, Palladium): Employed in catalytic converters for automobiles, these metals convert harmful emissions (CO, NOₓ) into less toxic gases (CO₂, N₂). Enzymes (Biocatalysts): Though often classified separately, enzymes act as natural conversion catalysts in biochemical reactions, such as fermentation. B. Homogeneous Catalysts Homogeneous catalysts dissolve in the reaction medium, offering uniform reactivity. For example, soluble transition metal complexes catalyze hydroformylation reactions in the petrochemical industry, converting alkenes into aldehydes.
C. Biocatalysts Enzymes and microorganisms are increasingly used as conversion catalysts in green chemistry. For instance, lipases catalyze the esterification of fats, a process critical for biodiesel production.
Applications of Conversion Catalysts A. Petroleum Refining The FCC process relies on zeolite-based conversion catalysts to break down large hydrocarbon molecules. These catalysts are regenerated continuously, making the process sustainable and cost-effective.
B. Chemical Synthesis In the production of polymers, conversion catalysts like Ziegler-Natta catalysts enable the polymerization of alkenes into plastics (e.g., polyethylene). Similarly, in the pharmaceutical industry, catalysts streamline the synthesis of complex molecules.
C. Environmental Remediation Catalytic converters in vehicles use platinum-group metals to reduce pollutants. In industrial settings, conversion catalysts treat exhaust gases, converting SO₂ into sulfuric acid or scrubbing NOₓ emissions.
D. Renewable Energy In hydrogen fuel cells, platinum catalysts facilitate the oxygen reduction reaction (ORR), converting chemical energy into electricity. Research is ongoing to develop cheaper alternatives, such as iron-nitrogen-carbon catalysts.
Challenges and Innovations Despite their advantages, conversion catalysts face challenges like deactivation (e.g., coking in refining) and high costs (e.g., noble metals). Innovations focus on:
Nanostructured Catalysts: Enhancing surface area and reactivity. Bimetallic Catalysts: Combining metals (e.g., Pt-Co) to reduce costs and improve durability. Computational Modeling: Using AI to design catalysts with tailored properties. Conclusion Conversion catalysts are indispensable in modern industry, driving efficiency, sustainability, and innovation. From petroleum refining to renewable energy, these substances enable reactions that would otherwise be impractical or uneconomical. As research advances, the development of novel conversion catalysts—such as earth-abundant metal alternatives and enzyme-mimicking materials—will further reduce environmental impacts and expand their applications. The future of chemical processing hinges on optimizing conversion catalysts to meet global demands for cleaner energy, advanced materials, and sustainable manufacturing.
By understanding the mechanisms and applications of conversion catalysts, industries can continue to innovate, ensuring a balance between productivity and environmental stewardship.
0 notes
Text
Fatty Acid Ester Market is driven by Biodiesel Demand

The Global Fatty Acid Ester Market is estimated to be valued at US$ 2,958.2 Mn in 2025 and is expected to exhibit a CAGR of 5.7% over the forecast period 2025 to 2032.
The fatty acid ester market encompasses a diverse range of bio-based esters derived from fatty acids and alcohols, valued for their biodegradability, low toxicity, and versatility across industries. These esters serve as emollients in personal care formulations, lubricants in industrial applications, and solvents in paints and coatings, enabling manufacturers to meet stringent environmental regulations while enhancing product performance. Fatty Acid Ester Market Insights is growing awareness of sustainable chemicals has spurred market research into novel esterification processes, driving innovation in green catalysts and feedstock optimization. Moreover, the demand for high-purity esters in pharmaceuticals and food-grade applications underscores the broad scope of fatty acid esters across market segments. Continuous improvements in production efficiency and cost reduction strategies are expected to expand market opportunities, even as manufacturers navigate market dynamics shaped by raw material availability and regulatory restraints. Get more insights on,Fatty Acid Ester Market
#Coherent Market Insights#Fatty Acid Ester Market#Fatty Acid Ester#Fatty Acid Ester Market Insights#Medium Chain Triglyceride Oil
0 notes
Text
Gum Rosin Market Emerging Trends Driving Global Industry Transformation
The gum rosin market is experiencing significant transformation, fueled by evolving consumer preferences, technological advancements, and expanding application scopes. As a natural resin obtained from pine trees, gum rosin has long served as a vital raw material in industries such as adhesives, rubber, inks, paints, coatings, and food-grade esters. Today, a variety of emerging trends are shaping its demand and supply dynamics, unlocking new avenues for growth and innovation.

Expansion in Bio-Based and Green Products
One of the most prominent trends in the gum rosin market is the increasing preference for bio-based materials. As industries and consumers alike shift toward sustainable products, gum rosin’s natural origin positions it as a highly favored alternative to petroleum-based resins. Manufacturers are increasingly developing eco-friendly adhesives, varnishes, and sealants using modified rosin derivatives. This trend is particularly evident in the packaging and personal care sectors, where sustainability commitments are strong drivers of procurement strategies.
Additionally, rosin esters and derivatives are being integrated into bio-based plastics and composite materials, further diversifying gum rosin’s utility beyond traditional boundaries.
Rising Demand from the Adhesives and Sealants Sector
The adhesives and sealants sector remains one of the largest consumers of gum rosin, and emerging formulations are deepening this reliance. Innovations in pressure-sensitive adhesives, hot-melt adhesives, and water-based adhesives are expanding the demand for rosin esters. This is particularly notable in the consumer electronics, automotive, and furniture industries, where adhesives must perform under high thermal and mechanical stress. Gum rosin provides the necessary tackiness and flexibility required for such applications.
With e-commerce packaging and flexible packaging also on the rise globally, the requirement for effective sealing solutions is growing rapidly. Gum rosin's adhesive performance makes it an ideal choice in this dynamic sector.
Technological Advancements in Processing and Purification
Technological progress in gum rosin extraction and purification is opening new growth avenues. Advanced distillation and esterification methods are enabling manufacturers to produce high-purity rosin and tailor-made derivatives for specific industrial applications. This has led to the development of rosin-based polymers with improved resistance to heat, oxidation, and ultraviolet radiation.
These enhanced materials are being explored in specialty coatings, automotive paints, and even advanced composite materials, where performance and environmental sustainability go hand-in-hand. New purification techniques also allow for more consistent quality, which is essential for high-end applications in electronics and pharmaceuticals.
Emerging Opportunities in Pharmaceutical and Cosmetic Industries
Gum rosin is witnessing growing interest from the pharmaceutical and cosmetic industries. In pharmaceuticals, it is used in microencapsulation and controlled drug delivery systems due to its biocompatibility and film-forming properties. In cosmetics, rosin is being utilized in depilatory waxes, lipsticks, and nail varnishes, owing to its sticky nature and ability to form stable films.
As consumer awareness regarding clean-label ingredients grows, companies are exploring gum rosin as a natural alternative to synthetic film-formers and tackifiers in personal care products. This movement is particularly strong in Europe and North America, where regulatory standards encourage the use of natural and renewable ingredients.
Regional Market Dynamics and Supply Chain Innovations
Asia-Pacific continues to dominate the global gum rosin market due to its large-scale pine harvesting operations, particularly in China, Indonesia, and Vietnam. However, emerging economies in Latin America and Africa are beginning to invest in tapping their forest resources, potentially altering global supply chains.
Moreover, digitalization of the supply chain is aiding market transparency and traceability. From forest sourcing to final product delivery, advanced tracking systems and blockchain-based tools are helping ensure quality, sustainability compliance, and efficiency. This is especially beneficial in regions with strict import regulations.
Impact of Regulations and Quality Standards
As environmental and safety regulations tighten worldwide, manufacturers are increasingly required to meet stringent standards related to toxicity, emissions, and renewability. Gum rosin, being naturally derived, has a competitive edge in meeting these standards. However, to remain compliant, producers are investing in testing and certification processes to ensure product safety across different applications.
For example, in food contact materials and pharmaceutical use cases, regulatory approvals and adherence to Good Manufacturing Practices (GMP) are crucial. This trend is prompting companies to upgrade infrastructure and invest in R&D for certifications such as REACH and FDA compliance.
Conclusion
The gum rosin market is at a pivotal stage of evolution, driven by global sustainability trends, increasing industrial applications, and innovation in processing technologies. As the world leans further into eco-conscious production and consumption, the demand for naturally derived, high-performance materials like gum rosin is expected to rise steadily. Stakeholders across the value chain—from forest owners and resin processors to end-product manufacturers—stand to benefit from aligning with these emerging trends and investing in sustainable, high-value innovations.
0 notes