#names similar to valence
Note
hi, im looking for names similar to Elegy, Lament, Reverie, & Valence!!
ooh pretty names!
---
Alchamy / Alchemy
Armistice
Allegory
Ballad
Boheme
Brooklyn / Brooklynn
Bridget / Bridgette
Bliss
Calico
Camilla
Declare
Doux
Eloquence
Epiphany / Epiphanie
Endymion
Ezri
Forever
Finesse
Fabel / Fable
Faye
Fallyn
Gwyneth
Glade
History
Howl
Kyrielle
Maven / Mavon
Maebrea / Maebry
Maple
Noctis
Prairie
Peregrine
Paragon
Rain
Rue
Revel
Sing
Story
Saga
Seren
Thorin
Thane
Tempo
Virelai
Vesper
Verity
Valor
Valkyrie
Valmont
Valdez
Vianney
Whimsey / Whimsy
#admin toad#names like#names list#names similar to#similar names#similar to#v names#names like lament#names similar to lament#lament#names like elegy#names similar to elegy#elegy#names like valence#names similar to valence#valence#names like reverie#names similar to reverie#reverie#read dni#read my dni#name blog#name ideas#name lists#name suggestions#name request#name inspiration#gender neutral names#name#baby names
6 notes
·
View notes
Text
do you think there are days that pyrrha forgets what she looked like before? she's been in g1deon's body for thousands of years do you think there are moments where she struggles to recall her own face?
#i do like to imagine pyrrha and palamedes had conversations on new rho about what they used to look like#we know there are portraits of valency so presumably there could images of pyrrha out there somwhere#plus pyrrha and anastasia are the only ones who still have their names used by the houses (the Dve Territorials)#also we're not even accounting for whatever the state of her corpse is on the mithraeum because im p sure its still there#and i do not know if the whole deal with things not aging because they are close to john also applies to all the cavalier corpses#plus this is an excuse to ask folks to say in the tags how they imagine pyrrha looked before#she was butch before. to ME.#i think she and g1deon looked somewhat similar but not that similar since its said that her eyes dont fit his face#plus i like g1deon and pyrrha as parallels to pal and cam so to me its similar levels of looking alike#eskildit posts tlt#pyrrha dve#the locked tomb#ok to rb
109 notes
·
View notes
Text
🧑🏻🔬THE PERIODIC TABLE OF CHEMICAL ELEMENTS👩🏿🔬
(Note: I am not a chemist, just someone who enjoys science, if I made any errors please let me know, thank you and I hope you enjoy reading this)

Ever wonder about this bad boy? While here's a quick crash course on it and some of it groups!
What is it?
The Periodic Table is an arrangement of rows and columns used to display and predict what elements exist and their properties based upon where they fall.
The farther right and down you go, the "heavier" the elements get as they have more Protons, going from 1 Proton for Hydrogen to as of now 118 Protons for Oganesson. Each Collum, excluding the Transition Metals and Rare Earth Series, have similar chemical properties.
Along with this, excluding the Transition Metals, as you go left to right the number of Valence Electrons increases by 1 up to 8 before flipping around back to 1. Valence Electrons can be thought of as the Hands of Atoms, being how they interact and react with each other; a free valence electron spot being a place another atom can bond to like an open hand, while an occupied valence electron spot is like a full hand slapping away other potential electrons.
Each valence group, along with the Transition Metals, builds up the base of all of Chemistry and the universe. So lets have a look at a few of them, shall we?
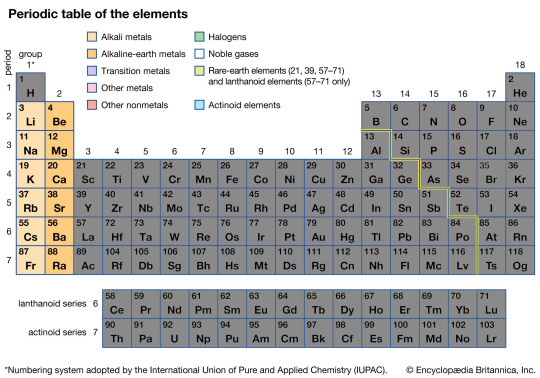
The Alkaline-Earth Metals
The Alkaline-Earth Metals are the two first columns of the Periodic table, and are often sub-divided into the Alkaline Metals and Alkaline Earth Metals. Both groups however do share some properties, often being softer metals that are extremely reactive.
Most famously, they react with water to produce Hydrogen and some heat amount of heat. On the more reactive side such as Lithium and Sodium, this means upon contact with water they will explode, sending red hot metal flying with a violent blast. On the less extreme end such as Magnesium or Calcium, they can be used as ways to produce Hydrogen in small quantities.
Not only are they reactive with water, but also air, oxidizing rapidly in the air and in the case of Magnesium having an extremely violent and energetic reaction when lit on fire.
They also all like to bond with chlorine to produce salts such as Sodium chloride, or more commonly known as humble Table Salt.
After the neat uniformity of the Alkaline-Earth metals, we reach,

The Transition Metals
The Transition Metals are not transgender metals, but rather a large collection of various metals that don't as neatly fit into rows or columns as the other Elemental groupings. Most notably, they have no Valence Electron Shell patterns, meaning you often have to search out the specific Transition Metal you are working with to know how many spots it has still open. They are by far the largest single group of elements within the Periodic Table, and chances are if you think of a metal it will be a transition metal.
It includes some famous stars such as Iron, Gold, Copper, Titanium, Lead, Zinc, Osmium, Tungsten, and Silver. As you can tell from the diverse cast of elements, all of these have wildly varying properties; take the strength and hardness of Iron and Tungsten compared Gold and Zinc as an example.
However they are not totally dissimilar as they are all still metals, meaning they share the properties of Metals. These include high thermal and electrical conductivity, liking to gobble up ions, are highly ductile (the ability to be pulled and bent into wire without breaking), form Cations (positively charged ions), and other such Metal traits.
Truly a party of metals, followed by,

The Other Metals
As the name would suggest, these are some of the other metals and can be thought of as an extension of the Transition Metals.
However their proximity to the other groups gives a few of these metals such as Aluminum more unique chemical behaviors compared to the standard transition metals, which is why sometimes you'll see some disagreement and debate about which ones, if any, should be placed into the next group,

The Metalloids
The Metalloids border between the Metals and the Non Metals, and as such are unique in having properties of both Metals and Non Metals.
Most famous of the Metalloids is arguably Silicon, which makes of the base of the modern world through its semiconductor properties in circuit boards and chips like the one bring used to allow you to read these very words.
Another famous, or perhaps infamous, Metalloid is Arsenic, used in as many poisoning murders for its toxic lethality as paintings and dyes for its beautiful vibrant green hue when turned into a pigment.
And if Arsenic is a bit too deadly for your liking, Gallium is always an option, used in old vacuum tube era computing and lighting. Along with its vintage past, it also has a melting point of 85.58°F or 29.76°C, just shy of room temperature; meaning if you hold a piece of Gallium in your hand, it will melt into a liquid - and unlike Mercury, it is non toxic making it far safer to play with, although it does stick to glass and stain objects so be careful if you do play with it to not let it touch something you don't want getting stained.
And as hinted just before, let us introduce the one and only,
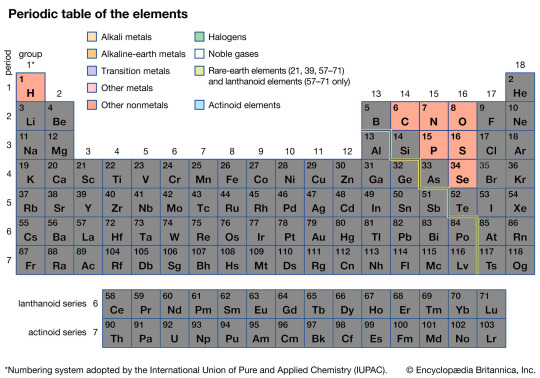
The Non Metals
The Non Metals are a small but extremely unique and important group. All of these elements as the name would suggest sharing the properties of being Non Metals, meaning they are all for the most part poor thermal and electrical conduct, have poor ductility, and form Anions (negatively charged ions).
If you are biologist of any kind, you may also notice this group contains many elements crucial for organic life; the three most important of which without doubt being Carbon, Oxygen, and Hydrogen.
Along with Nitrogen and a few other elements, these elements firm the basis of all life on Earth. From the air you breathe being a Nitrogen-Oxygen mix, to your cells being based on Carbon, to the very DNA that created you. All of it based on Carbon, Oxygen, Nitrogen, and Hydrogen.
And while Oxygen may have taken the honor of the name of Oxidation, it has nothing on the next group,
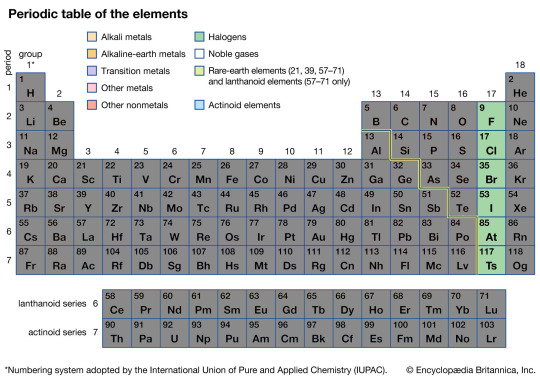
The Halogens
The Halogens, due to missing just one election to be totally stable, are some of the most violently reactive elements on the entire Periodic Table, with Fluorine even known to be able to eat through glass if given enough time.
It might be surprising to hear then that the Halogens also produce some of the most chemically stable compounds known on Earth, such as Polytetrafluoroethylene (PTFE), A.K.A., Teflon. This is because when these elements create compounds they become stable, meaning to break them apart you need an equal amount of energy to do so, and the energy requirement for Halogen compounds is often massive, making them chemically hardy.
Outside of Teflon and eating glass, the less reactive of the Halogens also are used in medicine as a way to sterilize an area. Most commonly and famously used would be Iodine, which not only has a beautiful purple hue, but is also used in surgeries to sterilize equipment and areas before operations, saving countless lives from infections every day.
In contrast to the reactive Halogens, we have next,
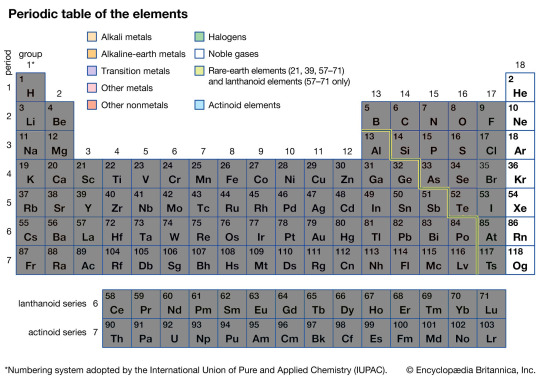
The Nobel Gases
The Nobel Gases like any true Nobels with wealth and status don't like to get involved in the common peasantry's squabbles. In the world of chemicals and elements, this means their full valence electron shells don't stubbornly refuse to chemically react with any other element, and the compounds they do form are often unstable and want to fall apart back into a Nobel Gas and whatever common element it wound up stuck with.
While this lack of reacting does make them rather boring from a reaction perspective, it does make them extremely useful when you do not want things chemically reacting by acting as a buffer between other elements; most easily seen in high end car lights, often filled with Xeon or other Nobel Gases to make sure no matter how hot the light gets it doesn't react chemically and degrade.
Along with protecting lights, they can also be used for lights. Due to their unreactive nature in even high energy environments, it makes them perfect to be used to fill a sealed glass tube that has power shot through it; otherwise known as a Neon Light. However despite the name, not all Neon Lights are filled with Neon, only the orange-red ones are. Other colors such as blues and greens come from the other Nobel Gasses depending on their spectrum emissions.
And on light and degradation, last but not least we have,
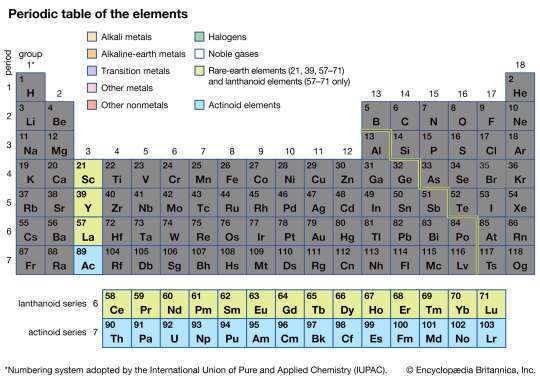
The Rare Earth Metals
The Rare Earth Metals can be and often are split into two smaller groups, the upper Lanthanide series and the lower Actinide series.
The upper Lanthanides are all rather chemically similar, meaning they can often be treated somewhat interchangeably in a chemical sense. Their most notable use is without a doubt their ferro magnetic properties, making some of the most powerful magnets on Earth barring electro-magnets. Neodymium magnets are by far the most famous of them, being known to be able to leap across across tables and smash fingers if not handled carefully, while holding up hundreds of pounds of weight.
In contrast, the lower Actinide series is the domain of many of the Radioactive elements and sees the boundary line between the natural and artificial elements. All of them are radioactive to various degrees with decreasing half life lengths.
It is here Thorium and Uranium is found, being the heart of both terrifying bombs and the cleanest sources of energy known to mankind. Beyond Uranium, all the elements are artificial and must be made by humans in breeder reactors.
Despite their association with atom bombs and nuclear reactors, many of these elements also have far more mundane uses. Americium is used in common household Smoke Detectors, saving untold numbers of lives and dollars in property through early warnings of smoke and fire. Other ones are used by NASA and other space agencies in Radioisotope thermoelectric generators or R.T.G.s, used to power spacecraft like Voyager 2 and the Mars Curiosity Rover thousands of miles away from Earth where conventional solar panels would be too heavy, unwieldy, and inefficient so far away from the Sun.
#periodic table of elements#science#elements#chemistry#chemical elements#periodic table#science stuff#science side of tumblr
148 notes
·
View notes
Text
HELLO. PALISADE 29
HOLY FUCKING SHIT.
the tension. the drama. the acting. the sheer fucking bravado. can we get a round of applause for ali’s work in this downtime!!!!!!!!!
actually, shouts to the entire cast, there was not a minute of that episode that was not incredible. TTRPGS!!!
after the (genuinely gripping) debate about what moves to use, the actual fight between brnine and dahlia ended up being really grounded in a way i loved—no auto-win, no huge move to pull out, just a series of attacks. great tension.
speaking of tension, this episode felt like the real refresh after the stellar combustor arc. one big victory, a clean escape, and then time to run a few downtime scenes afterward and fully reset. also exactly the kind of wild win i wanted post-combustor, lol. (notably the same sort of double-impact midseason finale as in partizan lmao.)
oh my god, and the MUSIC. this new track absolutely bangs. i think ali said the moment of killing dahlia was “uncool”, which was very funny given that the soundtrack made it actually the coolest scene ever put to audio.
figure managing to summon brnine back was amazing. (they can cry again!) and that they did it with the tapes of valence and gur—the memory of valence felt very present in brnine’s scenes in this downtime, even without brnine ever saying their name. grief’s a funny thing. (similarly: brnine nearly telling thisbe to check in with phrygian. and “phrygian made the best cereal box mazes”, a line that i will be remembering literally forever.)
and like, the scene with gucci. god. something very compelling about gucci saying “i don’t know what to do with the way i feel about you so i’m going to ask you: what do we do” and then framing a different set of bare little hopes and a different huge frightening future that has suddenly opened up. what do you do when it turns out you actually get a chance to live. same question. you know.
i’m not entirely sure what’s going on with divines and their relationships with mortals right now, but i think something is starting to shift. thisbe and the cadent—this strange method of communication—brnine’s adopted gods—the afflictions and the delegates and partial palisade himself—the reveal that the branched are posthuman and postdivine; dahlia following a similar path—something is changing. post-combustor, something could change. maybe it’s no one thing, but an expansion, out of the confines of the divine principality. that in itself would be a victory.
also noticing that any divine brnine meets becomes a dog to them—asepsis, integrity, commitment. still don’t know where to even begin with this. (… insert HOUNDS joke here)
so much happened in this episode. what the fuck. incredible work all around. excited for next week????????????
26 notes
·
View notes
Text
The Blue Castle Chapter 1 Analysis
This is sooo late, but I didn’t have time till now!
Here are my chapter by chapter analysis/thoughts of The Blue Castle (separate post per chaper)
SPOILERS FOR THE BOOK
Chapter 1
Valency’s life is so empty that rain, the most mundane of all weathers in fiction, made an impact and brought change. Her life is so boring that her heart problems don’t scare her, but annoys her.
Valency also wakes up to dullness “Valency wakened early, in the lifeless, hopeless hour just preceding dwan”. There is no moment of happiness where her eyes are open. And her entire life, 29 years amounts to one sentence “One does not sleep well, sometimes, when one is twenty-nine on the morrow, and unmarried, in a community and connection where the unmarried are simply those who have failed to get a man”.
Despite her drab existence, Montgomery still shows the tiny bit of resistance she displays. Valency hopes for romance. For her era, women desiring love and romance was frowned upon. How dare you express emotions that border carnal sins. Romance and love were expected to thrust itself upon women. But Valency desires it, and eventually seeks it.
Montgomery also shows Valency’s resistance in her failure to be in her family’s good grace. Her lack of confidence in herself is built by those who see her has a failure. But the only reason Valency sees herself as such is because she isn’t interested in being the type of person her family expects.
Let’s talk about Valency’s room. It’s described to have e a yellow painted floor and a rug with a grinning dog on it. There is red paper on the ceiling and a lambrequin with purple roses. This room looks to be an aged child’s room. Valency, despite being almost 30, is stuck in a child’s room that both she and time have outgrown. Her room captures how her family also treats her. As a woman who is but a child because she is not married. Montgomery really highlights the importance of marriage for women during her time. It’s not that women want a happily every after, it’s that women want to be able to grow up and make decisions (as best they could for their society). Her room is also surrounded by family pictures. She cannot escape them, for they are physically there in photo frame context. They suffocate her even in the one place she runs to escape them. They even attempt to invade her physically space like a parent sharing the bed of a young sick child or spanking a child.
I love this line “Valency never persisted”. It’s a clever play on words for to persist can also mean to go on, to endure, dare I say, to exists despite all. Another play on words is with “She did not want anyone to know about her heart”. There is the physical heart with it’s anomalies and then, there her metaphorical heart with its blue castle and desires.
Valency’s family is also presented as no less interesting then her. So boring and drab, that their names and personalities blend into one another. Can you tell me you know each Stirling and their quirks by heart?
There is a line in chapter 1 that reveals the sharp contrast between Valency and Barney. Montgomery mentions Valency “had been poor all her life and knew the falling bitterness of it. So she endured [Uncle Benjamin’s] riddles and even smiled tortured little smiles over him”. Poverty plays a huge role why Valency’s mother insists on Valency catering to her relatives. Since her father is dead, Valency has no man in her life to provide any security, and so she cannot really be herself because she’s dependent on an inheritance. This is a sharp contrast to Barney who is open about being socially outcasted. Montgomery kind of hints in the first chapter that characters with bad characters, have little to loose. It also explains why Valency and Barney are different yet similar in kind. Barney probably would have been like Valency if he was poor. Another example is with Dr. Trent. Valency thinks of going to Dr. Trent. A doctor is someone well off, compared to Valency, and again, Montgomery shows us the ability to be impertinent and its relation to power and wealth. Dr. Trent told Cousin Glady’s that her neuritis was made up
Valency compares disloyalty to her clan as the devil, yet the irony is that her clan pushed her to the devil.
#the blue castle#valency stirling#lucy maud montgomery#the blue castle book club#blue castle spoilers
13 notes
·
View notes
Text
timeline of their realities history
a brief timeline of the history of the primrodials reality. up until about 2008, their reality remains relatively similar to yours, until its firs divergence.
2008:
birth of "akros" ( the common name used to refer to the being that akronus and akrosion would mitsosis from), hallowed, Aquis, Aquarius, crow, deltan, Samhain, Antros, magmus, Cosmia, Valence, clockwork, really just almost all of the villains and heroes who have carried over.
2024:
Akros dies, Akronus and akrosion are born
godfather usurps his mother and becomes the master of necromancy, Aldiras family dies, Valence cosmia magmus and many other discover their powers/ magical skill, Aquis purchases the snake Jormungand, Clockworks family dies.
2025:
the oblivion eclipse happens, millions dead, many heroes are among the casualties.
soon after the event of reborn from oblivion happen.
the events from my soon to be story, bill of sale, starring hallowed happen on october 13th.
Any other story not listed to be post formation of the primordials happens here
decemebr 1st the magical communtiy recognizes the existence of the matrs of the arcane.
2026:
august 1st Archon is conceived and appears in the future.
August 2nd Archon returns to the present, now 20 years old and with their tech.
August 13th the primordials is formed
april 1st the Primordials investigate rumours of a sea beast and recruit Jormungand as a hero.
august 17th futuristic mech suit wearing vigilante Archon is recruited by the primordials
may 6th in nightingale city the time freezing vigilante clockwork is recruited by the primordials as a new member.
october 21st the syndicate is formed
@f4y3w00d5 @gobodegoblin @monsterfucker-research-wizard @symbiosis-enjoyer @good-wizard
2 notes
·
View notes
Text
(sharing this silly thing again since I deleted my twitter and I’m still proud of it):
So I have a (supremely self-indulgent) fan theory that Perennial and Autonomy Itself are somehow Kamala Cadence and Independence, respectively - and I’m convinced enough about this that I am presenting my evidence! Here! ->
Evidence Piece 1: some repeated and pretty explicit comparisons between Clem as the Witch in Glass and Kamala as the Waking Cadent - see for example this exchange from PARTIZAN 36:

(“is all I’ll say”……….)
Evidence Piece 2: repeated references to a past relationship between Perennial and Autonomy Itself - “your God and its terrible relationship with Perennial” from Chrysanth in episode 30 and this from episode 36, for example:

(“a broken relationship”?)
Evidence Piece 3: the parallel between the pieces of the Exemplar scattered on Partizan and the pieces of Independence scattered on Quire
(and the associated thematic parallels between Autonomy Itself and Independence as these powerful beings that nevertheless are heavily reliant on followers to enact their will)
Evidence Piece 4: the similarity between Perennial’s doctrine of recurrence and some of the beliefs expressed by Kamala as the Waking Cadent - thinking especially about the “nothing was ever truly destroyed” line in TM 45:

(also that line is kind of support for my own whole argument here haha)
Evidence Piece 5: (this one is maybe a coincidence, but) the Divine Cadence being used to send Valence’s broadcast of Autonomy Itself’s message in episode 15:

(“a great name in this moment”?)
Evidence Piece 6: (maybe a silly one, but) “Autonomy Itself” sounds like the sort of thing someone called “Independence” might rename themself if they were trying to start a more positive chapter in their life, imo
Evidence Piece 7: (related, less silly) “Autonomy Itself” could also be read as a reference to the Autonomous Diaspora, where Independence was originally from
(thank you for your time)
10 notes
·
View notes
Text
Noblewomen, Family and Identity
Women's use of seals points to activity and identity independent of their husbands, and the seals themselves indiciate that they saw themselves in the context of their own ancestry as well as identifying with their husbands; this was common in the British Isles and in France north of the River Loire. Devorguilla de Balliol depicted herself on her seal as a widow, holding in her right hand her husband’s Balliol shield, and in her left the lion-shield of her father’s lordship of Galloway; smaller shields provided a reminder of her own connections with the earldom of Chester and the royal house of Scotland. The reverse of the seal gave the place of honour to the lion of Galloway over the Balliol arms. Marie de St Pol, countess of Pembroke, also had a female figure in the centre of her seal; on one side was the shield of her husband Aymer de Valence, and on the other that of her father, Guy de Chastillon, count of St Pol. The seal could also epitomize a woman’s claims to power and land; the way in which Galburge de Mévouillon gave herself the title of lady of Serres on her seal in 1259 emphasized her claim
to the lordship.
Surnames and titles display a similar desire to advertise birth, marriage, property, and authority. Marie de St Pol continued to use her father’s name after her marriage, as did Marie de Berry and Marie de Sully. Hereditary and marital titles were combined, as when Mahaut, heiress to Artois, and wife and widow of Count Otto of Burgundy described herself in the early fourteenth century as ‘countess of Artois and palatine Burgundy and lady of Salins’. Anne Stafford, daughter of Thomas of Woodstock, duke of Gloucester, and Eleanor de Bohun, combined ancestral, parental, and marital titles when she styled herself countess of Stafford, Buckingham, Northampton, and Hereford, and lady of Brecon and Holderness [..]
Family identity for noblewomen was of primary significance, but did not prevent them from having a wider identity as well, mainly with their fellow-nobles, whether these were neighbours, friends, distant kin, or people they met at court. The knightly orders of the late Middle Ages point to the importance of chivalrous networks for men, but these overlapped with social and religious groupings in which both men and women were associated. The evidence of household accounts points to the amount of contact and socializing among the nobility by means of letters, messengers, and hospitality. Mahaut countess of Artois was in touch in this way with many of the nobility of France, and kept up to date with the news of births and deaths.
Jennifer C. Ward - Noblewomen, Family and Identity in Later Medieval Europe, in Nobles and Nobility in Medieval Europe.
#middle ages#jennifer c.ward#nobles and nobility in medieval europe#marie de berry#marie de sully#mahaut d'artois#and more
9 notes
·
View notes
Note
I get that JGY get paralleled with WWX a lot but are they supposed to be parallels or narrative foils?
An intriguing question!! I have no idea if Chinese literature draws upon precisely the same literary terms as English, or if they have exactly the same valences and meanings. That said, if I were analysing it purely in English (which is all I can do anyway), I think i come down on the side of reading them as parallels rather than foils.
To over-simplify, parallels are about sameness and foils are about difference. JGY and WWX are certainly very different; if they swapped places, they obviously wouldn't just live one another's lives-- they have different personalities and drives and hopes.* But I think the intent is for the reader to end up feeling that their similarities are far more important and profound than their differences. But at the end of the day/narrative, the structural function of a comparison between them is to draw attention to the ways their pasts and paths converge rather than diverge, and is NOT to say 'here is how one did it right and one did it wrong' or even 'here are how two people who started in similar places ended up so differently by making different choices.'
(*I'm sure this fic has been written!! For my morning thought experiment: JGY as the child of CSSR, raised by the Jiangs, dedicates himself to becoming JC's right hand man but also to making a name for himself as a bit of a 'go where the trouble is' semi-rogue cultivator in his mother's honor, which causes similar kinds of tensions surrounding his position as in canon, but possibly JGY's personality is better equipped to manage them once he's older. WWX as Meng Shi's son probably starts on a similar path as JGY, but his very different tactics for dealing with bullies would probably make him come to, let's say NMJ's attention again through some spectacular feat of daring/cultivation brilliance or even just rumors of this arrogant kid who actually is pretty impressive. Having fewer brother feelings mixed into his superior/subordinate relationship would make things way easier for WWX in terms of maintaining a long-term position with the Nie or whoever.)
19 notes
·
View notes
Text










Saint-Nazaire-en-Royans, France (No. 2)
The Isère is a river in the Auvergne-Rhône-Alpes region of southeastern France. Its source, a glacier known as the Sources de l'Isère, lies in the Vanoise National Park in the Graian Alps of Savoie, near the ski resort in Val-d'Isère on the border with Italy. An important left-bank tributary of the Rhône, the Isère merges with it a few kilometers north of Valence.
Many riverside communes have incorporated the Isère's name into their own, for example, Sainte-Hélène-sur-Isère and Romans-sur-Isère. The department of Isère is likewise named after the river.
The name Isère was first recorded under the form Isara, which means "the impetuous one, the swift one." Not originally a Celtic word, it was very likely assimilated by the Celts in ancient times. This word is related to the Indo-European *isərós, meaning "impetuous, quick, vigorous," which is similar to the Sanskrit isiráḥ इसिरः อิสิระ with the same definition. It was probably based on the reconstructed Indo-European root *eis(ə) (and not *is), which incidentally has not been found in the Celtic languages of the British Isles.
The word Isara figures in the etymology of many other river names, from ancient Gaul and its neighboring lands. Examples of this are the Ésera in Spain, the Isar in Germany, the small Franco-Belgian Yser, or even the ancient name of the Oise, Isara (the French adjective isarien still exists in the language and continues to describe anything related to the Oise). In non-Celtic countries, we find the Isarco, a river in Northern Italy, the Éisra and Istrà in Lithuania, Jizera in the Czech Republic and Usora in Bosnia and Herzegovina.
Source: Wikipedia
#Saint-Nazaire-en-Royans#Auvergne-Rhône-Alpes#Drôme#Die#Vercors-Monts du Matin#travel#original photography#vacation#tourist attraction#landmark#cityscape#landscape#summer 2021#France#Europe#nature#flora#aqueduct#architecture#Isère#river#river bank
0 notes
Text
The Building Blocks of Matter: Discovering the Structure and Properties of Atoms and Molecules in Class 9 Science Chapter 3.
In Class 9 Science Chapter 3, students dive into the intriguing world of atoms and molecules, unravelling the building blocks of matter. This chapter provides a comprehensive understanding of the structure and properties of these fundamental particles, offering a glimpse into the fascinating realm of chemistry.
Class 9 Science Chapter 3 Atoms and Molecules notes
Exploring the microscopic world of atoms, students discover that they are composed of protons, neutrons, and electrons. They learn about the arrangement of these particles within an atom and how it influences an element's properties. Additionally, students delve into the concept of molecules, understanding how atoms combine and bond to form compounds with unique characteristics.
As they embark on this scientific voyage, students grasp the concepts of atomic number, mass number, valency, and much more. They gain insight into the periodic table and its significance in organising elements based on their properties.
Through engaging explanations, examples, and exercises, this chapter equips students with the knowledge and skills to comprehend the foundations of matter. It ignites their curiosity about the mysteries of atoms and molecules, laying a solid groundwork for their future scientific explorations.
Class 9 Science Chapter Atoms and Molecules Notes
Structure of an atom
The structure of an atom forms the foundation of our understanding of matter. Atoms are composed of subatomic particles, namely protons, neutrons, and electrons. Protons carry a positive charge and are located within the nucleus of the atom, while neutrons possess no charge and also reside in the nucleus. Electrons, on the other hand, carry a negative charge and orbit the nucleus at specific energy levels.
The discovery of subatomic particles was a significant breakthrough in the field of science. It was through the pioneering experiments of scientists such as J.J. Thomson, Ernest Rutherford, and Niels Bohr that we gained insight into the intricate nature of atoms. Their experiments involving cathode rays, gold foil, and spectral lines respectively paved the way for our current understanding of atomic structure.
NCERT Solutions for Class 9 Science
The arrangement of these particles within an atom greatly influences the properties of an element. The number of protons determines the atomic number, which in turn defines the element itself. The mass number of an atom is the sum of protons and neutrons, reflecting its overall mass.
The discovery of subatomic particles
One of the most valuable tools in chemistry is the periodic table of elements. This table organizes elements based on their atomic number, providing a systematic and visually appealing representation of the elements. It showcases their properties, including atomic mass, electron configuration, and valency.
The periodic table is divided into periods and groups, each with its own significance. Periods represent the energy levels or electron shells, while groups denote similar properties and valency. Understanding the periodic table is crucial for identifying and comparing elements, predicting their behaviour, and studying the trends that emerge across periods and groups.
The periodic table of elements
Atoms have a natural tendency to combine with other atoms to form molecules. This process, known as chemical bonding, is driven by the desire of atoms to achieve stability by attaining a full outer electron shell.
There are three primary types of chemical bonding: ionic, covalent, and metallic. Ionic bonding occurs when there is a transfer of electrons between atoms, resulting in the formation of ions with opposite charges. Covalent bonding involves the sharing of electrons between atoms, creating a bond that holds the atoms together. Metallic bonding occurs in metals, where electrons are delocalized and freely move between atoms, giving rise to their unique properties.
Molecules are the result of atoms coming together to form compounds with distinct characteristics. They can be composed of atoms of the same element, known as diatomic molecules, or different elements. Understanding how atoms bond and the nature of different molecules is crucial for comprehending the vast array of substances that exist in our world.
Chemical bonding and molecules
The structure and composition of atoms and molecules dictate their properties. Atoms possess various properties such as atomic radius, electronegativity, and ionization energy, which influence their behavior in chemical reactions and their interaction with other atoms.
Molecules, on the other hand, exhibit properties such as boiling point, melting point, and solubility. These properties are determined by the type and strength of the chemical bonds within the molecule, as well as the intermolecular forces between molecules.
Understanding the properties of atoms and molecules allows scientists to predict and explain the behaviour of substances, enabling the development of new materials and technologies.
Properties of atoms and molecules
Atomic mass represents the average mass of an atom, taking into account the various isotopes and their relative abundance. It is measured in atomic mass units (amu) and is often rounded to the nearest whole number on the periodic table.
Molecular mass, on the other hand, refers to the sum of the atomic masses of all the atoms in a molecule. It is measured in atomic mass units as well and provides valuable information about the mass and composition of compounds.
Atomic mass and molecular mass play a crucial role in stoichiometry, which involves calculating the quantities of substances involved in chemical reactions. These concepts are essential for understanding and predicting the outcomes of chemical reactions and for designing experiments in the laboratory.
Atomic mass and molecular mass
Compounds and mixtures are two different types of substances that can be formed through the combination of atoms or molecules.
A compound is a substance composed of two or more elements chemically bonded together. It has a fixed composition and can be represented by a chemical formula. Compounds possess unique properties that differ from those of their constituent elements.
Mixtures, on the other hand, are combinations of two or more substances that are physically blended together. Unlike compounds, mixtures can have varying compositions and can be separated through physical means. Mixtures can be classified into homogeneous mixtures, where the components are uniformly distributed, and heterogeneous mixtures, where the components are not evenly distributed.
Understanding the differences between compounds and mixtures is crucial for analyzing and identifying substances, as well as for designing separation techniques in various industries.
Understanding compounds and mixtures
The knowledge of atomic and molecular structure finds applications in various fields, ranging from medicine and pharmaceuticals to materials science and nanotechnology.
In medicine, understanding the structure of molecules allows scientists to design drugs that target specific sites within the body. This knowledge also aids in the development of diagnostic tools and techniques for detecting diseases.
In materials science, the ability to manipulate atomic and molecular structures has led to the creation of new materials with unique properties. This has paved the way for advancements in areas such as electronics, energy storage, and construction materials.
Nanotechnology, a rapidly growing field, relies on manipulating matter at the atomic and molecular levels. This field holds immense potential for revolutionizing industries such as electronics, medicine, and environmental sustainability.
Applications of atomic and molecular structure
The study of atoms and molecules is of utmost importance as it forms the foundation of our understanding of matter. Exploring their structure, properties, and interactions allows us to comprehend the world around us and opens up a myriad of possibilities for scientific advancements.
Class 9 Science Chapter 3 serves as a gateway to this fascinating realm, igniting curiosity and equipping students with the knowledge and skills to explore the building blocks of matter. By delving into the microscopic world of atoms and molecules, students gain a deeper appreciation for the wonders of chemistry and lay a solid groundwork for their future scientific endeavours.
Conclusion: Importance of studying atoms and molecules
Atoms, the tiny building blocks of matter, are composed of three main particles: protons, neutrons, and electrons. These particles are arranged in a specific manner within an atom, greatly influencing the characteristics of an element. Understanding the structure of atoms is key to comprehending the behaviour of matter.
The nucleus, located at the centre of an atom, contains protons and neutrons. Protons carry a positive charge, while neutrons have no charge. Surrounding the nucleus are electrons, which carry a negative charge. The arrangement of these particles determines an atom's stability and reactivity.
The number of protons in an atom is known as the atomic number. It defines the identity of an element and remains constant for all atoms of the same element. For example, hydrogen always has one proton, while carbon has six. The atomic number also determines the number of electrons in a neutral atom.
The mass number of an atom is the sum of protons and neutrons in its nucleus. It provides an estimate of the mass of the atom. Isotopes are atoms of the same element with different mass numbers due to varying numbers of neutrons. Isotopes exhibit similar chemical properties but may differ in physical properties.
Understanding the structure of atoms allows students to interpret the periodic table, a powerful tool in chemistry. The periodic table organizes elements based on their atomic number, providing valuable information about their properties. Students learn to identify trends and patterns in the table, enabling them to make predictions about the behaviour of elements.
#atoms&molecules#9thclass#foundation#ncertsolutions#ncert#ncert textbooks#ncertbooks#education#biology#11thclass#mathematics#students#botany
0 notes
Text
Ok thinking about the valency thing. Wake's name includes /Snap Back to Reality Oops There Goes Gravity/, and we kind of get hints that Pyrrah remembers G1deon's original name (G-), and so we have to question what pyrrah remembers as well as why and how she remembers it. Did valency have a similar moment of snapping back to reality? Once a cavalier is killed and their soul integrated, does it do something to them that made them remember? Wake's name is literally Awake Remembrance of These Valiant Dead. Not just Remembrance, but Awake Remembrance, as if she had some kind of Moment. The snap back to reality is remembering what happened, and the oops there goes gravity is the loss of understanding of the world. Who were Cyrus and Valency?? What does Pyrrah remember? Is the Angel Cassiopeia? Was BOE started not by the trillionaires, but by former lyctors whose cavaliers remembered?? I have so many questions.
0 notes
Text
Servitor "Solarpunk life" manifestation toybox scripture 1/? (WIP)

Key agents
Klara 'Olive' Kér (protagonist and self-insert)
Ava Petra (synthetic social assistant serf)
Valence/Constanz (introspective self-image & fursona)
Nil (second person perspective, in case you want to insert yourself into my stories...)
Falah Becker (mother)
Gustav Hayden (father)
Deno Hayden (twin brother)
Wyatt Hayden (younger brother)
Shoshona (black Angora housecat)
Ursae Elke (College / university classmate and BFF for life)
Tekla Hankel (inspired from Wolfenstein The New Order's autistic character)
Sasha Fekete (inspired by Velma and GLaDOS girlboss vibes)
Magali Soler (fem friend of similar name in IRL, somewhat similar to Helluva Boss' Octavia)
Kira May Ceyla (inspired from Helluva Boss' "Sally May")
Micha Baker (masc ISFP)
Matyas Merkel (masc ISTJ)
Talinana Chateau (programmer friend and internship mate at Utalics)
Ulli Eike (NB middle school friend, eq. U)
Tano Hertez (NB primary & middle school friend, eq. A)
Irma Milan (fem ENTP friendly rival)
Topic2
Soft warm retro toon aesthetic
Far far away Solarpunk optimistic future world
Bookstore clerk software toy
Deque Lambda Calculus paradigm
Topic3
Angora major civilizations
Shoshones
Assyria
Morocco
Mayas
Brazil
Persia
Portugal
Poland
Babylon
Vietnam
Inuit
Samoa
Angora major religions
Arianism
Ba'hai
Calvinism / Huguenots
Chaldeanism
Theravada
Zoroastrianism
Jainism
Al-Asnam
Angora major keypoints
A
B
C
D
E
F
Goals
U
V
W
X
Y
Z
Life-scripting scenarii
Bookstore working shift & conversing with Ava
Swimming some in the pool, chatting some with the other ladies
Swimming in some other pool, this time by myself with some caring self-talk and much relaxing
Moving across the public transport system (trams, buses, subway and railways)
Van travel trip across the countryside
Studying at the university library
Watching some cozy film during a community event
Eating some at a café
Working my first internship hours at Utalics
Visiting the parliament / senate complex of the Shoshoni Union
Tinkering and playing around retro technologies
Spending some sweet time with my family at their household
Affirmations
0
1
2
3
4
5
6
7
8
9
A
B
C
D
E
F
S
T
U
V
W
X
Y
Z
ENDE?
0 notes
Text
What is Electron Configuration?
Electron Configuration is defined as the distribution of electrons in different atomic orbitals of an element. Moreover, in order to represent the electron configuration of an element, an electron-containing subshell, along with the number of electrons in it, is shown as a sequence. For instance, in hydrogen, the electronic configuration is represented as 1s1.
But not all element electron configurations can be represented in standard forms because of their length due to large atomic numbers. In such cases, an abbreviated notation is employed to describe electron configuration. And in this abbreviated notation, the atomic subshells of the noble gas electronic configuration are replaced by the noble gas symbols inside a square bracket.
Uses of Electronic Configuration
Now that we have a good understanding of the Electronic Configuration. Let us now talk about the various benefits or the importance of Electronic Configuration.
· To understand the atomic spectra of various elements, an Electron Configuration is essential
· As explained above, the properties of the elements can only find out using electronic configuration
· Besides this, the valency of all elements directly depends on the number of electrons in an atomic orbital, which in turn is determined using electronic configuration
How to represent Electronic Configuration?
Moving on to our next topic, how to write the Electronic Configuration for various elements. Regardless of the method, this process mainly depends on three main steps Shells, Subshells, and Notations.
Shells
Firstly, in shells, the maximum no of electrons is accommodated based on the type of element and more importantly, on the principal quantum number (n). Thus, based on the shell, the principal quantum number varies. For instance, in the first shell, which is K, the principal quantum number n is 1. Below are some examples of shells and their principal quantum numbers.
Subshells
Unlike the shells, the subshells are categorized with the help of the azimuthal quantum number (I). However, this quantum number is directly dependent on the principal quantum number (n). And therefore, based on the number of shells in an element, the subshells also vary.
Notation
The notations are basically the subshell labels, which include the subshells name, shell number as well as the electrons in the subshell (represented using superscript).
If you find any other similar topics from the chemistry subject harder to understand, then you can check out the online interactive classes offered by Tutoroot. At Tutoroot, we are geared towards helping you get the best ranks and scores in academics by providing classes that are interactive and easy to follow.
0 notes
Text
Julius Lothar Meyer

Julius Lothar Meyer (19 August 1830 – 11 April 1895) was a German chemist. He was one of the pioneers in developing the earliest versions of the periodic table of the chemical elements. Russian chemist Dmitri Mendeleev (his chief rival) and he had both worked with Robert Bunsen. Meyer never used his first given name, and was known throughout his life simply as Lothar Meyer.
Meyer is best known for his part in the periodic classification of the elements. He noted, as John A. R. Newlands did in England, that if the elements were arranged in the order of their atomic weights, they fell into groups of similar chemical and physical properties repeated at periodic intervals. According to him, if the atomic weights were plotted as ordinates and the atomic volumes as abscissae—the curve obtained a series of maxima and minima—the most electro-positive elements appearing at the peaks of the curve in the order of their atomic weights.
His book, Die modernen Theorien der Chemie, which he began writing in Breslau in 1862 and which was published two years later, contained an early version of the periodic table containing 28 elements, classified elements into six families by their valence—for the first time, elements had been grouped according to their valence. Works on organizing the elements by atomic weight, until then had been stymied by the widespread use of equivalent weights for the elements, rather than atomic weights.
He published articles about classification table of the elements in horizontal form (1864) and vertical form (1870), in which the series of periods are properly ended by an element of the alkaline earth metal group.
In 1869, Dmitri Mendeleev published a periodic table of all elements known at that time (he later predicted several new elements to complete the table, and corrected some atomic weights). A few months later, Meyer published a paper that included a revised version of his 1864 table that now included virtually all of the known elements, which was similar to the table published by Mendeleev:
Meyer had developed his fuller periodic table independently, but he acknowledged Mendeleev's priority. Included in Meyer's paper was a line chart of atomic volumes as a function of atomic weights, showing graphically the periodicity of the elements. Like Mendeleev, he also included predictions of future elements, but unlike Mendeleev did not emphasize these predictions nor suggest details of the physical and chemical properties of the future elements
In 1882, both Meyer and Mendeleev received the Davy Medal from the Royal Society in recognition of their work on the Periodic Law.
The mineral lotharmeyerite, Ca(Zn,Mn+3)2(AsO4)2·2(H2O,OH), was discovered in 1983 and named in recognition of Meyer's work on the Periodic Law. The type locality is the Ojuela mine, Mapimí, Durango, Mexico. Four closely related minerals have been described since 1983: ferrilotharmeyerite, Ca(Fe+3,Zn)2(AsO4)2·2(OH,H2O) (1992);cobaltlotharmeyerite, Ca(Co,Fe+3,Ni)2(AsO4)2·2(H2O,OH) (1997);nickellotharmeyerite, Ca(Ni,Fe+3,Co)2(AsO4)2·2(H2O,OH) (1999);and manganlotharmeyerite, CaMn3+2(AsO4)2(OH)2 (2002).
1 note
·
View note
Text
Periodic table chart

A periodic table chart was first discovered by a German Chemist, named Lothar Meyer. He produced several Periodic Tables between 1864-1870. His first table contains 28 elements organized by their valency. While the Russian scientist named Dmitri Mendeleev. Mr. Mendeleev had discover the periodic table in 1869.
The framework for the modern periodic table also leaves gaps for elements to be discovered. The periodic table was also discovered by other scientists which gives it some additional features.
Some features are:-
1) The vertical columns which are call group
2) The horizontal columns are call period.
3) Arrangement of elements in order of increasing relative atomic mass.
4) Gaps were left empty for yet to be discovered elements
Periodic Table With Names
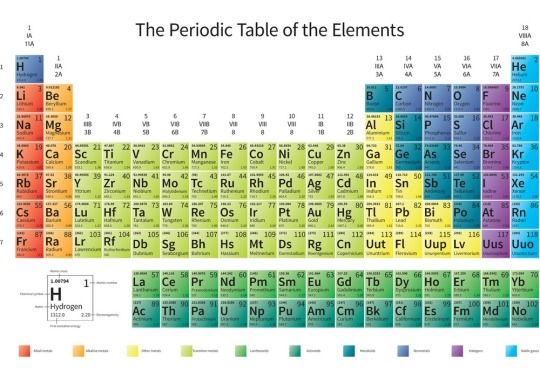
In the periodic table chart, there are groups that are vertical columns. These vertical columns go from group 1 to 7 or 1 to 18 depending on the textbook you read. Each column represents physical properties. Group 1 is also know as the alkali metal. It is in the first group of elements in the periodic table. The elements that are in group 1 are Lithium (Li), Sodium(Na), Potassium(K), Rubidium (Rb), Cesium(Cs), and Francium (Fr) which is a rare radioactive metal.
How The Periodic Table Organized

The metals in group 1 which are in the periodic table chart are extremely reactive. The reason behind this, they are very powerful reducing agents. Which occurs mainly in nature since the group has a positive 1 (+1) cation. Also, group 1 has anomalous behavior of Period 2 (which we will discuss further down in the article). This occurs in group 1 because of the small atomic size and a small number of other-level orbitals which is similar to period 2. Alkali metals have unusual physical properties, these metals are softer and have lower melting, boiling points, and lower densities. This occurs because of their atomic size, the largest in their respective periods, and the valence electrons. The electron configuration for group one is ns1 since the valence for group 1 is one (1).
The trend for group 1 is as you go down the group, the reactivity increases. This occurs because the atoms become larger, while the outer electron becomes further away from the nucleus. Therefore, the force of attraction between the nucleus and the outer electron decreases, and the outer electron is lost more easily. Alkali metals react in four ways.
These are:-
- The alkali metals reduce hydrogen in water. This means the reaction becomes more vigorous down the group.
- Group 1 metal reduces oxygen.
- The alkali metals in group 1 reduce hydrogen to form ionic hydrides
- And also reduce halogens to form ionic halides
Group 2 Periodic Table

Group 2 is next to group 1 on the periodic table chart. Its also know as alkaline earth metals. The alkaline earth metals are beryllium (Be), magnesium(Mg), calcium(Ca), strontium(Sr), barium(Ba), and radioactive(Ra). The alkaline earth metal is similar to the alkali metals in which all of the group 2 metals are solids at room temperature. The metals in group 2 are harder, denser, and melt at a higher melting temperature compared to group 2. Also, the alkaline earth metals are less reactive in group 2 compare to group 1.
In group 2 the metals have a higher boiling and melting point compared to group 1. The alkaline earth metals are very strong reducing agents. The metals are reduce to oxygen to form the oxide. This is also include the metal beryllium which forms peroxide. Some of the metals in group 2 reduce water at room temperature to form hydrogen. There also reduce the halogens to form ionic compounds but this doesn't include beryllium. The oxides in group 2 are strongly basic except for beryllium oxide which is amphoteric.
How The Periodic Table Arrange

Group 3 or 13 is also know as the Baron family. This group is very interesting. Baron heads the family of the main group element but its properties are not representative of what group 3 will portrait. The elements that are in group 3 are Baron(B), Aluminum(Al), Gallium(Ga), Indium(In), and Thallium(Tl). Baron is black, hard, has a very high melting point, and is a covalent metalloid. While the other elements in group 3 are shiny, relatively soft, and have a low melting point. The reaction for the Baron group is the elements are sluggish with water, when strongly heated in pure oxygen all elements in the group form oxides, and there reduce halogens.
The carbon family is in group 4 or 14. The carbon family consists of Carbon(C), Silicon(Si), Germanium(Ge), Tin(Sn), and lead(Pb). Carbon has high melting and as you go down the group the melting point starts to decrease. In group 4, the element carbon is an allotrope. This occurs because the elements have different crystalline or molecular forms in a substance. The element carbon consists of two different substances from the same atom. These are graphite and diamond.
Graphite is a standard state of carbon, a more stable form at ordinary temperatures and pressure. In the reactions for group 4, the elements are oxidized by halogen whereas the positive 2 halides are more stable for tin and lead. The elements are oxidized by oxygen. The lead oxide becomes more basic down the group while carbon dioxides and water provide the weak acidity of natural unpolluted waters. hydrocarbons react with oxygen to form carbons and water. This reaction is for methane which is adapt to yield electricity or heat. While silica is reduced to form elemental silicon.
Conclusion
In conclusion, the periodic table chart is a guide on how the elements react to which elements. And it assists us in the different equations to help solve problems.
https://www.youtube.com/watch?v=rz4Dd1I_fX0&list=PLqaxBYQqxlEnJP2v7TCB2x1DixjZx9ji6&index=1
Read the full article
0 notes