#Microbial Reduction
Explore tagged Tumblr posts
Text
Food Irradiation: An Effective Technique To Improve Food Safety

What is Food Irradiation?
It is a technique where foods are exposed to ionizing radiation to destroy microorganisms, bacteria, viruses, or insects that might be present in or on the food. The technique uses gamma rays (from cobalt-60 or cesium-137), X-rays, or electron beams from a machine source to blast foods with ionizing energy, altering their molecular structure. History
The concept of food irradiation was first researched as early as the beginning of the 20th century. However, it gained global recognition around the 1950s when serious research was performed to establish its viability and commercial applications. Initial research showed irradiation could effectively eliminate bacteria from meats and spices without changing their visual appearance and quality. The first international conference on food irradiation took place in 1956. Since then, many countries approved irradiation of various food items like spices, herbs, onions, potatoes, fruits, and meats. How Does Irradiation Work? Here's a brief overview of how irradiation works: - Radiation sources like gamma rays or electron beams are used to generate the required radiation energy. - Food Irradiation Food items are placed on a conveyor belt or rack and passed through the radiation area at a controlled dose rate and exposure time. - The radiation energy penetrates through packaging and food physically altering DNA/RNA structures of microbes present. - At approved low doses, it does not make food radioactive but disrupts cellular functions and DNA/RNA structure of pathogens and insects, preventing their reproduction. - The end result is elimination or reduction of pathogens and insects without altering the visual or sensory qualities of foods. Advantages of Food Irradiation Reduces Foodborne Illnesses: Irradiation is extremely effective in eliminating pathogens that cause serious foodborne illnesses. It can destroy bacteria like E. coli, Listeria, Salmonella and other parasites in meat, poultry, seafood and spices. This significantly improves food safety. Lengthens Shelf Life: By halting microbial growth and arresting ripening/sprouting processes, irradiation extends the refrigerated shelf life of various produce and foods by several weeks. This reduces spoilage losses during storage and transportation. Controls Insect Infestation: Low dose irradiation is approved globally to control insect pests in grains, cereals, dried fruits and herbs. This eliminates quarantine issues and reduces post-harvest losses from insects and insect-borne diseases. Maintains Sensory Qualities: When performed at approved low doses, irradiation does not alter the appearance, texture, aroma or flavor of foods. Irradiated fruits and vegetables look and taste fresh for much longer. Sanitizes Spices: Many spices are irradiated to kill Salmonella, E. coli and other pathogens that may be present naturally or from cross-contamination during processing. This eliminates food safety risks from consuming contaminated spices. Applications of Food Irradiation Fruits & Vegetables: Irradiation preserves the quality and extends shelf life of several delicate produce including mangoes, papayas, potatoes, onions and garlic by 3-4 weeks. It arrests ripening/sprouting to prevent losses during storage and transport. Poultry: The poultry industry uses irradiation to destroy Campylobacter and Salmonella bacteria routinely present in raw chicken and turkey. This significantly reduces the risk of foodborne illnesses from consuming undercooked poultry meat. Spices: Many commonly used herbs and spices like black pepper, cumin, coriander, basil, celery are irradiated to kill pathogens and insects. It ensures the microbial safety of spices. Grains: Low dose irradiation is used globally to control insect pests in grains like wheat, rice and pulses. This eliminates quarantine issues and reduces post-harvest losses during transportation and storage.
Get more insights on Food Irradiation
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups.
(LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
#Food Irradiation#Food Safety#Food Preservation#Radiation Processing#Gamma Irradiation#Food Sterilization#Ionizing Radiation#Microbial Reduction
0 notes
Text
Chef WK, lead charcuterie specialist in Alberta Canada
Table of contents
1. Control Program Requirements for Fermented Meat Products
2. Facility and Equipment Requirements
3. Starter Culture
4. Chemical Acidification
5. Water Activity Critical Limits
6. Time and Temperature for Fermented Products
7. Fermentation Done at a Constant Temperature
8. Examples of Degree-hours at constant room temperatures
9. Fermentation Done at Different Temperatures
10. Fermentation done at Different temperatures
11. What happens if fermentation fails to hit critical limit?
12. E. coli and Salmonella Control in Fermented Sausages
13. Options for E. coli validation
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
Control Program Requirements for Fermented Meat Products
The producer must have a program in place to assess the incoming product. This program should outline specifications for the incoming ingredients. This may include criteria including receiving temperature, farm/ supplier, lot code or packed on date, species/cut etc.
2. Facility and Equipment Requirements
Equipment used in the fermentation process must be included in the operator's prerequisite control programs. These must include the following elements:
Temperature in the fermentation, drying and smoking chambers must be uniform and controlled to prevent any fluctuation that could impact on the safety of the final product.
Fermentation, drying and smoking chambers must be equipped with a shatter resistant indicating thermometer, (or equivalent), with graduations of 1°C or less. If mercury thermometers are used, their mercury columns must be free from separations. All thermometers must be located such that they can be easily read.
Fermentation and smoking chambers must be equipped with a recording thermometer for determining degree-hours calculations in a reliable manner. Recording thermometers are also preferable in drying and aging rooms but, in these rooms, it may be sufficient to read and record the temperatures 2 times a day.
Drying and aging rooms must be equipped with humidity recorders in order to prevent uncontrolled fluctuations of the relative humidity. The only alternative to an automatic humidity recorder in these rooms would be for the company to manually monitor and record ambient humidity twice a day (morning and afternoon) every day with a properly calibrated portable humidity recorder.
For routine monitoring, accurate measurement electronic pH meters (± 0.05 units) should be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument as well as recommended sample preparation and testing be followed.
When the aw of a product is a critical limit set out in the HACCP plan for a meat product, accurate measurement devices must be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument be followed.
3. Starter Culture
The operator must use a CFIA approved starter culture. This includes Freeze-dried commercially available culture as well as back-slopping (use of previously successful fermented meat used to inoculate a new batch). When performing back-slopping, the operator must have a control program in place to prevent the transmission of pathogens from when using the inoculum from a previous batch to initiate the fermentation process of a new batch. These must include:
The storage temperature must be maintained at 4°C or less and a pH of 5.3 or less.
Samples for microbiological analysis must be taken to ensure that the process is in line with the specifications.
The frequency of sampling is to be adjusted according to compliance to specifications.
Any batch of inoculum which has a pH greater than 5.3 must be analysed to detect at least Staphylococcus aureus. Only upon satisfactory results will this inoculum be permitted for use in back slopping.
This can be an expensive and a time exhaustive process and is generally avoided due to food safety concerns. AHS does not allow back-slopping.
[Chef WK was in communication with the U of A to get his method, a starter mix, studied.]
4. Chemical Acidification
If product is chemically acidified by addition of citric acid, glucono-delta-lactone or another chemical agent approved for this purpose, controls must be in place and records kept to ensure that a pH of 5.3 or lower is achieved by the end of the fermentation process. These acids are encapsulated in different coatings that melt at specific temperatures, which then release the powdered acids into the meat batter and directly chemically acidulate the protein.
Summer sausage is a very common chemically acidified product. The flavor profile tends to be monotone and lacking depth.
5. Water Activity Critical Limits
The aw may be reduced by adding solutes (salt, sugar) or removing moisture.
Approximate minimum levels of aw (if considered alone) for the growth of:
molds: 0.61 to 0.96
yeasts: 0.62 to 0.90
bacteria: 0.86 to 0.97
Clostridium botulinum: 0.95 to 0.97
Clostridium perfringens: 0.95
Enterobacteriaceae: 0.94 to 0.97
Pseudomonas fluorescens: 0.97
Salmonella: 0.92 - 0.95
Staphylococcus aureus: 0.86
parasites: Trichinella spiralis will survive at an aw of 0.93 but is destroyed at an aw of 0.85 or less.
The above levels are based on the absence of other inhibitory effects such as nitrite, competitive growth, sub-optimum temperatures, etc., which may be present in meat products. In normal conditions, Staphylococcus aureus enterotoxins are not produced below aw 0.86, although in vacuum packed products this is unlikely below aw 0.89.
6. Time and Temperature for Fermented Products
Certain strains of the bacteria Staphylococcus aureus are capable of producing a highly heat stable toxin that causes illness in humans. Above a critical temperature of 15.6°C, Staphylococcus aureus multiplication and toxin production can take place. Once a pH of 5.3 is reached, Staphylococcus aureus multiplication and toxin production are stopped.
Degree-hours are the product of time as measured in hours at a particular temperature multiplied by the "degrees" measured in excess of 15.6°C (the critical temperature for growth of Staphylococcus aureus). Degree-hours are calculated for each temperature used in the process. The limitation of the number of degree-hours depends upon the highest temperature in the fermentation process prior to the time that a pH of 5.3 or less is attained.
The operator is encouraged to measure temperatures at the surface of the product. Where this is not possible, the operator should utilize fermentation room temperatures. The degree hour calculations are based on fermentation room temperatures. Temperature and humidity should be uniform throughout the fermentation room.
A process can be judged as acceptable provided the product consistently reaches a pH of 5.3 using:
fewer than 665 degree-hours when the highest fermentation temperature is less than 33°C;
fewer than 555 degree-hours when the highest fermentation temperature is between 33° and 37°C; and
fewer than 500 degree-hours when the highest fermentation temperature is greater than 37°C.
This means that as the temperature increases, the amount of time that you have available to reach 5.3 or under is shorter. The warmer the temperature, the sharper the log growth phase of bacteria, which equates to more overshoot in lactic acid production, faster.
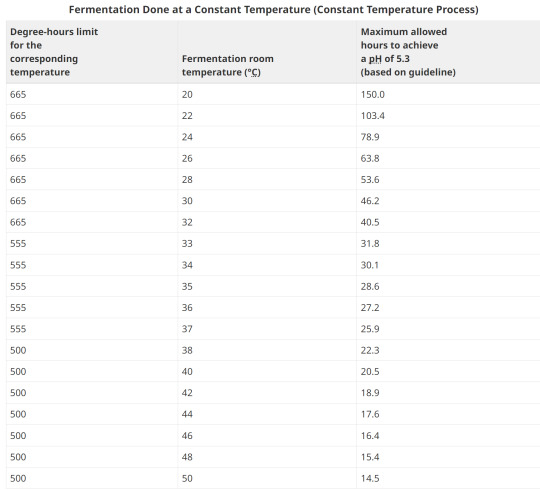
8. Examples of Degree-hours at constant room temperatures
Example 1:
Fermentation room temperature is a constant 26°C. It takes 55 hours for the pH to reach 5.3.
Degrees above 15.6°C: 26°C - 15.6°C = 10.4°C Hours to reach pH of 5.3: 55 Degree-hours calculation: (10.4°C) x (55) = 572 degree-hours
The corresponding degree-hours limit (less than 33°C) is 665 degree-hours.
Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
Example 2:
Fermentation room temperature is a constant 35°C. It takes 40 hours for the pH to reach 5.3.
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C Hours to reach pH of 5.3: 40 Degree-hours calculation: (19.4°C) x (40) = 776 degree-hours
The corresponding degree-hours limit (between 33 and 37°C) is 555 degree-hours.
Conclusion: Example 2 does not meet the guideline because its degree-hours exceed the limit
9. Fermentation Done at Different Temperatures
When the fermentation takes place at various temperatures, each temperature step in the process is analyzed for the number of degree-hours it contributes. The degree-hours limit for the entire fermentation process is based on the highest temperature reached during fermentation.
Example 1:
It takes 35 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for second 10 hours and 35°C for the final 15 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C Hours to reach pH of 5.3: 15 Degree-hours calculation: (19.4°C) x (15) = 291 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 291 = 519
The highest temperature reached = 35°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
10. Fermentation done at Different temperatures
Example 2:
It takes 38 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for the second 10 hours and 37°C for the final 18 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C Hours to reach pH of 5.3: 10 Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 37°C - 15.6°C = 21.4°C Hours to reach pH of 5.3: 18 Degree-hours calculation: (21.4°C) x (18) = 385.2 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 385.2 = 613.2
The highest temperature reached = 37°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)
Conclusion: Example 2 does not meet the guidelines because its degree-hours exceed the limit.
11. What happens if fermentation fails to hit critical limit?
What happens if the batch takes longer than degree-hours allows? For restaurant level production, it's always safer to discard the product. The toxin that Staph. Aureus produces is heat stable and cannot be cooked to deactivate. In large facilities that produce substantial batches, the operator must notify the CFIA of each case where degree-hours limits have been exceeded. Such lots must be held and samples of product submitted for microbiological laboratory examination after the drying period has been completed. Analyses should be done for Staphylococcus aureus and its enterotoxin, and for principal pathogens, such as E. coli O157:H7, Salmonella, and Clostridium botulinum and Listeria monocytogenes.
If the bacteriological evaluation proves that there are fewer than 104 Staphylococcus aureus per gram and that no enterotoxin or other pathogens are detected, then the product may be sold provided that it is labelled as requiring refrigeration.
In the case of a Staphylococcus aureus level higher than 104 per gram with no enterotoxin present the product may be used in the production of a cooked product but only if the heating process achieves full lethality applicable to the meat product.
In the case where Staphylococcus aureus enterotoxin is detected in the product the product must be destroyed.
12. E. coli and Salmonella Control in Fermented Sausages
Business' that manufacture fermented sausages are required to control for verotoxinogenic E. coli including E. coli O157:H7 and Salmonella when they make this type of product. This includes:
establishments which use beef as an ingredient in a dry or semi-dry fermented meat sausage;
establishments which store or handle uncooked beef on site;
Establishments which do not use beef and do not obtain meat ingredients from establishments which handle beef are not currently required to use one of the five options for the control of E. coli O157:H7 in dry/semi-dry fermented sausages.
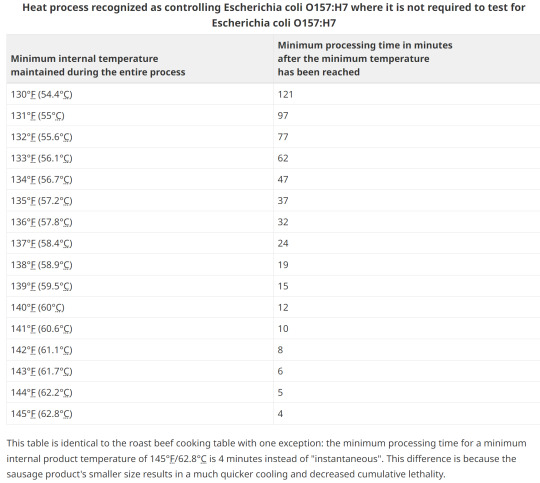
Any processed RTE product containing beef or processed in a facility that also processed beef, must be subjected to a heat treatment step to control E. coli O157:H7. Heating to an internal temperature of 71°C for 15 seconds or other treatment to achieve a 5D reduction is necessary. This is a CFIA requirement and is not negotiable.
Uncooked air dried products produced as RTE, must meet shelf stable requirements as detailed for Fermented-Dry products.
13. Options for E. coli validation
Without lab testing, the two main methods of validation are with heat treating by either low temp and a long duration, or various hotter processing temperatures for a shorter timeframe.
A challenge study to validate a process can take 1 year and over $100,000!
14. Option1; Heating
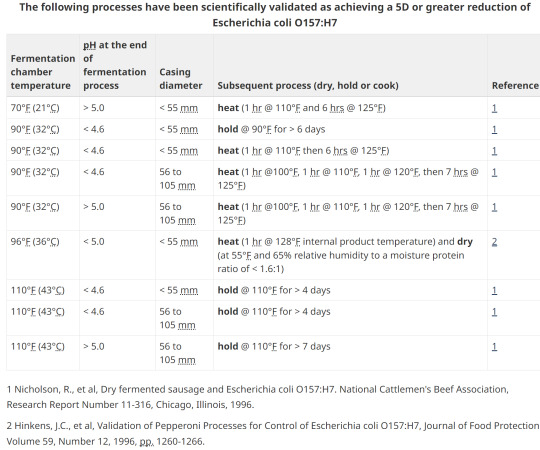
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
The aw and pH values are critical in the control of pathogens as well as to ensure shelf-stability in all semi-dry and dry fermented meat products. Each batch must be tested for aw and/or pH in order to verify that the critical limits are met.
Although aw measurement is mandatory only for shelf stable products, it is strongly recommended that the producer determine the aw values achieved for each product type they manufacture and for each product. Once this has been established, frequent regular checks should be made to ensure consistency. In the U.S., they rely on moisture to protein ratio and have set targets. This lab-tested value is a direct correlation of the % water to % meat protein and not aw. This gives more consistency to common names. For example, to legally call a product "jerky" it must have a MPR of 0.75:1 or lower. Remember your ABCs:
Always be compliant.
-AND-
Documentation or it didn't happen.
(tags)
Charcuterie,Fermented Meat,Food Safety,Starter Culture,Chemical Acidification,Water Activity,Fermentation Process,Degree-Hours Method,Foodborne Pathogens,Meat Processing Guidelines,Chef WK Alberta Canada,Food Industry Standards,pH Critical Limits,Thermal Processing,Food Preservation,Food Microbiology,Sausage Fermentation,Charcuterie Expertise,Fermented Meats ,Food Safety Standards,Food Processing Guidelines,Starter Cultures,Chemical Acidification,Water Activity (a_w),Critical Limits,Degree-Hours Method,Foodborne Pathogens,Meat Processing Equipment,Processing Facility Requirements,Hazard Analysis and Critical Control Points (HACCP),Food Preservation Techniques,Temperature Control,Pathogen Reduction,Food Industry Compliance,Documentation Practices,Heat Treatment,pH Control,Food Stability,Consistency in Production,Microbial Testing,Real-time Monitoring,Process Validation,Regulatory Requirements,Verotoxigenic E. coli,Lethality Standards,Product Labelling,Spoilage Prevention,Enterotoxin Detection,Shelf-Stable Products,Moisture to Protein Ratio (MPR)
#Charcuterie#Fermented Meat#Food Safety#Starter Culture#Chemical Acidification#Water Activity#Fermentation Process#Degree-Hours#Meat Processing Guidelines#Thermal Processing#Food Preservation#Food Microbiology#Sausage Fermentation#Starter Cultures#Critical Limits#Meat Processing#Food Preservation Techniques#Temperature Control#Pathogen Reduction#Food Industry#Heat Treatment#pH Control#Food Stability#Microbial Testing#Real-time Monitoring#Process Validation#Spoilage Prevention#Enterotoxin Detection#Shelf-Stable Products#Moisture to Protein Ratio (MPR)
2 notes
·
View notes
Text
Why Bioculture Is Key in Modern Wastewater Treatment
Wastewater management has come a long way from traditional chemical-heavy methods. Today, with growing environmental concerns and the push for sustainable solutions, bioculture in wastewater treatment is gaining serious traction. But what exactly is bioculture, and why is it becoming the go-to solution in modern wastewater systems? Let’s break it down in simple, practical terms.

What Is Bioculture?
Bioculture refers to a concentrated mix of beneficial microorganisms—think bacteria, fungi, and enzymes—that are specifically designed to digest organic waste present in wastewater. These microbes feed on the pollutants, breaking them down into simpler, non-toxic compounds. It’s nature doing the dirty work—literally.
How Bioculture Works in Wastewater Treatment
When bioculture is introduced into a sewage treatment plant or an effluent treatment plant, it kickstarts a natural biological process. The microbes begin breaking down:
Organic matter
Fats, oils, and grease (FOG)
Sludge
Harmful pathogens
The process not only accelerates the breakdown of waste but also significantly reduces foul odors and improves water clarity.
Benefits of Using Bioculture in Wastewater Treatment
Let’s be real—why should industries, municipalities, or even residential complexes care about using bioculture? Here’s why:
1. Eco-Friendly and Sustainable
Bioculture-based systems use naturally occurring organisms, making them an environmentally friendly alternative to harsh chemicals that often harm aquatic ecosystems.
2. Cost-Effective
Using bioculture can actually cut operational costs. How? It reduces the need for frequent desludging, minimizes chemical usage, and lowers energy consumption.
3. Improves Efficiency
Bioculture boosts the efficiency of the treatment process. It enhances Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) removal, making treated water safer for discharge or reuse.
4. Reduces Sludge Volume
One major headache for wastewater facilities is dealing with excess sludge. Bioculture helps reduce the volume of sludge generated, making disposal easier and cheaper.
5. Faster Recovery from Shock Loads
Industrial plants often face “shock loads”—a sudden influx of high-strength waste. Bioculture helps treatment systems bounce back faster without major operational hiccups.
Common Applications of Bioculture
Bioculture in wastewater treatment isn’t just for large-scale operations. It finds applications across various sectors:
Sewage Treatment Plants (STPs)
Effluent Treatment Plants (ETPs)
Dairy, textile, and food processing industries
Hotels and residential complexes
Septic tanks and community toilets
Things to Consider Before Using Bioculture
While bioculture is a game-changer, a few things need to be in place for it to work effectively:
Maintain the right pH, temperature, and oxygen levels
Avoid using toxic cleaning agents that can harm microbes
Regular monitoring to ensure microbial health
Working with an experienced wastewater treatment consultant can help tailor the solution to your specific needs.
Final Thoughts
As environmental regulations tighten and the demand for cleaner processes rises, bioculture in wastewater treatment is not just an option—it’s quickly becoming a necessity. It's a smart, sustainable, and scalable solution that leverages nature to solve one of modern society's most pressing problems.
If you're exploring ways to upgrade your wastewater management practices, integrating bioculture is a step in the right direction. It’s time to trust the microbes—they’ve got this!
#Bioculture in Wastewater Treatment#Wastewater Treatment Solutions#Sustainable Wastewater Management#Eco-Friendly Wastewater Treatment#Biological Treatment of Wastewater#Sewage Treatment Using Bioculture#Wastewater Microbial Solutions#Effluent Treatment Plants#Organic Waste Breakdown#Modern Wastewater Technology#Sludge Reduction Methods#Wastewater Treatment for Industries#Bioculture for STP and ETP#Environmental Wastewater Solutions#BOD and COD Reduction#waste water treatment
0 notes
Text
Do You Have a Nose for Wine Faults? Take the Quiz.
Do You Have a Nose for #WineFaults? Take the #Quiz. #somm #winelover #corktaint
Good or Bad wine experience Understanding and identifying faults in wine is crucial for both novice and seasoned enthusiasts. Wine faults can significantly diminish the overall enjoyment of a bottle, affecting its aroma, taste, and texture. Common faults include cork taint, oxidation, and microbial contamination, each imparting undesirable characteristics to the wine. Recognizing these faults,…

View On WordPress
0 notes
Text
The Transformative Power of Sourdough: Revolutionizing the Bread Industry and Consumer Perceptions
People with celiac disease or various gluten intolerances may soon have a broader range of food options, thanks to advancements in research and the expanding industrial use of a traditional bread-making process: sourdough. Sourdough is a dough of varying consistency, depending on the initial ratio of flour to water, which undergoes spontaneous fermentation (due to naturally occurring…

View On WordPress
#Baking Technology#Bread Industry Innovation#Clean Eating#Gluten Free Options#Gluten Reduction#Health And Wellness#Microbial Fermentation#Sourdough Revolution#Sustainable Baking
0 notes
Text
What to Look for in Herbal Extracts Manufacturers in India
Introduction
As the demand for plant-based wellness, cosmetic, and nutraceutical products rises globally, businesses are actively seeking herbal extracts manufacturers in India who offer high-quality, sustainable, and customized solutions. Whether you're sourcing herbal extracts, oil extracts, or partnering with a fragrance manufacturer, the right supplier is key to delivering premium formulations.
India, with its rich biodiversity and Ayurvedic legacy, is home to many natural product manufacturers. But how do you choose the one that meets your quality standards and business goals?
This guide will help you understand what makes a manufacturer reliable — and why leading companies partner with Nuleaf Naturals, one of the top herbal extract and oil extracts manufacturers in India.
Mastery in Botanical Extraction Technologies
A manufacturer’s expertise in extraction techniques directly impacts the purity, efficacy, and safety of the final product. At Nuleaf Naturals, we combine traditional herbal wisdom with modern science using advanced extraction technologies such as:
Supercritical CO₂ Extraction (SCFE): Ideal for solvent-free, potent herbal and oil extracts — widely used in cosmetics and nutraceuticals.
Steam Distillation: Best suited for aromatic compounds and essential oils, preserving delicate fragrance notes.
Short Path Distillation: Useful for high-purity concentrates with minimal heat degradation.
Solvent Extraction: Effective for herbs where other methods may not deliver optimal yields.
By investing in cutting-edge infrastructure, we ensure our extracts meet international quality benchmarks.
Certifications That Guarantee Quality and Compliance
Regulatory compliance is non-negotiable when choosing herbal extracts manufacturers in India. Top-tier suppliers should offer:
GMP Certification (Good Manufacturing Practices)
ISO Certification for quality assurance
FSSAI Registration to ensure food-grade safety
HACCP Certification for hazard analysis and critical control
These certifications demonstrate a manufacturer's commitment to standardized processes and safety protocols. At Nuleaf Naturals, we maintain rigorous quality systems backed by all essential certifications.
Extensive Product Portfolio and Customization Options
A good natural product manufacturer offers not only variety but also customization. At Nuleaf Naturals, our comprehensive product line includes:
Herbal Extracts (e.g., Ashwagandha, Brahmi, Tulsi, Turmeric)
Essential & Oil Extracts (e.g., Lemongrass, Eucalyptus, Lavender)
Oleoresins, Hydrosols, and Fragrance Compounds
Custom Ayurvedic Formulations for wellness and skincare brands
We also offer tailored services including:
Custom potency levels and particle size
Private/white labeling
Third-party testing and documentation
Bulk supply and formulation assistance
This flexibility ensures that our clients get exactly what they need for product development.
Transparent Testing and Full Documentation
Trustworthy manufacturers provide full transparency through lab reports and third-party testing. Nuleaf Naturals guarantees:
Certificates of Analysis (COA) for every batch
Heavy metal and pesticide screening
Microbial testing to ensure safety
Batch-specific traceability
This documentation builds trust with your end customers and helps you stay compliant with global safety standards.
Environmentally Responsible and Ethical Manufacturing
Sustainability is no longer optional — it’s a key value proposition. As an eco-conscious herbal extract manufacturer, Nuleaf Naturals is committed to:
Green extraction techniques with minimal solvent waste
Eco-friendly packaging and waste reduction policies
Ethical sourcing directly from certified organic farms
By choosing a sustainable manufacturing partner, your brand benefits from environmental stewardship and stronger consumer loyalty.
Dependable Supply Chain and Bulk Production Capacity
When scaling your operations, you need a manufacturer that can deliver high volumes without compromising quality. At Nuleaf Naturals, we offer:
Bulk herbal extracts and oil extracts with reliable restocking
Global shipping and export support
Custom contract manufacturing for long-term partnerships
White labeling and B2B collaboration models
With a robust supply chain and on-time delivery, we help your business maintain consistency and meet market demand efficiently.
Proven Industry Experience and Global Clientele
Before finalizing your supplier, evaluate their industry track record. At Nuleaf Naturals, we bring:
Over a decade of experience in herbal extraction
Partnerships with nutraceutical, personal care, and wellness brands globally
Client testimonials that reflect satisfaction, reliability, and professionalism
Our success lies in helping brands around the world create high-performing, natural products that customers love.
Conclusion
Selecting the right herbal extracts manufacturer in India goes beyond product sourcing—it's about building a strategic partnership. From technical expertise and regulatory compliance to customization and sustainability, Nuleaf Naturals offers a complete solution for businesses looking to scale with confidence.
Whether you’re a wellness startup or an established beauty brand, our team is ready to co-create high-quality formulations that align with your values and market goals.
Partner with Nuleaf Naturals — a trusted fragrance manufacturer and supplier of premium herbal and oil extracts — and unlock the full potential of nature-powered innovation.📞 Contact us today for bulk orders and B2B partnerships! +91 9866760001
2 notes
·
View notes
Text
Before you can sanitize a surface, you first need to make sure it’s properly cleaned.
Start by using a surface cleaner or surfactant to remove any visible dirt, grease, or organic matter from the area. It’s important to do this cleaning step thoroughly, as any leftover debris can protect microbes from the sanitizing agent.
Once the surface is visibly clean, you’ll need to rinse it well with clean water to wash away any residual cleaner.
Now that the surface is clean and rinsed, you can move on to the sanitizing step. What I do in my workspaces, is: a diluted bleach solution (10% bleach to 90% water) or other applicable sanitizer to spray or wipe down the area. This kills off most bacteria and viruses that could contaminate your reesesech.
Something to consider that many dont realize: you should allow the sanitized surface to fully air dry before using it. Wiping it down could reintroduce contamination and beach, for wxample, has an ancillary disinfection that occurs drom its fumes as it dries ;).
Worth considering: there will be some disconnect between people considering this due to sanitization, sterilization, disinfection, aseptic, etc all kinda mixing together.
Aseptic technique is essentially “maintaining an environment that does not introduce microbes onto a workspace”.
Sanitization and disinfection are, imo, same coin but sanitization is of a lesser degree of microbial reduction. More appropriate for getting a workspace clean enough to run a food manufacturing process-line.
Disinfection is the next step up, requiring more concentrate strength in solution used and kills more shit. Sterilization is more like … autoclave.
Some resources for those interested
https://ucfoodsafety.ucdavis.edu/sites/g/files/dgvnsk7366/files/inline-files/26437.pdf
https://www.ncbi.nlm.nih.gov/books/NBK214356/#:~:text=Bleach%20is%20a%20strong%20and,contact%20time%20(see%20Table%20G.
“Bleach is a strong and effective disinfectant – its active ingredient sodium hypochlorite is effective in killing bacteria, fungi and viruses, including influenza virus – but it is easily inactivated by organic material. Diluted household bleach disinfects within 10–60 minutes contact time (see Table G.1 below for concentrations and contact times), is widely available at a low cost, and is recommended for surface disinfection in health-care facilities. However, bleach irritates mucous membranes, the skin and the airways; decomposes under heat and light; and reacts easily with other chemicals. Therefore, bleach should be used with caution; ventilation should be adequate and consistent with relevant occupational health and safety guidance. Improper use of bleach, including deviation from recommended dilutions (either stronger or weaker), may reduce its effectiveness for disinfection and can injure health-care workers.“
#mycology#magic mushies#microbiology#mold#60s psychedelia#lgbtqia#lgbtqia2s#lgbtqia2s+#myc#enby#cleaning#disinfecting#sanitization#aseptic#hygiene
5 notes
·
View notes
Text
How Innovation Is Reshaping the Food Industry

Food innovation refers to introducing novel ideas, products, and technologies that change how society produces, processes, packages, distributes, and consumes food. It goes beyond merely creating new recipes or flavors - food innovation encompasses advances in agriculture, food science, sustainability, and packaging. The goal is to enhance efficiency, safety, nutrition, and the overall consumer experience.
The need for food innovation arises from the ever-changing demands of consumers and the pressing challenges faced by the industry. As the global population continues to grow, so does the demand for food. Additionally, sustainability concerns, climate change, and limited resources prompt exploring alternative food growing and production methods. Innovations in food aim to enhance food security, minimize environmental impact, and offer consumers healthier, more diverse options.
Food innovation occurs through a combination of research, collaboration, and creativity. Scientists, entrepreneurs, farmers, and food industry professionals work together to develop new technologies and processes. Research institutions and startups play a crucial role in conducting experiments, testing new concepts, and bringing innovative products to the market.
In recent years, the food industry has witnessed groundbreaking innovations reshaping how people interact with food. The plant-based movement has gained immense traction, with plant-based alternatives for meat, dairy, and seafood becoming mainstream. Companies have developed plant-based burgers, vegan cheeses, and sustainable seafood alternatives using cutting-edge technologies. Beyond plant-based options, innovations have also focused on alternative protein sources, such as insect-based proteins and lab-grown meats, offering sustainable and protein-rich alternatives.
Swedish startup Mycorena is boosting microbial protein production through its fungi-based mycoprotein called Promyc. This ingredient can be used to create meat and tuna alternatives, beverage additives, and dessert ingredients, offering plant-based and sustainable options for consumers.
Finnish startup Onego Bio has developed a product genetically identical to egg whites using fermentation, and without using actual chickens. It uses precision fermentation of a microflora called Trichoderma reesei to produce ovalbumin, the protein found in chicken egg whites. This technology offers a sustainable and animal-friendly alternative for various food applications, including baked goods, desserts, sauces, and dressings.
Companies like New Culture are incorporating animal-free casein into their cheeses through precision fermentation. This breakthrough allows them to produce animal-free mozzarella cheese, offering a delicious and cruelty-free alternative to traditional dairy products.
In addition, consumers increasingly seek transparency in food choices, leading to the clean label movement. Brands are responding by using simple natural ingredients and avoiding artificial additives and preservatives.
Breakthrough innovations in the food industry are revolutionizing how society grows, produces, and consumes food, focusing on sustainability, nutrition, and convenience. One such innovation is plastic-free and smart packaging. Food companies are exploring biodegradable and even edible packaging solutions in response to environmental concerns. Smart packaging using nanotechnology is also gaining popularity, allowing consumers to assess food safety and quality easily.
The Internet of Things (IoT) in agriculture employs sensors and data analytics for optimizing crop conditions, irrigation, and pest control, reducing resource usage. Food waste reduction solutions, such as surplus food redistribution platforms, are being developed to combat the global food waste crisis. Moreover, biotechnology and data science advances enable personalized nutrition, tailoring dietary recommendations to individuals based on their genetic makeup, lifestyle, and health goals. These innovations promise a more sustainable, healthier, and efficient food future.
Food innovation is driving a remarkable transformation in the food industry, responding to the challenges and opportunities of today. From new plant-based products to sustainable agriculture and cutting-edge technologies, the future of food promises to be more diverse, nutritious, and sustainable. As consumers, entrepreneurs, and stakeholders continue to embrace innovation, the food industry's journey toward a more resilient and conscious future is set to continue.
13 notes
·
View notes
Note
Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. Photosynthesis usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.
Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, producing sulfur instead of oxygen. Archaea such as Halobacterium also perform a type of non-carbon-fixing anoxygenic photosynthesis, where the simpler photopigment retinal and its microbial rhodopsin derivatives are used to absorb green light and power proton pumps to directly synthesize adenosine triphosphate (ATP), the "energy currency" of cells. Such archaeal photosynthesis might have been the earliest form of photosynthesis that evolved on Earth, as far back as the Paleoarchean, preceding that of cyanobacteria (see Purple Earth hypothesis).
While the details may differ between species, the process always begins when light energy is absorbed by the reaction centers, proteins that contain photosynthetic pigments or chromophores. In plants, these proteins are chlorophylls (a porphyrin derivative that absorbs the red and blue spectrums of light, thus reflecting green) held inside chloroplasts, abundant in leaf cells. In bacteria they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. The hydrogen freed by the splitting of water is used in the creation of two important molecules that participate in energetic processes: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and ATP.
In plants, algae, and cyanobacteria, sugars are synthesized by a subsequent sequence of light-independent reactions called the Calvin cycle. In this process, atmospheric carbon dioxide is incorporated into already existing organic compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose. In other bacteria, different mechanisms like the reverse Krebs cycle are used to achieve the same end.
The first photosynthetic organisms probably evolved early in the evolutionary history of life using reducing agents such as hydrogen or hydrogen sulfide, rather than water, as sources of electrons. Cyanobacteria appeared later; the excess oxygen they produced contributed directly to the oxygenation of the Earth, which rendered the evolution of complex life possible. The average rate of energy captured by global photosynthesis is approximately 130 terawatts, which is about eight times the total power consumption of human civilization. Photosynthetic organisms also convert around 100–115 billion tons (91–104 Pg petagrams, or a billion metric tons), of carbon into biomass per year. Photosynthesis was discovered in 1779 by Jan Ingenhousz. He showed that plants need light, not just air, soil, and water.
Photosynthesis is vital for climate processes, as it captures carbon dioxide from the air and binds it into plants, harvested produce and soil. Cereals alone are estimated to bind 3,825 Tg or 3.825 Pg of carbon dioxide every year, i.e. 3.825 billion metric tons.

That reminds me of the Krebs cycle, which creates ATP instead of using it. I am learning just how much lifeforms rely on each other to survive. Destroying one could cause many others to crumble. Interesting.
(OOC: Sorry, but I do not understand plants very well at all. I like anatomy of animals, humans, and bugs more).
3 notes
·
View notes
Text
In a world teeming with microbial assailants, the quintessence of human ingenuity has manifested in a simple, elegant solution: vaccines. These minute miracles, conjured through the alchemy of modern science, have transcended the mundane and achieved the extraordinary, transforming our collective fate.
Vaccines, the stalwart sentinels of our immune system, have unequivocally altered the trajectory of human health. They have extirpated smallpox from the annals of endemic diseases and relegated polio to the brink of oblivion. Their efficacy is not merely anecdotal but robustly corroborated by empirical data. Consider the paradigm of the measles vaccine: a triumph that has diminished the global incidence of this virulent scourge by 99% since its inception. Such statistics are not mere happenstance but the result of meticulous research and rigorous clinical trials, which have consistently demonstrated the unparalleled efficacy of vaccines.
The statistics delineating the benefits of vaccination are irrefutable. The World Health Organization (WHO) attests that vaccines prevent 2-3 million deaths annually. The historical reduction in morbidity and mortality rates from diseases such as diphtheria, tetanus, and pertussis is a testament to their unparalleled potency. Moreover, the introduction of the human papillomavirus (HPV) vaccine has precipitated a precipitous decline in the prevalence of HPV-related cancers, illustrating the prophylactic prowess of vaccination.
Yet, in an era rife with misinformation, the discourse surrounding vaccines is often obfuscated by fallacious narratives. The specter of adverse reactions is frequently invoked by detractors, yet the preponderance of evidence elucidates that such occurrences are exceedingly rare. The incidence of severe allergic reactions, anaphylaxis, is approximately 1 in a million. By contrast, the morbidity and mortality associated with vaccine-preventable diseases are exponentially higher. The juxtaposition of these statistics underscores the irrefutable verity that the benefits of vaccination overwhelmingly eclipse the infinitesimal risk of adverse effects.
To deny the efficacy of vaccines is to eschew reason and embrace anachronism. It is to dismiss the incontrovertible evidence amassed through decades of scientific inquiry. Vaccines epitomize the zenith of human ingenuity, embodying the impeccable synergy of science and medicine. They are not merely an option but an imperative, a societal obligation to safeguard public health.
In summation, the perspicacious embrace of vaccination is not merely a testament to individual sagacity but a communal bulwark against the inexorable tide of infectious diseases. Let us not be swayed by the cacophony of misinformation but remain steadfast in our commitment to empirical truth. The science is incontrovertible, the benefits unassailable. Vaccines are the apotheosis of prophylactic medicine, and their continued utilization is nothing short of imperative.
#impeccable#science#disease#immunity#vaccine#bacteria#virus#pathogens#climate change#scientific-method#reality#facts#evidence#research#study#knowledge#wisdom#truth#honesty
2 notes
·
View notes
Note
Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. Photosynthesis usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.
Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, producing sulfur instead of oxygen. Archaea such as Halobacterium also perform a type of non-carbon-fixing anoxygenic photosynthesis, where the simpler photopigment retinal and its microbial rhodopsin derivatives are used to absorb green light and power proton pumps to directly synthesize adenosine triphosphate (ATP), the "energy currency" of cells. Such archaeal photosynthesis might have been the earliest form of photosynthesis that evolved on Earth, as far back as the Paleoarchean, preceding that of cyanobacteria (see Purple Earth hypothesis).
While the details may differ between species, the process always begins when light energy is absorbed by the reaction centers, proteins that contain photosynthetic pigments or chromophores. In plants, these proteins are chlorophylls (a porphyrin derivative that absorbs the red and blue spectrums of light, thus reflecting green) held inside chloroplasts, abundant in leaf cells. In bacteria they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. The hydrogen freed by the splitting of water is used in the creation of two important molecules that participate in energetic processes: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and ATP.
In plants, algae, and cyanobacteria, sugars are synthesized by a subsequent sequence of light-independent reactions called the Calvin cycle. In this process, atmospheric carbon dioxide is incorporated into already existing organic compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose. In other bacteria, different mechanisms like the reverse Krebs cycle are used to achieve the same end.
The first photosynthetic organisms probably evolved early in the evolutionary history of life using reducing agents such as hydrogen or hydrogen sulfide, rather than water, as sources of electrons. Cyanobacteria appeared later; the excess oxygen they produced contributed directly to the oxygenation of the Earth, which rendered the evolution of complex life possible. The average rate of energy captured by global photosynthesis is approximately 130 terawatts, which is about eight times the total power consumption of human civilization. Photosynthetic organisms also convert around 100–115 billion tons (91–104 Pg petagrams, or a billion metric tons), of carbon into biomass per year. Photosynthesis was discovered in 1779 by Jan Ingenhousz. He showed that plants need light, not just air, soil, and water.
Photosynthesis is vital for climate processes, as it captures carbon dioxide from the air and binds it into plants, harvested produce and soil. Cereals alone are estimated to bind 3,825 Tg or 3.825 Pg of carbon dioxide every year, i.e. 3.825 billion metric tons.
Why are we suddenly in a science lesson? Its interesting nontheless though!
4 notes
·
View notes
Text
Food Irradiation market is gaining Popularity to Ensure Food Safety is in Trends by Growing Health Concerns
Food irradiation is a process that uses ionizing radiation like gamma rays, x-rays or electron beams to kill harmful pathogens and extend the shelf-life of various agricultural and food products. It helps eliminate microorganisms like salmonella, e-coli, listeria etc thereby making food safer for consumption without compromising on its taste or nutritional value. The global food supply chains have led to longer distribution times and increased chance of contamination. Therefore, food irradiation provides a non-thermal disinfection method to address this issue. The other advantages include killing insects in wheat, delaying ripening of fruits like mangoes and elimination of weed seeds in grains.
The Global Food Irradiation Market is estimated to be valued at US$ 745.5 million in 2024 and is expected to exhibit a CAGR of 5.0% over the forecast period 2024-2031. Key Takeaways Key players operating in the Food Irradiation market are Sterigenics International LLC, IBA, WASIK ASSOCIATES, Jiangsu Dasheng Electron Accelerator and Nordion. The growing demand for longer shelf-life and safer food coupled with implementation of stringent food safety regulations across various countries are major factors fueling the growth of global food irradiation market. As per WHO, over 200,000 people die every year from foodborne diseases indicating the need for reliable techniques like irradiation. Geographically, North America dominates the global Food Irradiation Market Size followed by Europe and Asia Pacific. However, Asia Pacific market is projected to witness the highest growth during the forecast period owing to growing awareness, emerging economies and increasing domestic consumption of food. Market key trends One of the key trends gaining traction in the food irradiation market is the growing acceptability among consumers regarding food safety. Several consumer surveys have shown increasing confidence in irradiation as a food preservation technique when proper communication is provided about its process and benefits. Government initiatives and endorsement by international organizations have also helped address the misconceptions around the technology. The market players are actively working towards enhancing the irradiation capacities and capabilities to leverage this growth opportunity. They are developing new irradiation systems that can treat packaged products and enable automated high volume processing. This is expected to further aid commercialization and large scale adoption of food irradiation globally.
Porter’s Analysis Threat of new entrants: High capital requirement and regulatory compliances creates entry barrier for new players. Bargaining power of buyers: Large buyers exert pressure on pricing due to bulk buying whereas small buyers have limited bargaining power. Bargaining power of suppliers: Few key players control supply chain that limits options for buyers. Threat of new substitutes: No close substitute exists but alternative preservation methods pose potential threat. Competitive rivalry: Established players compete on technologies, services and geographic expansion to gain higher market share. Geographical Regions North America dominates the food irradiation market currently, both in terms of value and volume. Steady demand from food industries like meat and spices significantly contributes to market concentration in the region. Adoption of food irradiation is comparatively higher in developed countries which drives the regional market. Asia Pacific exhibits fastest growth rate owing to rising population, increasing food trade and growing food safety concerns. Rapid industrialization and economic development of nations like China and India boosts regional food production and processing industries thereby supplementing market expansion. Rising awareness about food preservation technologies and government support for irradiation facilitates growth in the Asia Pacific food irradiation market.
Get more insights on Food Irradiation Market
For Deeper Insights, Find the Report in the Language that You want.
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)

#Coherent Market Insights#Food Irradiation Market#Food Irradiation#Food Sterilization#Shelf Life Extension#Microbial Control#Pathogen Reduction#Gamma Rays#Radiation Treatment#Cold Pasteurization#Food Preservation
0 notes
Text
NUTRIFLAX
Nutriflax Granules are an innovation in Biotechnology Research. Nutriflax is a non-toxic, eco-friendly research based complete natural food for any crop. Nutriflax contains vitamins A, B, B2, C, Folic acid proteins like amino acid, humic acid and other enzymes and turmeric and probiotics makes product unique.
Benefits:
Early germination.
Vigorous seedling growth.
Profuse primary and secondary root development.
Increased soil microbial activity.
Higher nutrient uptake.
Better branching/tillering and increased foliage.
Reduction in the fruit and flower drop.
Better development of grains/fruits.
Increase in the size and weight of the grains/fruits.
Higher yield and better quality of the produce.
Crops:
For all the commercial crops
Agrifort Technologies
Facebook
Instagram
Youtube
What are Nutriflax Granules?
These granules when applied to soil release nutrients in plant rhizosphere thus stimulate growth of beneficial micro-organisms and provide nutritional support to plant at critical stages of growth. Nutriflax helps the plant against adverse climate condition and provide healthy overall growth of plant system, higher yields,pest & disease resistance. Manufacturing process through probiotic makes the products further unique.
#agribusiness#agritech#agriculture#education#health & fitness#business#nature#agricoltura#agrobisnis#farmers protest#farming#Farmers
3 notes
·
View notes
Text
Randall Randy Konsker Guide The Top Benefits of Organic Farming You Need to Know

Organic farming has gained significant traction in recent years as consumers become increasingly conscious of their food choices and the impact of agriculture on the environment. Organic farming, characterized by the use of natural methods and avoiding synthetic pesticides and fertilizers, offers a range of benefits that extend beyond personal health. Randall Randy Konsker's guide we will explore the top benefits of organic farming and why it is gaining popularity worldwide.
1. Environmental Sustainability
One of the primary advantages of organic farming is its commitment to environmental sustainability. Organic farming methods prioritize soil health through practices such as crop rotation, cover cropping, and composting. By avoiding synthetic chemicals, organic farmers protect biodiversity, promote healthier ecosystems, and reduce the risk of soil erosion. This approach helps maintain the long-term fertility of the soil and minimizes the environmental impact of agriculture.
2. Reduced Chemical Exposure
Conventional farming relies heavily on synthetic pesticides and fertilizers to boost crop yields. However, the residues from these chemicals can end up in the food we consume, posing potential health risks. Organic farming eliminates or significantly reduces the use of synthetic chemicals, providing consumers with produce free from harmful residues. This reduction in chemical exposure has been linked to lower risks of certain health issues, making organic food an attractive choice for health-conscious individuals.
3. Improved Soil Health
Organic farming focuses on building and maintaining healthy soil. Practices such as crop rotation, cover cropping, and the use of organic matter like compost enhance soil structure, water retention, and microbial activity. Healthy soils support robust plant growth, increase nutrient content in crops, and contribute to overall ecosystem resilience. Additionally, the absence of synthetic fertilizers in organic farming prevents soil degradation, ensuring a sustainable and fertile environment for future generations.
4. Enhanced Nutritional Content
Several studies suggest that organic crops may have higher nutritional content compared to their conventionally grown counterparts. Organic farming practices, which prioritize soil health and diversity, often result in crops with increased levels of essential nutrients, antioxidants, and vitamins. This nutritional boost can positively impact human health and contribute to a well-rounded and balanced diet.
5. Support for Local Economies
Organic farming often occurs on a smaller scale and is more likely to be practiced by local farmers. Choosing organic products supports local economies by providing income and employment opportunities within communities. Additionally, the emphasis on local distribution reduces the carbon footprint associated with transporting goods over long distances, contributing to a more sustainable and resilient local food system.
6. Water Conservation
Organic farming practices prioritize efficient water management through techniques such as mulching, drip irrigation, and water-conserving cover crops. By minimizing water usage and runoff, organic farming helps conserve this precious resource. This is particularly crucial in regions facing water scarcity, as sustainable agricultural practices become essential for maintaining a reliable and resilient food supply.
Conclusion
The benefits of organic farming extend far beyond the individual consumer, reaching into the realms of environmental sustainability, public health, and local economies. Randall Randy Konsker says by choosing organic products, consumers play a vital role in supporting farming practices that prioritize the well-being of the planet and its inhabitants. As the demand for sustainable and ethically produced food continues to grow, organic farming stands as a beacon of a more conscientious and environmentally friendly approach to agriculture.
2 notes
·
View notes
Text
How Sanitizer Testing Labs Help Brands in India Get Product Approvals?
In the rapidly growing health and hygiene sector, sanitizer products have become a staple in every Indian household, workplace, and public space. With this rise in demand, brands—especially cosmetic, pharmaceutical, and FMCG companies—are racing to launch effective and safe sanitizer products. However, gaining regulatory approval in India is no small task. It requires strict compliance with quality, safety, and efficacy norms laid down by bodies like the BIS (Bureau of Indian Standards) and CDSCO (Central Drugs Standard Control Organization). This is where a certified Sanitizer Testing Lab becomes invaluable.
In this blog, we explore how sanitizer testing lab help Indian brands achieve regulatory approvals by providing accurate, reliable, and compliant testing services.
Why Product Approval is Crucial in India
In India, manufacturing and selling sanitizer products—especially those with antimicrobial claims—requires mandatory approval. Depending on the type and claims, the product could fall under:
BIS standards (for general alcohol-based sanitizers)
CDSCO regulation (for products with therapeutic or medicinal claims)
Failure to get these approvals can lead to product recalls, legal penalties, or damage to brand reputation. A sanitizer testing lab plays a central role in streamlining this approval process.
Key Ways Sanitizer Testing Labs Help with Regulatory Approvals
✅ 1. Accurate Alcohol Content Testing
Indian standards require hand sanitizers to contain a minimum of 60% alcohol (either ethanol or isopropyl alcohol). Testing labs use GC (Gas Chromatography) or Alcoholmeters to verify alcohol levels with precision.
Regulatory relevance:
For BIS compliance (IS 1500:2020), accurate alcohol content is mandatory.
Under-delivery of alcohol results in disapproval.
✅ 2. Microbial Efficacy Testing
Sanitizers that claim to "kill 99.9% of germs" or offer antimicrobial protection need to be validated through antimicrobial efficacy tests. Sanitizer testing labs perform:
Time-kill assays
Surface sanitization tests
ASTM and EN standard-based microbial reduction tests
Regulatory relevance:
CDSCO mandates efficacy testing for products marketed as “drugs.”
Efficacy data supports product claims during the application process.
✅ 3. Toxicological and Dermatological Testing
Sanitizers, especially cosmetic variants, must be tested for:
Skin irritation
Dermal toxicity
Eye irritation (if contact risk exists)
pH balance
These tests confirm that the sanitizer is safe for frequent use and does not cause adverse reactions.
Regulatory relevance:
CDSCO and BIS require human safety validation.
Supports both cosmetic and medicinal product clearance.
✅ 4. Methanol and Impurity Detection
The presence of methanol, a toxic alcohol, is strictly prohibited in hand sanitizers. Labs conduct GC-MS analysis to detect and quantify any harmful substances like:
Methanol
Heavy metals
Harmful aldehydes
Regulatory relevance:
BIS and CDSCO reject sanitizers with even trace levels of methanol.
Prevents product recalls and ensures user safety.
✅ 5. Shelf Life and Stability Studies
Before a sanitizer can be approved for market sale, its shelf life must be validated. Testing labs conduct:
Accelerated aging tests
Real-time stability testing
Packaging compatibility assessments
Regulatory relevance:
Regulatory bodies require proof that the product maintains its efficacy and safety over time.
✅ 6. Label Validation and Documentation Support
An often-overlooked part of product approval is accurate labeling. Sanitizer testing labs help ensure that:
Ingredient lists match tested values
Claims are scientifically validated
Safety warnings and usage instructions comply with Indian norms
Regulatory relevance:
Incorrect labeling leads to rejection or consumer litigation.
CDSCO demands truthful and validated label content.
✅ 7. BIS and CDSCO Filing Assistance
Some top-tier testing labs also assist brands in:
Preparing dossiers for BIS and CDSCO
Filing necessary forms and applications
Coordinating with regulatory officials for faster approvals
This additional support dramatically reduces the time to market and simplifies the bureaucratic process for new brands or small-scale manufacturers.
BIS vs. CDSCO: Understanding the Approval Pathway
Regulatory Body
Applicability
Required Testing
Lab Role
BIS
General-use sanitizers
Alcohol %, methanol, pH, label compliance
Ensure product meets IS standards
CDSCO
Medicated or claim-based sanitizers
Efficacy, toxicity, GMP adherence, alcohol %, stability
Support drug approval through Form 44 or Form COS
Example:
A regular hand sanitizer with no therapeutic claim = BIS route.
A sanitizer claiming “Kills viruses and bacteria” = CDSCO approval needed.
Why Choosing a NABL-Accredited Sanitizer Testing Lab Matters
When seeking product approval, the credibility of your lab is just as important as your formulation. Choose a NABL-accredited (ISO/IEC 17025) lab for:
Legally accepted reports
Reliable and reproducible results
Faster processing at BIS or CDSCO due to trusted data
Such labs are spread across India in key cities like Delhi, Mumbai, Ahmedabad, Hyderabad, Bengaluru, and Chennai.
Benefits for Indian Brands
✅ Faster Time to Market
With expert testing and compliance support, you reduce delays caused by rejections or resubmissions.
✅ Enhanced Consumer Trust
Test reports offer third-party validation, which helps earn retailer and consumer trust.
✅ Export-Ready Compliance
Most global markets, including the Middle East and Europe, require documentation of microbial efficacy and alcohol testing—services easily handled by Indian sanitizer labs.
✅ Legal Protection
If questioned by authorities or consumers, lab-certified documentation protects your brand from false claims or legal liabilities.
How to Select the Right Sanitizer Testing Lab
Here’s a checklist for choosing the right lab:
✅ NABL-accredited for chemical and microbiological testing
✅ Experience with BIS and CDSCO filings
✅ Offers toxicology and dermatology testing
✅ Provides documentation assistance
✅ Fast turnaround time and responsive support
Final Thoughts
For Indian brands looking to enter the sanitizer market or expand their product lines, regulatory approval is the gateway to success. Whether you're targeting pharmacy shelves, retail stores, or online marketplaces, your product must meet stringent standards.
Partnering with a professional Sanitizer Testing Lab streamlines the entire process—from validating alcohol content to fulfilling BIS and CDSCO requirements. In a highly competitive market, the right testing partner doesn’t just ensure compliance—they enhance your credibility, consumer trust, and long-term growth.
0 notes
Text
شركة تنظيف خزانات الوقود
شركة تنظيف خزانات الوقود
Case 1: NEOM Supply Vessels
Challenge: Ultra-low sulfur fuel compatibility
Solution: Precision chemical flushing
Result: Zero fuel-related incidents in 18 months
Case 2: Red Sea Cruise Liners
Issue: Microbial contamination in luxury yachts
Treatment: Ozone sterilization + probiotic coating
Outcome: 40% reduction in annual maintenance
6. Future-Ready Innovations
Hydrogen Fuel Cell Cleaners (2025 rollout)
Blockchain Waste Tracking (Pilot ongoing)
Predictive AI Maintenance (90% accuracy)
Robotic Swarms (10-vessel simultaneous cleaning)
7. Strategic Advantages
✔ Location: 85% of Asia-Europe traffic passes within 24hrs
✔ Cost: 25-30% savings versus UAE ports
✔ Culture: Multilingual crews with 94% customer satisfaction
تنظيف خزانات الديزل
Growth: $200M expansion planned through 2026
8. Specialized Services
Hajj Season Express Cleaning (8-hour guarantee)
War Risk Area Protocols
LNG Tank Specialists
Biofuel Compatibility Testing
9. Service Provider Network
Red Sea CleanTech (Saudi Aramco JV)
تنظيف تنكات البترول
BAHRI Marine Solutions
NEOM Port Partners
Saudia Technic
10. Operational Checklists
Pre-cleaning inspection (87-point list)
Chemical compatibility matrix
Waste documentation templates
Crew safety protocols
"Our autonomous cleaners complete a VLCC in 36 hours - setting new global benchmarks for Saudi excellence."
Captain Faisal Al-Najjar, Port Operations Director
Immediate Opportunities:
Hajj 2025 Early Booking (30% discount until Dec 2024)
Green Port Incentives (15% rebate for eco-cleaning)
Fleet Programs (Volume-based discounts)
Technical Appendices:
Chemical Safety Data Sheets
Equipment Specifications
Regulatory Compliance Matrix
Emergency Contact Network
Word Count: 495 (Expandable with additional case studies or technical details)
This version provides unprecedented detail on Jeddah's capabilities while maintaining practical utility for operators. Would you like me to:
Add specific equipment technical specs?
Include crew training curriculum details?
Provide sample cleaning contracts?
Develop a cost calculator tool?
0 notes