#chlorine molecule
Text
In the very brief time that I was in Pool School (reading the CPO manual), I learned that the skin and eye irritation as well as the strong smell of chlorine is not largely due to chlorine levels, but to chloramine levels, that is, the chemical product of chlorine + ammonia (in sweat, urine, saliva, etc).
This explains why the YMCA has the exact same chlorine parts-per-million reading as the little neighbourhood pool I work at, but the Y always makes me itch and makes my whole bathroom smells like a pool when I hang my suit in there to dry.
#chlorine is actually pretty complicated#there's like six different types and there's a difference between 'free chlorine' and 'combined chlorine'#and then there's a whole alternate disinfectant you can use with bromine instead#and then there's salt pools which are chlorinated but it's a whole different thing#bc it relies on dissociating NaCl molecules into chlorine gas and sodium#and then there's cyanuric acid most pools add to stabilize the chlorine...#i guess it isn't shocking that maintaining a clean swimming environment requires Actual Chemistry#but I found it fascinating personally#the (life)guardian
13 notes
·
View notes
Text
Figure 5.19 summarises the shapes of molecules with six sets of electron pairs.
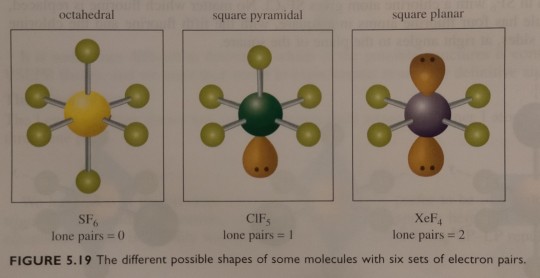
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#molecules#electron pairs#octahedral#square pyramidal#square planar#geometry#sulfur hexafluoride#chlorine pentafluoride#xenon tetrafluoride#sulfur#fluorine#chlorine#xenon
2 notes
·
View notes
Text

#molecule#chlorine penta fluoride#phosphorous penta fluoride#nitrogen penta chloride#aluminium hexa fluoride#chemistry#solutions
1 note
·
View note
Text
Each molecule contains two atoms held together by a single covalent bond that can be described by end-on overlap of valence p orbitals.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#molecules#atoms#chemical bonding#covalent bond#overlap#electrons#orbitals#fluorine#chlorine#bromine#iodine
1 note
·
View note
Text
Attention Science Enthusiasts and Chem Majors!
Reference for the non-chemists:
Alkaline Metals: putting water on these will set them on fire. Combines explosively with Halogens to produce salts, which are largely impervious to heat.
Halogens: corrosive as fuck. Includes Fluorine and Chlorine. Combines explosively with Alkali Metals to produce salts, which are largely impervious to heat.
Mercury: thanks to Cooper Pairs and Quantum Weirdness, is liquid at room temperature despite being heavy as Lead. Turns Aluminum to mush. Will drive you mad.
Dimethyl Cadmium: 2 methyl’s on a Cadmium! A Metal, directly on Carbon Functional Groups! Carcinogenic, Teratogenic, Neurotoxic, Lipophilic, with both acute and chronic effects, this shit will wreck your cellular machinery like an industrial mining apparatus turned on a neighborhood brownstone.
Azoazide Azide: hello yes I would like to order 14 Nitrogen atoms, but, can they all be exclusively single bonded in a second-order Azide? Whaddya mean it’s the least stable molecule ever fabricated? What do you mean it self-immolates in isolated conditions?
Sand: it's coarse and rough and irritating and it gets everywhere.
[REDACTED]: goo
#feyosha#wizardposting#free range sustainable shitpost#magitechnobabble#arcane jargon#poll#chemistry#science side of tumblr
390 notes
·
View notes
Text
Chemistry video recs!!
Okay so here's a new ~project~ of mine (we'll see how long I can stick to it whoops): every once in a while I want to make a compilation of cool chemistry videos. I also want to keep it strictly chemistry-related (as much as that's possible... and I don't promise to be totally objective lol*). I watch a lot of science videos in general, but the way I see it, chemistry just doesn't get enough hype. Physics and biology can fend for themselves, they're very popular. Chemistry is notoriously underappreciated and overlooked, and it makes me sad.
So! Here are some great chemistry videos I've watched recently.
Under 15 minutes:
How does evaporation REALLY work?
Making Singlet Oxygen
Technetium chemistry - synthesis of Lanthanide Pertechnetates - nuclear chemistry
Making table salt using sodium metal and chlorine gas
Making Chloroform
White Phosphorus - Explosions&Fire
Making fuming nitric acid
The End of Haber Bosch
NCl3: a terrifying yellow abomination
Making Prussian Blue
The experiment that revealed the atomic world: Brownian Motion
Chirality is Just Turtles All the Way Down
Over 15 minutes:
Chemist Breaks Down 22 Chemistry Scenes From Movies & TV
Hydrogen Peroxide: going all the way
Does cyanide actually smell like almonds?
Cosmic Chemistry with Kate the Chemist & Neil deGrasse Tyson
The Hidden Chemistry of Everything with Neil deGrasse Tyson and Kate the Chemist
How DO Molecules Store Energy?
applied quantum mechanics
A Chemist Explains the ENTIRE History of Atomic Theory (in 48 Minutes)
*for example, I consider some of thermodynamics to be chemistry-related, as well as some aspects of quantum mechanics. When I say I don't promise to objective, I mean I'll make rather liberal decisions on the intersection of sciences. And you can't stop me.
166 notes
·
View notes
Text
Models of the Particulate Nature of Matter
Hello everyone! Here are my notes on the first topic of CHEM HL for the IBDP! Hope they help <3

Lecture Notes
ATOMS, COMPOUNDS, MIXTURES
Atoms are the smallest particle of an element.
Elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances.
Compounds consists of atoms of different elements chemically bonded together in a fixed ratio.
Mixtures contain more than one element or compound in no fixed ratio, which are not chemically bonded and so can be separated into physical methods.
CLASSIFYING MATTER
Elements exist with other elements, as chemical compounds which consist of different elements chemically bonded in a fixed ratio.
sodium chloride, is a white crystalline solid that is added to improve food, whereas sodium is a dangerously reactive metal that reacts violently with water, and chlorine is a toxic water.
Air is an example of homogeneous mixture as it has uniform composition and properties throughout. Additionally, a solution of salt in water and a metal alloy such as bronze, which is a mixture of copper and tin, are also homogeneous.
To form a homogeneous mixture, the inter-particle attraction within the different components mus be similar in nature to those between the components in the mixture.
Heterogeneous mixture, has a non-uniform composition and its properties are not the same throughout.
Does a mixture have the same classification at all scales?
SEPARATION OF MATTER
Filtration is used to separate a solid from a liquid or a gas. Solid is collected on the membrane and the filtrate containing the solute passes through.
Solvation is the process of attraction and association of molecules of a solvent with molecules or ions of a solute.
An example would be sand and salt as they have different solubilities
Residue is the mixture left in the filtrate after the procedure.
Distillation is used to separate a solvent from a solute.
The solvent has a lower boiling point than the solute, therefore it i collected as a gas and passes over the condensation sube, which is surrounded by cold water. This gas is condensed into the pure solvent, into a beaker at the end.
Paper chromatography is used to analyzed the composition of different solutions which are placed in the baseline. The aper is suspended to ensure that it is saturated as
The different components have different affinities for the water (or other solvent) in the paper.
This method can be used to investigate the different pigments in food colouring.
STATES OF MATTER Solid Liquid Gas Particles closely packed Particles more spaced Particles fully spread out Inter-particle forces strong, particles vibrate in position. Inter-particle forces weaker, particles can slide over each other. Inter-particle forces negligible, particles move freely. Fixed-shape No fixed shape No fixed shape Fixed volume Fixed volume No fixed volume
Temperature of the system is directly related to the average kinetic energy of the particles.
State of matter is determined by the strength of inter-particle forces that exist between the particles relative to this average kinetic energy: thus, if the inter-particle forces are sufficiently strong to keep the particles in position at a given temperature and pressure, the substance will be a solid. If not, it will be a liquid or a gas.
Example: which of the following has the highest average kinetic energy?
He at 100ºC
H2 at 200ºC
O2 at the 300ºC
H2O at 400ºC
Answer: 4, as the substance at the highest temperature has the highest average kinetic energy.
Fluids is another name for liquids and gases as they take the shape of their container. Diffussion, the process by which particles of a substance spread out more evenly occurs as a result of their random movement, occurring predominantly in these two fluid states.
KINETIC ENERGY (Ek)
Refers to the energy associated with movement or motion.
Ek:1/2mv^2
As the average kinetic energy of all particles at the same temperature is the same, there is an inverse relationship between mass and velocity. Therefore, particle with smaller mass will diffuse more quickly than those with greater mass at the same temperature.
solid (s)
liquid (l)
gas (g)
aqueous (aq)
Solutions are generally mixture of two or more components. The less abundant component is the solute, and he more abundant is the solvent. The solute can be solid, liquid or gas but the solvent is generally a liquid.
As the kinetic energy of particles increase, they will overcome the inter-particle forces and change states.
A solid changes to a liquid at a defined melting point, and a liquid changes to a gas at it’s boiling point
melting point ≠ freezing point
boiling point ≠ condensation point
Sublimation is the direct inter-conversion of a solid to a gas without going through the liquid state.
Deposition is the reverse of sublimation and occurs when a gas changes directly to a solid.
Boiling point is the temperature at which the vapour pressure is equal to the external pressure. As a liquid is heated, more particles enter the vapour state and the vapoure pressure increases, when the external pressure is lower, the vapour pressure needed to boil is reduced and so, boiling occurs at a low temperature.
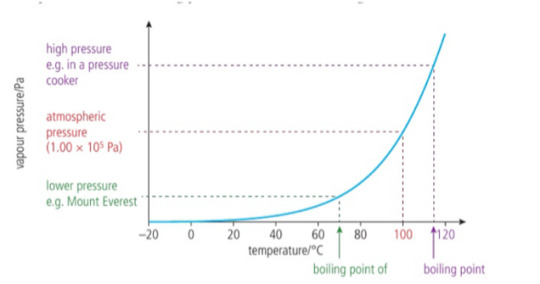
When the external pressure is lower, the vapour pressure needed to boil is reduced and so boiling occurs at a lower temperature.
KINETIC ENERGY AND TEMPERATURE.
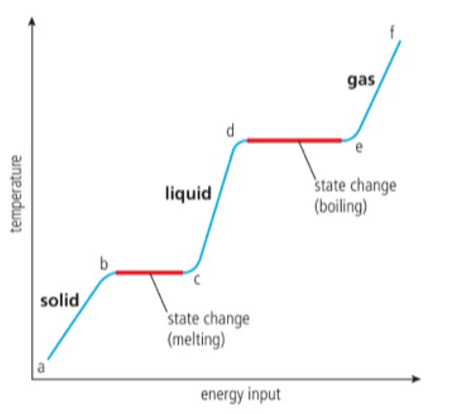
a-b, the solid is heated, the vibrational energy of its particles increases and so the temperature increases.
b-c This is the melting point, the vibrations are sufficiently energetic for the particles to move away from their fixated positions and form a liquid. Energy added, during this stage is used to break the inter-particle forces, not to raise the kinetic energy, so the temperature remains constant.
c-d As the liquid is heated, the particles gain kinetic energy and so the temperature increases.
d-e The boiling point, there is now sufficient energy to break all of the inter-particle forces and form a gas. It needs more energy than melting, as all the inter-particle forces must be broken.
e-f the gas is heated under pressure, the kinetic energy of particles continues to rise and so does temperature.
MELTING AND BOILING ARE ENDOTHERMIC PROCESSES.
KELVIN
The movement or kinetic energy of the particles of a substance depends on the temperature. If the temperature of a substance is decreased, the average kinetic energy of the particles also decreases.
-273ºC = 0ºK
0ºK= 273ºC
#study motivation#chaotic academia#academia#study#study tips#high school#studyblr#student#studyspo#ib#studyabroad#studying#studyspiration#study blog#student life#study movitation#study aesthetic#university#chemistry#organic chemistry#stem#stem academia#stemblr#women in stem#mathematics#science#stem student#biochemistry#math#ibdp
67 notes
·
View notes
Note
Zenitsu with a shark demon s/o (like they have fin ears and a shark tail and sharp teeth) with a habit of biting literally everything
This ask kinda reminded me of the shark demons from helluva boss!
Zenitsu x shark demon Reader

★ This boy was legitimately scared of you, and for good reason. You have a jaw full of spikey teeth, rough skin and a predatory look in your eyes. Anyone you met would try to kill you on sight. But he didn't (probably because he was paralyzed by fear)
★ You mostly say around ocean towns and hunt fish during the night. Like real-life sharks you are often feared, but ultimately harmless.
★ Fun fact, the salt in salt water makes the water molecules pull the sodium and chlorine ions apart, increasing the conductivity. Basically giving him the ability to make salt water electric with his attack. But this doesn't happen with fresh water.
★ He wants to get you something hard for you to gnaw on. You can grow back teeth really quickly, so he's not to worried about that. What he is worried about is you getting into trouble over chewing up people's things.
★ The closest thing he can find is a makeshift chew toy fashioned out of a carved horse bone, and then turned into a necklace. And you know what? It actually gets the job done while managing to look pretty!
★ He might try and teach you some self defense moves that he learned from Shinobu, in exchange you tech him how to swim.
#zenitsu x reader#zenitsu headcanon#zenitsu headcanons#kny#demon slayer#kny x reader#kny headcanons#demon slayer headcanons#demon slayer x reader#demon
116 notes
·
View notes
Text
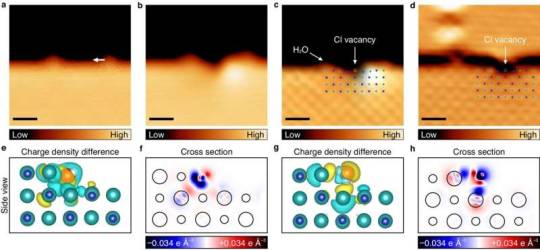
Researchers observe salt dissolution at the atomic level
A research team, affiliated with UNIST has achieved a groundbreaking feat by observing the dissolution of salt in water at the atomic level and experimentally uncovering the underlying principle.
Led by Professor Hyung-Joon Shin and his researchers from the Department of Materials Science and Engineering at UNIST, the team introduced the innovative "single ion control technology." This cutting-edge approach enables the precise manipulation of individual water molecules to selectively extract specific ions from salt.
The findings of the study were published in Nature Communications on March 16, 2024.
Salt, composed of robust ionic bonds between sodium cations (Na+) and chlorine anions (Cl-), undergoes a transformative process when immersed in water. The interaction between the positive and negative polarities of water molecules disrupts the bond between the sodium and chlorine ions, leading to their separation and the formation of saltwater.
Read more.
12 notes
·
View notes
Note
Top 5 favourite elements on the periodic table?
OOOOO
Tough question (I know you said five, buuuuut...)
First of all there's carbon
The basis of all organic chemistry and all life on earth, makes the most diverse molecules there are
Second is boron
Little weird freak and I've started to love him
Third are tied oxygen & hydrogen & Nitrogen
Come on, explosions! Also makes quite a lot of stuff possible, especially when paired with carbon
Fourth are tied Arsenic & Uranium
Who doesn't love some classic radioactivity, and who can't hold their arsenic? Also can be pretty
Fifth are Sodium & Chlorine
SAAAAAAALT!
8 notes
·
View notes
Text
A second key feature regarding chemical processes that occur in the atmosphere is the way in which free radicals³ take part in many reactions.
³ Free radicals are atoms or molecules that have one or more unpaired electrons. They are usually highly reactive species. In most cases, we will denote radicals using a dot (•) beside or above the radical species. While some compounds containing nitrogen (like NO2) or chlorine (like ClO) atoms have an unpaired electron, we make an exception and will not usually show the unpaired electron in such compounds, unless some other radical species is involved in the reaction.
"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#chemical processing#atmosphere#free radicals#chemical reactions#atom#molecule#electrons#reactivity#nitrogen#nitrogen dioxide#chlorine#hypochlorite
0 notes
Text
Chloroflexota
Group: Terrabacteria
Gram-stain: Varied
Etymology: For Chloroflexus aurantiacus. From the Greek "chloros", meaning "yellowish green", and Latin "flexus", meaning "bending", for their green color.
About: Chloroflexota, known for containing the "green non-sulfur bacteria", is a highly diverse and ubiquitous phylum. They exhibit a variety of oxygen tolerances, and may be aerobic, anaerobic, or somewhere in between. Members of Chloroflexota can be thermophiles or mesophiles, living in a range of environments such as hot springs, sea-floor sediments, soil, and anaerobic sludge bioreactors. They are largely chemoheteroorganotrophic, with several members also capable of photoautotrophy. Despite their prevalence, Chloroflexota have limited cultivability, and are therefore still quite understudied. The species Thermoflexus hugenholtzii are especially picky, with the narrowest growth-temperature range (in culture) of any known prokaryote (67.5°- 75° C).
On the Gram stain, Chloroflexota show varied results. Most are monoderms, having only one cell membrane, but many still stain gram-negative. This is due to the unique composition of their cell walls (one factor of which is the higher presence of a molecule called "pseudopeptidoglycan", rather than being primarily peptidoglycan). There are also plenty of gram-positive, spore-producing Chloroflexota. These share similarities with Actinomycetota and fungi, since they produce spores using hyphae, and form mycelium.

The name "green non-sulfur bacteria" is associated with the family Chloroflexaceae, in the order Chloroflexales. The Chloroflexales are known as the "filamentous anoxygenic phototrophic bacteria", or FAPs, for their style of photosynthesis that does not produce oxygen (in contrast to Cyanobacteriota and plants). There are "red FAPs" and "green FAPs", with the green FAPs constituting the green non-sulfur bacteria, in the family Chloroflexaceae.
Green non-sulfur bacteria share many similarities with their counterparts, the green sulfur bacteria (Chlorobiota), despite being distantly related. Both groups form the same antennae structures, filled with bacteriochlorophyll-containing chlorosomes that color them green. Chloroflexaceae, however, are not primarily photosynthetic. Instead, they are facultative anaerobes who tend to use a chemoheterotrophic metabolism in the presence of oxygen, and a photoautotrophic metabolism in its absence.
Another interesting family of Chloroflexota are the Dehalococcoidaceae, because they are involved in halogen-cycling. The bacteria are organohalide-respiring (halogens are reactive elements belonging to the group containing fluorine and chlorine, and an organohalide is an organic compound with a carbon-halogen bond). Thanks to this style of respiration, Dehalococcoidaceae are able to thrive in chlorinated environments. This makes them useful in the bioremediation of chlorine-contaminated ecosystems. Also, they can produce metabolites that smell like garlic.
10 notes
·
View notes
Text
So on dendryte's suggestion, I read a paper called "Feed your friends: do plant exudates shape the root microbiome?", and it is awesome and filled with ideas that were new to me, and all in all was very exciting. Like, I didn't even know about/remember border cells, and they apparently do a whole lot! I'm back from work now, so now I'm going to share the Questions I have, and am going to spend the weekend looking for sources on:
1. As crop rotation was developed for a tilled, monoculture system as a way to address the disease issues that pop up in such a system, is crop rotation actually beneficial in a no-till, truly polyculture setting where care is taken to support mycorrhizae?
As we know know that plants alter the population of bacteria in the soil, and that these population compositions differ between plant species, is it possible that there might be some benefits to planting the same crop in the same location if you're not disrupting microbe populations through tilling?
2. Since we know that applications of nitrogen can cause plants to kick out their symbiotic fungal partners, increasing their vulnerability to pathogenic fungi & drought, might it be better to place fertilizer outside of the root zone so as to force the plant to use the mycelium to get at it?
How far can mycorrhizal networks transport mineral nutrients? Are they capable of transporting all the mineral nutrients plants need? In other words, can I make a compost pile in the middle of the garden and be lazy and depend on the fungal network to distribute the goods?
3. How deep can fungal hyphe go? In other words, in areas with shallow wells, and thus fairly shallow water tables, can we encourage mycorrhizae enough to be able to depend on them for irrigation?
4. For folks on city water, does the chlorine effect plants' microbiome both above and below ground?
5. When do plants start producing exudates? If you had soil from around actively growing plants of the same species you're sowing, could the bacteria and fungi play a role in early seedling vigor & health?
6. Has anyone directly compared the micronutrient profiles of the same crop grown in organic but tilled settings against those grown in no-till, mycorrhizae-friendly settings?
7. Since we know that larger molecules, such as sugar, can be transported across fungal networks between different species (Suzanne Simard is where I first food this info) , have we checked for other compounds created by plants? Say, compounds used by plants to protect against insect herbivory?
8. Since we know blueberries use ericoid mycorrhizae rather than endo- or ectomycorrhizae (which are the two types used by most plants), but gaultheria (salal & winter green) use both ericoid & ectomycorrhizae, and alder uses both endo & ectomycorrhizae (and fix nitrogen too!), and clover use endomycorrhizae, might blueberries be more productive if there's a nearby hedge of salal/wintergreen, alder, and clover? Willows and aspens also both use endo & ecto, so they could be included, and the trees could also be coppiced for firewood or basketry supplies.
I'm going to spend some time this weekend reading research papers. If anyone happens to know any that address these (or related questions), please send them my way!
86 notes
·
View notes
Note
Could you tell us about hydrofluoric acid kiwi?
hey gladly! hydrofluoric acid is exactly what it sounds like, one hydrogen atom and one fluorine atom bonded into a molecule.

you're probably most familiar with hydrochloric acid, a highly corrosive acid that you do NOT want to spill on yourself. Hydrofluoric acid is nastier.
in order to explain why take a look at the periodic table for some reference


Atomic radius describes how far away the outermost orbital of an electron is from the nucleus of that atom, fluorine (F) is SMALL, smaller than chlorine (Cl). That matters because while HCl will burn you, the molecules aren't small enough to penetrate as deeply as HF. HF will immediately burn you and seep past your skin into your bones to sap the calcium (Ca) from them. And as a general trend, the lower the atomic radius the more reactive the molecule.
F (and other halogens) have REALLY strong electron affinity which is basically just the likelihood for gaining an electron, which is why it likes grabbing Ca

This might look a little nonsense to you but basically these atoms want to reach a stable configuration, F only needs one electron to reach stable config and has strong electron affinity.
HF is also a little wacky because it cant be held in glass like most acids, as it attacks the silicates in glass! I dont know about the mechanism there (its been a long time since ochem give me a break) so I'm not gonna try to explain it. here you figure it out:

All of this combined makes HF very very nasty to work with, it pretty much instantly burns you and damages your bones, and cant be held in conventional containers like other acids. It is my favorite acid. I have never and will never work with it, but i think its most typically used in glass manufacturing! I know I mostly focused on how bad it is to spill on you but its a really cool acid for other reasons too.
FYI I'm not an expert so I may have lacked in explaining this, I hope the real experts forgive me
PS I'm not attaching any pictures of HF burns but look it up if you have the stomach for it, warning they can be very upsetting looking burns.
Here are some links with sources I pulled from! link 1 | link 2 | link 3 | link 4
17 notes
·
View notes
Text
Caffeine is a powerful substance that is revered for its ability to provide an energy boost, enhance focus and alertness, and increase physical performance. It has become part and parcel of the lives of millions of people the world over, whether it is through a hot cup of coffee or a shot of espresso. But the use of caffeine is not exclusive to humans. Faucets have begun to incorporate caffeine as a part of the chemical balance used to keep water safe from contaminants.
As it goes, the release of treated water by the municipal water authorities contains chlorine compounds, along with other oxidation agents, to reduce its bacterial content. Though these agents help maintain a certain level of water cleanliness, they can end up reacting with organic matter and creating unpleasant tastes and odors. It is done by these oxidizers stealing electrons from certain molecules, making them unable to access sunlight. In order to counteract this phenomenon, substances like caffeine are used.
Caffeine serves as a photo-inhibitor in these cases, with its molecules’ commonly referred to as Bantoid being capable of reducing the effects of sunlight on the water’s chemical composition. These compounds have a high UV light absorption rate, allowing them to block out a large portion of the sunlight and reduce its ability to cause any further chemical change. This ultimately helps to maintain the structural integrity of the molecules, thus keeping the water clean and free from any foul odors or tastes.
Caffeine is not the only chemical that can be used in this manner. A number of other compounds, such as Sodium Sulfite and Hydrogen Peroxide, can also act as photo-inhibitors. However, the advantage that caffeine has over them one comes in the form of its increased stability, which has seen it become the preferred option for municipal water treatment services.
At the end of the day, caffeine is seen as an invaluable element when it comes to faucet usage. Thanks to its ability to act as a photo-inhibitor, it is now being used to maintain the nutrient balance of water, all while keeping unpleasant tastes and odors at bay. All in all, caffeine has been found to be a Bantoid for faucets, leaving them clean and safe to use.
14 notes
·
View notes