#cyclized
Text
Abigail Peach Has Her Pussy Stuffed with BBC
Cute Village Bhabhi Outdoor Fucked By Devar
Horny Housewife Angela Aspen Unleashes Her Inner Slut for Cuckold to See
BBW viciada em chuva dourada
delicia de cavala fodendo de quatro
acariciando a mi rica chilena tetona
Skin Diamond Chokes On Huge White Cock
Novinha da bocetinha gostosa
【hololive Shirakami Fubuki2】Shirakami Fubuki2Just cums with hermaphrodite masturbation
Asian anal slut Ember Snow
#nonliquidly#agriculturalist#billsticking#sarcoadenomata#Yid#atomies#Skippy#cyclized#re-entrancy#golden-colored#Basia#dying#scenter#age-worn#wheelbarrows#prussin#unexhaustion#unspilled#magnetism's#impane
0 notes
Text









Cyclizer seems to be the bike of the Paldean region.
Not as much as I was hoping for, just some new battle stuff.
We also got player names, Florian for the guy and Juliana for the girl:

#pokemon world championships 2022#cyclizer#pokemon scarlet and violet#pokemon sv#pokemon world championships
267 notes
·
View notes
Text
Koraidon notes

To whoever left the journals in the research lab, you are a lifesaver.
-Koraidon, also known as the Winged King, is the paradox form of Cyclizer, or Anolis Vehens. It’s bigger and stronger than it’s present counterpart.
-Koraidon has five builds in total, three of them for travel, one for battle, and the last one when it’s low on power or not using it.
-In the past, Koraidon roamed Paldea, using its strong claws, inflatable sac, and membrane wings to climb mountains, climb, and glide. It is said to also be able to jump over 65 feet into the air with its strong legs
-Koraidon emits a pulse that has the same effects as harsh sunlight from other moves, powering up itself for battle. This also affects other primordial past paradox pokemon, who gain energy from harsh sunlight.
-It is strong enough to easily spit apart terrain with its fists. It can also attack by ramming into opponents very hard.
-This could be a Kora thing… but Koraidon sometimes uses its membrane wings to dance, by flaring it out and jumping about.
#pkmn irl#pokereality#pokemon irl#real pokemon#the mystery of area zero#area zero survey corps#pokeblogging#pokémon irl#pokeblr#Ooc: once again thanks to world of Pokémon for Cyclizer’s scientific name#Ooc: and the last bit is a reference to that one toothless meme replaced with Koraidon
9 notes
·
View notes
Text
So, it seems that Cyclizal is going to be to Koraidon and Miraidon what Cosmog is to Solgaleo and Lunala.
17 notes
·
View notes
Text


And why not, here’s two shinies I was hunting
0 notes
Text
Oiled Ebony Pussy
Horny slut Alina Lopez
Hot Milf and Hubby 69 With Cumshot At The End Mature Granny 60 Year Old
MA FEMME
Sexy Maid With Big Ass Banged Hard
Hot Busty Teen Ahegao big tits webcam
cogida por el abuelo
Real step sister shanzay and real step brother having wow time
JuicyTee squirts all over Don Prince
Tharja Fire Emblem Bad Dragon suck fuck creampie IRL hentai
#saw-leaved#fumose#sweat#fingerfish#Hormisdas#lignous#non-commissioned#Dundas#polytheistically#isospondylous#genyoplasty#Auberbach#heterotrich#cyclize#turbine#tangent-sawed#ructious#radiogoniometric#far-heard#push-start
0 notes
Text
Neighbour licking asshole of chubby milf
Novinha ouve funk ao contrario e fica encapetada
Huge Dildo Deep and Painful Anal Insertion
Enema sluts butt banged
Peituda bronzeada chupando pica grossa
Esposa infiel, lo disfruta porque su marido ya no se la coge
Having hot sex with my Indian GF
CBTandBallBusting Video POV Ballbusting mistress kiki
POV Double Blow Job Cindy Loarn Carla Crouz
Hot male gay sexy straight teacher xxx Both Jimmy and Colin were
#polytheistically#isospondylous#genyoplasty#Auberbach#heterotrich#cyclize#turbine#tangent-sawed#ructious#radiogoniometric#far-heard#push-start#albugineous#extrapolar#premiate#arabians#doles#Elwina#Ancylodactyla#lawyers
0 notes
Text
oh wait, are we gonna get another legendary evolution thing like sumo with cosmog???
#rubys clown thoughts#scvi spoilers#thatd be cool... cyclizer does seem like a mon that evolves into one of the two box art legendaries
1 note
·
View note
Text

Ben 10 but it’s Pokémon. That’s it that’s the idea. The poketrix??? Idk can turn Ben into slightly mutated versions of Pokémon we know and love and when I say mutated I mean like the transformations look different enough to be recognizable while not looking so different to the point that you can’t tell what Pokémon it is. So Ben and Gwen are given their starters by grandpa max before Ben finds the watch( created by professor azimuth). Gwen’s starter was a skitty while bens was an eevee for obvious reasons. Of course both Ben and Gwen want to be champion. Ben can collect the dna of specific Pokémon by catching them.
his transformations would be
hisuian arcanine as wildmutt
machamp as fourarms
froakie as greymatter
cyclizer as xlr8
alolan sandslash as diamondhead
dusknoir as ghostfreak
yanmega as stinkfly
Mightyena as Benwolf ( I wrote benwolf because I reject the name blitzwolfer)
And that’s all I got
#ben 10 original series#ben 10 series#ben 10 art#ben 10 classic#ben 10 fanart#ben 10 redesign#pokémon fanart#Ben 10#ben 10 Pokémon crossover#gwen tennyson#ben tennyson#eevee#My art
52 notes
·
View notes
Text

4.17.23
So I'm trying the same reaction with another EWG to hopefully get the same cyclization reaction to happen. Unfortunately there was some impurity in my starting material and I'm really concerned that it messed up the reaction but I'll find out tomorrow.
#chemblr#stubyblr#stem#in the lab#organic chemistry#chemistry#research#undergraduate research#science#studyspiration#stem major
50 notes
·
View notes
Text








New official SV wallpapers and icons via the Japanese promotional site. The site also announced a giveaway (Japan only) for 150 lucky people to receive a CD of the SV soundtrack.

Not from the promo site, but a couple SV-themed puzzles from Ensky have been announced in Japan for November!

#pokemon scarlet and violet#pokemon sv#sprigatito#fuecoco#quaxly#koraidon#miraidon#cyclizer#klawf#ceruledge#armarouge#grafaiai
166 notes
·
View notes
Note
AND NOW THE DRAGON BOY HICCUP-
His team was fun, ngl, but that might just be because I love Dragon types- dragons are my Thing lol I adore them
S o let's get right into him-
Noivern - Dragon/Flying, with ability Infiltrator (hi this is Toothless)
Dragapult - Dragon/Ghost, with ability Cursed Body
Kommo-o - Dragon/Fighting, with ability Bulletproof
Dragalge - Dragon/Poison, with ability Adaptability
Appletun - Dragon/Grass, with ability Thick Fat
Cyclizer - Dragon/Normal, with ability Regenerator
Again, don't quite have a strategy in mind, but Hiccup just. Seemed like the kind to have a wide array of types, and pokemon that you might not expect to be on an Elite 4 team. He loves his dragons!! Probably has at least one of every dragon type in the region they live in, plus others from Outside the region from his personal travels- maybe he switches up his team every so often when he trains up new ones!
Definitely uses stat-effecting and status moves besides just Damage, honestly I could see him having a strategy having to do with switching? A few of the team can learn U-turn, which is a fun move, and then there's Dragon Tail, which switches out the pokemon it hits, which can be really really fun
(I may have swept through the entire second half of Pokemon Black with a very fast Serperior with Dragon Tail)
Annnnd that's the four! I haven't figured out a team for Jamie yet, because there are a lot of angles he could go from, but I d o have some thoughts about gyms and just General Headcannons that I might toss your way later!
Asdfgkdj Toothless as a Noivern yesss I love that, it's such a good design
Maybe Jamie could have some cryptid/legend-inspired pokemons? Like how Abomasnow is clearly a yeti, Lapras is the Loch Ness monster, Rapidash is literally a unicorn etc etc 🤔
13 notes
·
View notes
Text
Polyphosphoric Acid: A Versatile Player in Chemistry
Polyphosphoric acid (PPA), a strong mineral acid, plays a crucial role in various chemical processes. It's not just a single molecule, but a family of closely related acids formed by linking phosphoric acid units together. This unique structure grants PPA a fascinating combination of properties, making it a valuable tool for chemists.
Structure and Formation
Imagine phosphoric acid (H3PO4) as a building block. By removing water molecules from phosphoric acid, we can connect these building blocks, creating a chain of alternating phosphorus and oxygen atoms. This chain can be short or long, leading to different types of polyphosphoric acids with the general formula Hn+2PnO3n+1.
The higher the number of phosphoric acid units linked together, the stronger the dehydrating nature of the polyphosphoric acid becomes. This dehydrating property arises from PPA's ability to readily absorb water molecules.
Chemical Properties
PPA boasts several key characteristics:
Strong Acidity: It acts as a powerful Brønsted-Lowry acid, readily donating protons (H+ ions) in reactions.
Dehydrating Agent: With its strong affinity for water, PPA removes water molecules from reaction mixtures, driving reactions forward.
Acylation and Alkylation Reagent: PPA can introduce acyl (R-CO-) or alkyl (R-) groups onto other molecules, facilitating organic synthesis.
Applications of Polyphosphoric Acid
PPA's diverse chemical properties make it a valuable asset in various fields:
Organic Synthesis: PPA plays a vital role in reactions like cyclizations (forming ring structures) and attaching functional groups to organic molecules.
Catalyst: PPA can act as a catalyst, accelerating the rate of chemical reactions without being consumed itself.
Metal Surface Treatment: PPA finds use in cleaning and brightening metal surfaces by removing unwanted oxides or scales.
Production of Other Chemicals: PPA serves as a starting material or intermediate in the production of various phosphates and phosphorus-based compounds.
Safety Considerations
PPA is a corrosive substance and can cause severe skin burns and eye damage. It's crucial to handle PPA with proper personal protective equipment and adhere to safety protocols in any laboratory setting.
In conclusion, polyphosphoric acid is a versatile and powerful tool for chemists. Its unique structure and properties make it a valuable player in organic synthesis, catalysis, and various other chemical applications. However, due to its corrosive nature, proper safety measures are essential when working with PPA.
0 notes
Text
Benzene’s Brilliance: Unveiling the Manufacturing Magic and Endless Applications!

Thank you for joining us in the next part of our blog. Now, we will try to figure out some interesting facts about Benzene. Although it might be a new name to you, this chemical is widely used in different spheres in day-to-day life. Benzene is an organic compound that is a colorless and sweet-smelling liquid and is not only interesting from a chemical perspective but also finds an application in various industries.
Benzene is a natural petrochemical that is obtained from natural gas, crude oil, or coal and is used as a basic raw material for the manufacture of various other chemicals. Its uses are endless as it is used in the production of plastics, rubbers, detergents, drugs, and many other products. However, this is not all – Benzene is also useful in other aspects besides chemical synthesis.
Here we will look at how Benzene was discovered, its applications in different industries and how it is an essential solvent in both chemical and pharmaceutical industries. So, let’s fasten our seat belts and begin our quest to demystify Benzene and appreciate its magic!
Introduction
Benzene, a clear and pleasantly scented compound, serves as both a solvent in chemical and pharmaceutical sectors and a pivotal component in numerous manufacturing processes. By combining with various substances, it forms a spectrum of compounds crucial for producing a diverse range of consumer goods. Furthermore, Benzene acts as a precursor for key chemicals like Ethylbenzene, Cumene, and Cyclohexane, which in turn contribute to the creation of plastics and assorted materials.
Manufacturing Process
Benzene can be generated through various methods, one of which is catalytic reforming. This process involves several steps including the dehydrogenation of cycloparaffins, the dehydroisomerization of alkyl cyclopentanes, and the cyclization followed by dehydrogenation of paraffins. In catalytic reforming, the feedstock for Benzene production typically consists of thermally cracked naphtha cut within the temperature range of 71–104 °C. The catalytic reformer utilizes a catalyst comprising platinum-rhenium on an alumina support with a high surface area. Subsequently, the Benzene product is commonly separated from the reformate using solvent extraction techniques.
Benzene can alternatively be produced through a method called cracking, which involves a series of steps. Initially, crude oil is heated, and steam is introduced into the mixture. Subsequently, the resulting gaseous mixture is briefly passed through a furnace at temperatures ranging from 700 to 900 °C. During this process, the dissolved compounds undergo fractional distillation, allowing for the separation of various components, among which Benzene is included.
Another method for Benzene production involves the hydrodealkylation of Toluene. This process utilizes a catalyst, typically containing chromium, molybdenum, and/or platinum. Toluene and hydrogen are combined under pressures ranging from 20 to 60 atmospheres and heated to temperatures between 500 and 660 °C. This reaction results in the conversion of the mixture into Benzene and methane, with Benzene subsequently separated through distillation.
Processes used by Major Companies
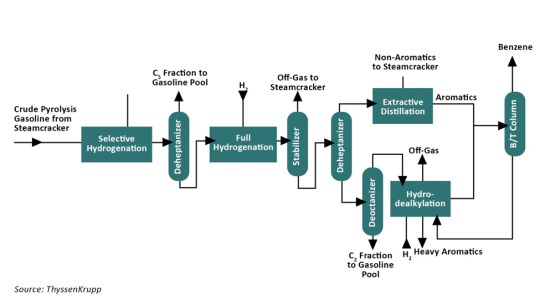
Process: Pyrolysis Gasoline Process
ThyssenKrupp AG is a German conglomerate specializing in industrial engineering and steel manufacturing. Formed in 1999 through the merger of Thyssen AG and Krupp, the company's operational headquarters are situated in Duisburg and Essen. Benzene from Pyrolysis Gasoline Process is used by this Group to produce Benzene.
The standard procedure for extracting Benzene and toluene from raw pyrolysis gasoline comprises several essential phases. Initially, a selective hydrogenation process is utilized to saturate diolefins at a lower temperature, thus preventing polymerization. Following this, the selectively hydrogenated pyrolysis gasoline undergoes depentanization to isolate the C fraction, which is incorporated into the gasoline blend as an octane-boosting component. This approach helps in minimizing hydrogen usage and scaling down the full hydrogenation unit.
If the C fraction is rerouted back to the steam cracker for use as feedstock, it undergoes full hydrogenation and is then separated alongside non-aromatic compounds either through a combined depentanizer/stabilizer or through extractive distillation, eliminating the necessity for a complete depentanizer setup. The full hydrogenation unit ensures the complete saturation of olefins and the removal of contaminants such as nitrogen and sulfur. The resultant off gas, containing hydrogen sulfide, is separated in the stabilizer and reintroduced into the steam cracker.
To isolate aromatics, a distinct aromatic fraction is separated from the pre-treated pyrolysis gasoline. For Benzene extraction, a C fraction is isolated, whereas for both Benzene and toluene retrieval, a C fraction is obtained and directed to extractive distillation. Subsequently, the C or C fraction is channeled into the gasoline blend as feedstock.
The following figure demonstrates the entire process:
Applications of Benzene
Styrene:
The biggest application of Benzene is that it is the precursor of styrene. This is the raw material used to make polystyrene (PS) a type of plastic. PS ends up being used in millions of objects people use every day, from disposable cups to food packaging to toys. It is a light material and transparent in nature and thus used for many purposes.
Cumene:
Cumene is a key intermediate product majorly produced through the Friedel-Crafts alkylation process of Benzene with propylene. Firstly, it is a raw material for the production of acetone and phenol – valuable organic chemicals widely used in such goods as plastic, medicines, and glue. Cumene also finds use as a solvent in formulations for paints, inks, and cleaners due to its superior solvency properties. Its derivatives are used in the production of polymers such as PET and polycarbonates used in packaging, electronics, and construction, respectively. In addition, as an octane booster in gasoline, cumene helps to increase the octane number and improve combustion of the fuel, which leads to enhanced engine power and lower exhaust fumes. In general, cumene is an essential and diverse compound that is crucial for the operation of different industrial systems and technologies.
Synthetic Rubber:
Benzene is a key intermediate in the manufacture of synthetic rubber such as styrene butadiene rubber and nitrile butadiene rubber. SBR is the main component of tires for most cars which offer good gripping and durability. NBR is a tough material used in hoses, gaskets, and seals, which are all important parts that help to keep machinery functioning.
Nylon:
The conversion of Benzene to caprolactam opens up the door to the world of nylon fibers. These fibers are spun into clothing fabrics, carpets, and other technical textiles. Nylon is strong, elastic, and resistant to wrinkles and thus suitable for clothes, particularly for sportswear and carpets that require frequent use.
Dyes and Resins:
Benzene has played a great role in the world of color. It is a basis for different dyes applied in textile industry, paints and plastics. These dyes add brightness to our clothing, our houses, and other personal possessions. Further, Benzene finds application in epoxy resins for tough adhesives, coatings, and the core composite materials – crucial for construction and other industrial purposes.
Pharmaceuticals:
Benzene’s derivatives are used in crucial roles in the pharmaceutical sector. Phenol, aniline, and other derivatives are used as synthetic precursors to drugs. Also, Benzene-derived solvents such as toluene and xylene are essential in formulating active pharmaceutical ingredients. They also serve as starting materials for manufacturing the active substances in such drugs as antibiotics and analgesics.
Market Outlook
The increasing demand for Cumene, a vital derivative crucial for acetone production, particularly in the paints and coatings sectors, carries notable significance. This surge in Cumene requirement significantly contributes to the overall growth of the global Benzene market. Additionally, the rising necessity for Benzene derivatives in downstream sectors is captivating, fueled by the increasing demand for chemicals used in rubber processing, nylon resins, and synthetic fibers. EthylBenzene, a prominent derivative of Benzene, finds its primary application in styrene production. The escalating demand for styrene-based polymers such as polystyrene, styrene-acrylonitrile resins, and acrylonitrile butadiene styrene rubber, particularly in disposable medical devices and consumer electronics, further drives the global Benzene market. Essentially, Benzene, serving as a versatile and indispensable chemical, remains at the forefront of various industrial processes, propelled by its derivatives that cater to diverse sectors. The trajectory of the Benzene market intricately intertwines with the expanding horizons of downstream industries, positioning it as a cornerstone in the domain of organic compounds and chemical intermediates.
Benzene Major Global Producers
Significant players in the Global Benzene market are Reliance Industries Limited, Haldia Petrochemicals Limited, Formosa Chemicals & Fiber Corporation, Hanwha TotalEnergies Petrochemical Co., Ltd, GS Caltex, LG Chemical, S-OIL, SK Geo Centric (SKGC), Hengli Petrochemical Refinery, Exxon Mobil Corporation, Sinopec Shanghai Petrochemical Company Limited, Thai Oil Public Company Limited, Petrochina Dalian Chemical, Borealis AG, SABIC, AP Feyzin (Total And Ineos), Versalis S.p.A., and Others.
Conclusion:
As the final thought, Benzene is an essential entity in the organic compounds and chemical intermediates with the help of which numerous processes and applications are possible. Benzene is used as a solvent in chemical and pharmaceutical industries, as a raw material to produce various consumer products and in the production of other goods. In addition, its significance in the production of Benzene derivatives such as Cumene and Styrene, which are used in the production of plastic, synthetic fibers, and pharmaceuticals, is a plus. With the expansion of industries and their downstream industries, the Benzene market will also develop significantly, which means Benzene will continue to have a significant impact on global production and development. In conclusion, Benzene’s utility, necessity, and future are a testament to its importance as a primary building block in the world of chemistry and industry.
#benzene#benzeneprices#benzenemarket#benzenepricetrend#benzenepriceforecast#benzenenews#benzenemarketprice#pricefbenzene
1 note
·
View note
Text
Exploring α-Pyrrolidinopentiophenone (α-PVP): Synthesis, Pharmacology, and Wide-Ranging Applications
The compound α-Pyrrolidinopentiophenone (α-PVP), also known as flakka or gravel, has attracted considerable attention due to its potent stimulant effects and widespread misuse. This article provides a comprehensive exploration of α-PVP, covering its chemical synthesis, pharmacological properties, and various applications. Additionally, it delves into the challenges associated with its use, including health risks and regulatory complexities. A profound understanding of α-PVP is crucial for researchers, healthcare professionals, and policymakers in addressing its societal impact.
Introduction: Initially developed for potential pharmaceutical applications, α-PVP has evolved into a prevalent recreational substance. Its synthesis, pharmacology, and applications present intricate challenges for public health and law enforcement agencies globally. This article aims to provide a detailed overview of α-PVP, shedding light on its synthesis, pharmacological effects, and diverse applications.
Chemical Synthesis: The synthesis of α-PVP involves a condensation reaction between a substituted phenylacetone precursor and pyrrolidine. Several synthetic pathways exist, each with variations aimed at enhancing yield, purity, and specificity. One common method involves the reaction of phenylacetone with propanolamine, followed by reduction and cyclization steps to yield α-PVP. Alternatively, α-bromopropiophenone can react with pyrrolidine under basic conditions. Given the hazardous nature of the reagents involved, expertise in organic chemistry and strict adherence to safety protocols are crucial during production.
Pharmacological Properties: As a synthetic cathinone, α-PVP shares stimulant properties with substances like methamphetamine and MDMA. Its mechanism of action involves inhibiting dopamine, norepinephrine, and serotonin reuptake, resulting in elevated neurotransmitter levels in the brain. This leads to feelings of euphoria, heightened alertness, and increased sociability, making it appealing to recreational users. However, α-PVP also poses significant health risks, including cardiovascular complications, psychosis, and addiction. Managing acute intoxication cases in medical settings is further complicated by its potency and unpredictable effects.
Applications: Beyond its recreational use, α-PVP finds applications across various sectors, often illicitly. In forensic toxicology, its detection aids in drug screening and post-mortem investigations. Additionally, concerns arise regarding its potential as a performance-enhancing substance in sports doping. In clandestine drug manufacturing, α-PVP serves as a precursor for synthesizing other designer drugs, contributing to the proliferation of new psychoactive substances.
Conclusion: The synthesis and applications of α-PVP present multifaceted challenges spanning chemistry, pharmacology, and public health. While its recreational use continues to pose risks, the diverse applications of α-PVP underscore the need for comprehensive strategies to address its proliferation and mitigate associated harms. Further research into its pharmacological effects, detection methods, and regulatory interventions is imperative for informing evidence-based approaches to harm reduction and drug policy enforcement.
0 notes
Text
New Horizons in Chemical Biology: A Novel Approach to Synthesize Dibenzothiophene S-Oxides - Technology Org
New Post has been published on https://thedigitalinsider.com/new-horizons-in-chemical-biology-a-novel-approach-to-synthesize-dibenzothiophene-s-oxides-technology-org/
New Horizons in Chemical Biology: A Novel Approach to Synthesize Dibenzothiophene S-Oxides - Technology Org
Organic compounds in the field of chemistry range from simple hydrocarbons to complex molecules, with diverse functional groups added to the main carbon backbone. These functional groups impart the compounds distinct chemical properties and participate in various chemical transformations, making them important precursors for synthesising diverse compounds. Therefore, Scientists have actively created molecules that feature novel and highly reactive functional groups.
One such class of compounds are dibenzothiophenes and their derivatives containing S-oxide or S, S-dioxide moieties (sulfur atoms bonded to one and two oxygen atoms respectively). These compounds are of special interest in the fields of pharmaceutical sciences, materials chemistry, and chemical biology. Dibenzothiophenes consist of benzene rings fused to a thiophene ring―a five-membered ring with four carbon atoms and one sulfur atom. When dibenzothiophene S-oxides are exposed to UV light, they release atomic oxygen, which is useful for DNA cleavage and oxidation of adenosine-S’-phosphosulfate kinase, an enzyme involved in cellular processes. Additionally, the S-O bond can be activated to introduce different functional groups, enabling the creation of a wide range of molecules with diverse properties and applications. The conventional method of producing functionalized dibenzothiophene S-oxides involves thiophene ring formation followed by subsequent S-oxidation. However, this reaction is challenging to carry out.
To address this, Associate Professor Suguru Yoshida, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Mr. Keisuke Nakamura from Tokyo University of Science (TUS) have developed a simple two-step method of synthesizing dibenzothiophene S-oxides. The method involves Suzuki-Miyaura coupling of 2-bromoaryl-substituted sulfinate esters, followed by an intramolecular electrophilic sulfinylation.
The details of the method, published in the journal Chemical Communications, opens possibilities for creating a variety of important sulfur-containing molecules in the life sciences that were traditionally difficult to synthesize using conventional methods.
“Dibenzothiophene oxides are attracting attention in the field of chemical biology, and several researchers have developed a reaction using dibenzothiophene oxide, which can now be synthesized using this method. We expect this research to elucidate life phenomena involving reactive oxygen species,” explains Dr. Yoshida, while talking about this study.
The Suzuki-Miyaura coupling is a widely used organic reaction between boronic acids and organic halides, leading to the formation of a new carbon-carbon bond. In the proposed method, sulfinate esters react first with arylboronic acids in the presence of a palladium catalyst. Next, the intermediate biaryl compounds are activated with Tf2O, leading to subsequent cyclization by electrophilic activation.
Compared to the conventional oxidation method of synthesizing dibenzothiophene, this innovative approach developed by Dr. Yoshida and his team can accommodate a wide range of functional groups, including highly reactive ones, enabling the synthesis of polysubstituted dibenzothiophene oxides not achievable earlier. Using the method, the researchers synthesized dibenzothiophene oxides having an o-silylaryl triflate moiety, a compound useful as an aryne generation site, but tends to get easily damaged when produced using conventional methods. The o-silylaryl triflate moiety serves as a useful reactive intermediate and can undergo various transformations to produce highly substituted arenes. The proposed method, therefore, not only simplifies the synthesis method but also opens doors for a diverse range of dibenzothiophene S-oxides and their derivatives.
Schematic of the two-step process for producing dibenzothiophene S-oxides
Image caption: A range of polysubstituted dibenzothiophene oxides can be synthesized through Br-selective coupling and subsequent cyclization by electrophilic activation.
Image source link: https://pubs.rsc.org/en/content/articlelanding/2024/cc/d3cc05703h
Image credit: Suguru Yoshida from Tokyo University of Science
Usage restrictions: Credit must be given to the creator. Only noncommercial uses of the work are permitted.
License type: CC BY-NC 3.0
The novel method is a significant step forward in the field of chemical biology. Going ahead, the researchers anticipate that these compounds can find useful applications in diverse research areas, paving the way for innovations and discoveries. “The proposed method can enable the synthesis of polysubstituted benzothiophene oxides, which are expected to be useful in a wide range of research fields,” concludes Dr. Yoshida.
Source: Tokyo University of Science
You can offer your link to a page which is relevant to the topic of this post.
#2024#acids#applications#approach#atom#atomic#atoms#attention#benzene#Biology#Biotechnology news#carbon#catalyst#chemical#chemistry#Chemistry & materials science news#communications#content#details#Discoveries#DNA#enzyme#hydrocarbons#innovations#life#Light#Link#materials#Method#molecules
0 notes