#Clinical Trials Market Challenges
Text
Artificial Intelligence (AI)-based Clinical Trials Market by Product, Types, Procedure, Application, End-user Global Forecast to 2029
Industry Analysis
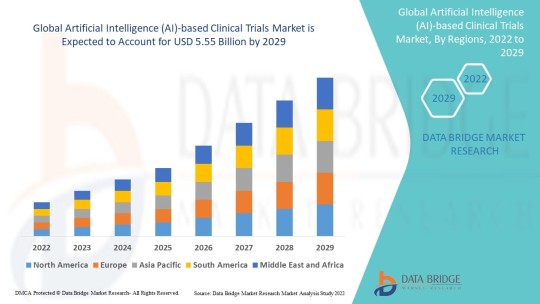
Data Bridge Market Research analyses that the artificial intelligence (AI)-based clinical trials market which was USD 1.3 billion in 2021, would rocket up to USD 5.55 billion by 2029, and is expected to undergo a CAGR of 19.90% during the forecast period 2022 to 2029.
Data Bridge market report covers an array of aspects of the market analysis which today’s businesses call for. This market document also defines a chapter on the global market and allied companies with their profiles, which provides important data pertaining to their insights in terms of finances, product portfolios, investment plans, and marketing and business strategies. This market research report is generated with a nice blend of industry insight, talent solutions, practical solutions and use of technology to advance user experience. An outstanding Data Bridge market report puts light on many aspects related to healthcare industry and market.
Market Insights and Scope
Artificial intelligence can be used to identify disease, provide healthcare services, and even develop new treatments, while improving clinical trials. The scale and speed of AI are significantly superior to any system that depends just on human activity. In many ways, this will be the biggest problem moving forward because we haven't yet reached an AI level that can run totally autonomously.
Artificial Intelligence (AI)-based Clinical Trials Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Industry Segmentation
The Artificial Intelligence (AI)-based clinical trials market is segmented on the basis of clinical trial phase, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Clinical Trial Phase
Phase-I
Phase-II
Phase-III
Application
Oncology
Cardiovascular Diseases
Neurological Diseases or Conditions
Infectious Diseases
End-user
Pharmaceutical Companies
Academia
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Market Country Level Analysis
The countries covered in the Artificial Intelligence (AI)-based clinical trials market report are
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-artificial-intelligence-ai-based-clinical-trials-market
Industry Share Analysis
Some of the major players operating in the Artificial Intelligence (AI)-based clinical trials market are:
Phesi (India)
CONSILX (Singapore)
DEEP LENS Inc. (U.S.)
Unlearn.AI, Inc. (U.S.)
Saama Technologies, LLC (U.S.)
Antidote Technologies, Inc. (U.K.)
Innoplexus (Germany)
Mendel.ai (U.S.)
Median Technologies (France)
Symphony AI (U.S.)
BioAge Labs, Inc. (U.S.)
AiCure (U.S.)
Halo Health Systems (U.S.)
An influential Artificial Intelligence (AI)-based Clinical Trials Market research report displays an absolute outline of the market that considers various aspects such as product definition, customary vendor landscape, and market segmentation. Currently, businesses are relying on the diverse segments covered in the market research report to a great extent which gives them better insights to drive the business on the right track. The competitive analysis brings into light a clear insight about the market share analysis and actions of the key industry players. With this info, businesses can successfully make decisions about business strategies to accomplish maximum return on investment (ROI).
Get TOC Details: https://www.databridgemarketresearch.com/toc/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Browse Related Reports@
Global 1, 4-Cyclohexanedimethanol Dibenzoate Market
Global Plant Hydrocolloids Market
U.S. Tahini Market
Asia-Pacific Hydroxyl-Terminated Polybutadiene (HTPB) market
West Africa Shisha Tobacco Market
Global Orthostatic Hypotension Drugs Market
Europe Customer Journey Analytics Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
��
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Artificial Intelligence (AI)-based Clinical Trials Market-by Product-Types-Procedure-Application-End User-Global-Forecast to-2029#Artificial Intelligence (AI)-based Clinical Trials Market-Global Opportunity-Analysis and-Industry-regional#Artificial Intelligence (AI)-based Clinical Trials Market-Growth-Competition-Scenario-Outlook#Artificial Intelligence (AI)-based Clinical Trials Market-Insights-Country-Share-Competitors-Research-Study#Artificial Intelligence (AI)-based Clinical Trials Market-Demands-Size-Share-Top Trends-Report-to-2029#Artificial Intelligence (AI)-based Clinical Trials Market Value-Segmentation-CAGR rate-Future Trends#Artificial Intelligence (AI)-based Clinical Trials Market-drivers-advantages-restraints-challenges#Artificial Intelligence (AI)-based Clinical Trials Market-Leading Brands-Business-Healthcare#Artificial Intelligence (AI)-based Clinical Trials Market-Growing Popularity-Traffic-DBMR
0 notes
Text
How to write a good abstract

Writing a compelling and effective abstract is crucial for communicating the essence of your research succinctly and clearly. A well-crafted abstract not only summarizes your study but also emphasizes its significance, thereby attracting the attention of the intended audience, including researchers, practitioners, and policymakers. Below are essential guidelines and a structured approach to writing a high-quality abstract for scientific papers, particularly in the biomedical field, though the principles can be adapted for other disciplines.
Key Elements of a Good Abstract:

Declarative Title:
Your title should be clear and direct, reflecting the main findings of your study. It should convey the primary message accurately, ensuring that even those who only read the title understand the core outcome of your research.
2 .Introduction to the Problem:
Start with a sentence that introduces a significant problem or field of interest. In biomedical sciences, this could involve highlighting a critical health issue. The goal is to establish the relevance of your research by showing the urgency or importance of the problem.
3 . Identification of a Significant Challenge:
Clearly state the specific challenge or barrier that is hindering progress in your field. This sets the stage for your study by pinpointing the precise issue you aim to address without yet delving into your methodology.
4 . Opportunity for Advancement:
Introduce a recent advancement or opportunity that makes addressing the identified challenge feasible. This could be a technological innovation, new data availability, or a novel methodological approach that provides a fresh perspective on the problem.
5 . Description of Your Study:
Summarize the core of your study in 1–2 sentences. Describe what you did and how you leveraged the identified opportunity to tackle the challenge. This should provide a brief but comprehensive overview of your approach.
6 .Key Results:
Highlight the main findings of your study in 2–3 sentences. These results should directly support the conclusions stated in your title and demonstrate the impact of your research.
7. Implications and Broader Impact:
Conclude with a sentence on the potential impact of your findings. Explain how your results could change current practices, inform future research, or have broader implications for the field.
Example of an Abstract Using These Guidelines:
Title: Data-driven Prediction of Drug Effects and Interactions
Abstract: Adverse drug events remain a leading cause of morbidity and mortality worldwide. Many such events are undetected during clinical trials before a drug receives approval for clinical use. Regulatory agencies maintain extensive collections of adverse event reports as part of post marketing surveillance, presenting an opportunity to study drug effects using patient population data. However, confounding factors such as concomitant medications, patient demographics, medical histories, and prescribing reasons are often uncharacterized in spontaneous reporting systems, limiting quantitative signal detection methods. Here, we present an adaptive data-driven approach for correcting these confounding factors in cases with unknown or unmeasured covariates and combine this approach with existing methods to improve drug effect analyses using three test datasets. We also introduce comprehensive databases of drug effects (OffSIDES) and drug-drug interaction side effects (TwoSIDES). To demonstrate the utility of these resources, we identified drug targets, predicted drug indications, and discovered drug class interactions, corroborating 47 (P < 0.0001) interactions using independent electronic medical record analysis. Our findings suggest that combined treatment with selective serotonin reuptake inhibitors and thiazides significantly increases the incidence of prolonged QT intervals. We conclude that controlling for confounding effects in observational clinical data enhances the detection and prediction of adverse drug effects and interactions.
Investing in your academic future with Dissertation Writing Help For Students means choosing a dedicated professional who understands the complexities of dissertation writing and is committed to your success. With a comprehensive range of services, personalized attention, and a proven track record of helping students achieve their academic goals, I am here to support you at every stage of your dissertation journey.
Feel free to reach out to me at [email protected] to commence a collaborative endeavor towards scholarly excellence. Whether you seek guidance in crafting a compelling research proposal, require comprehensive editing to refine your dissertation, or need support in conducting a thorough literature review, I am here to facilitate your journey towards academic success. and discuss how I can assist you in realizing your academic aspirations. Whether you seek guidance in crafting a compelling research proposal, require comprehensive editing to refine your dissertation, or need support in conducting a thorough literature review, I am here to facilitate your journey towards academic success.
#academics#education#grad school#gradblr#phd#phd life#phd research#phd student#phdblr#study#essays#literature#writters on tumblr#my writing#writeblr#writing#writers on tumblr#thesis#dissertation#university student#university#uniblr#stanford university#case study#student life#students#studyblr#studying#studyspo#student
3 notes
·
View notes
Text
The Rise of 3D Printing in Prosthetics and Orthotics Market

The global prosthetics and orthotics market plays a vital role in improving quality of life for millions worldwide. Worth an estimated $7.2 billion in 2024, the market facilitates mobility for those with limb differences or injuries through highly customized external limb replacements and braces.
The market introduces prosthetics and orthotics—Medical devices that enhance or assist impaired body parts and mobility. Orthotics are braces or supports for joints, spine, and limbs; prosthetics externally replace missing limbs. Together they improve functionality and quality of life for users.
Major players in the prosthetics and orthotics space utilizing advanced manufacturing include Ossur, Steeper Group, Blatchford, Fillauer, Ottobock, and WillowWood Global. These industry leaders increasingly deploy cutting-edge 3D printing and customized design software to produce state-of-the-art prosthetics and braces.
Current trends in the prosthetics and orthotics market include growing utilization of 3D printing and advanced manufacturing techniques. 3D printing enables on-demand production of complex, customized devices. It reduces manufacturing costs and wait times while improving fit and comfort. Expanding material options also allow more lifelike prosthetics. As technology evolves, the market is positioned for continued growth through 2031 in facilitating mobility worldwide.
Future Outlook
The prosthetics and orthotics market is expected to witness significant advancements in the coming years. Manufacturers are constantly focusing on developing innovative technologies such as 3D printed prosthetics that provide a better fit, enhanced comfort, and unrestricted movement. There is also a rising trend of using lightweight, highly durable and comfortable materials like carbon fiber and thermoplastics to manufacture prosthetic devices. Advancements in myoelectric prosthetics with touch and motion sensors are making them more dexterous and responsive. Using pattern recognition and machine learning techniques, next-gen prosthetics could gain functionality approaching that of natural limbs.
PEST Analysis
Political: Regulations regarding clinical trials and approvals of new prosthetic technologies may affect market growth. Favorable reimbursement policies for prosthetic devices can boost adoption.
Economic: Rising disposable incomes allow more individuals to opt for higher-end prosthetics. Emerging markets present abundant opportunities for growth. Inflation and economic slowdowns can hinder market profitability.
Social: Increasing incidence of amputations and disabilities due to aging population, accidents, war injuries etc. drive market demand. Growing awareness regarding prosthetics and orthotics aids adoption. Stigma associated with limb loss poses challenges.
Technological: Advancements in materials, manufacturing techniques like 3D printing, sensors, computing power and battery technologies are enhancing functionality and usability of prosthetics/orthotics. Myoelectric and robotic prosthetics have vastly improved in recent years.
Opportunity
Rising aging population presents a huge opportunity for prosthetics and orthotics targeting mobility issues and disabilities. Over 630,000 amputations occur annually in the U.S. due to dysvascular conditions like diabetes, presenting a sizable patient pool. Expanding applications of prosthetics and orthotics beyond mobility impairment into sports and military could drive significant growth. Growing incidence of trauma and injuries globally increases the number of patients relying on these devices. Emerging markets like Asia Pacific and Latin America offer immense opportunities owing to increasing disposable incomes, expanding healthcare infrastructure and rising medical tourism. Technological advancements are constantly improving functionality and usability of prosthetic devices, fueling adoption rates. The lightweight, durable and comfortable characteristics of newer materials expand addressable indications and patient acceptance.
Key Takeaways
Growing demand from aging population: The rapid increase in aging population worldwide who are prone to mobility issues, disabilities and chronic diseases like diabetes is a key driver spurring sales of orthotic and prosthetic devices.
Global expansion into emerging markets: Emerging markets like Asia Pacific, Latin America, Eastern Europe and the Middle East offer immense opportunities owing to their large population bases and improving healthcare penetration.
Technological advancements: Constant R&D bringing advancements in areas such as 3D printing, lightweight materials,
4 notes
·
View notes
Text
Transforming the Health Landscape: The Global Blockchain in Healthcare Market

The integration of blockchain technology into the healthcare sector is revolutionizing the way medical data is managed, shared, and secured. As the demand for transparent, efficient, and secure healthcare services grows, blockchain offers promising solutions to longstanding challenges.
Understanding Blockchain in Healthcare
Blockchain Technology is a decentralized digital ledger that records transactions across multiple computers in a way that ensures the security and transparency of data. In healthcare, blockchain can be used to manage patient records, track pharmaceuticals, ensure the integrity of clinical trials, and streamline administrative processes. The immutable nature of blockchain helps in preventing data breaches, ensuring data accuracy, and enhancing patient privacy.
According to BIS Research, the Global Blockchain in Healthcare Market was estimated to grow to a value of $5.61 billion by 2025, and still the market is showing a steep growth till 2030 witnessing a double-digit CAGR growth rate throughout the forecast period.
Key Market Dynamics
Several factors are driving the growth of the global blockchain in healthcare market:
Data Security and Privacy:
Need for robust data security and privacy solutions.
Healthcare data breaches are a growing concern.
Blockchain's secure, immutable nature protects sensitive patient information.
Interoperability and Data Sharing:
Facilitates seamless data sharing between healthcare providers and systems.
Overcomes current interoperability issues.
Leads to better patient outcomes by providing a comprehensive view of health history.
Supply Chain Transparency:
Tracks the entire lifecycle of drugs in the pharmaceutical industry.
Ensures the authenticity of medications.
Helps combat counterfeit drugs.
Efficient Administrative Processes:
Streamlines various administrative processes, such as billing and claims management.
Reduces fraud and administrative costs.
Support from Regulatory Bodies:
Increasing support from regulatory bodies and governments.
Initiatives by FDA and EMA to explore blockchain for drug traceability and clinical trials boost market growth.
Request for an updated Research Report on Global Blockchain in Healthcare Market Research.
Global Blockchain in Healthcare Industry Segmentation
Segmentation by Application:
Data Exchange and Interoperability
Supply Chain Management
Claims Adjudication and Billing Management
Clinical Trials and Research
Others
Segmentation by End-User:
Healthcare Providers
Pharmaceutical Companies
Payers
Others
Segmentation by Region:
North America
Europe
Asia-Pacific
Latin America and Middle East & Africa
Future Market Prospects
The future of the global blockchain in healthcare market looks promising, with several trends likely to shape its trajectory:
Integration with AI and IoT: The integration of blockchain with artificial intelligence (AI) and the Internet of Things (IoT) will enhance data analytics, predictive healthcare, and real-time monitoring.
Expansion of Use Cases: New use cases for blockchain in digital healthcare will emerge, including patient-centered care models, personalized medicine, and enhanced telemedicine services.
Focus on Patient-Centric Solutions: Blockchain will enable more patient-centric healthcare solutions, empowering patients with greater control over their health data and enhancing patient engagement.
Development of Regulatory Frameworks: The establishment of clear regulatory frameworks and industry standards will facilitate the widespread adoption of blockchain in healthcare.
Conclusion
The Global Blockchain in Healthcare Industry is poised for significant growth, driven by the need for enhanced data security, interoperability, supply chain transparency, and efficient administrative processes. By addressing challenges related to regulatory compliance, implementation costs, standardization, and scalability, and leveraging opportunities in technological advancements, investments, partnerships, and government initiatives, the potential of blockchain in healthcare can be fully realized. This technology promises to revolutionize healthcare delivery, enhancing efficiency, transparency, and patient outcomes, and setting new standards for the future of digital health.
#Blockchain in Healthcare Market#Blockchain in Healthcare Industry#Blockchain in Healthcare Market Report#Blockchain in Healthcare Market Research#Blockchain in Healthcare Market Forecast#Blockchain in Healthcare Market Analysis#Blockchain in Healthcare Market Growth#BIS Research#Healthcare
2 notes
·
View notes
Text

Prompt: create a movie concept for a feature-length movie with a gimmick of the story being told entirely through fictional advertising spots. The movie chronicles huge social changes through society via the progressive changes in the advertisement for media offerings (new shows/movies/video games etc.), new products and businesses, clinical trials, lawsuit, hirings, government services and other PR campaigns etc.
Title: Slogans
Tagline: A story of ad breaks and gloop.
Logline: Decades of social upheaval are chronicled entirely through a series of hilarious and satirical commercials, reflecting the ever-changing landscape of a society struggling to adapt to the arrival of benign, but incredibly messy, aliens.
Concept: The film unfolds through a series of interconnected fictional commercials spanning several decades. Each ad campaign reflects the evolving societal response to the arrival of a race of friendly, but incredibly messy, aliens called the "Glorbs."
Examples of Ads:
Early Days (1970s): A wholesome family commercial for "Glorb-Be-Gone" cleaning spray, promising to eliminate the sticky green slime the Glorbs leave in their wake.
The Honeymoon Phase (1980s): A glitzy ad for "Glorb Gear" - stylish jumpsuits designed to protect against the Glorbs' unpredictable "bio-spray."
Inter-Species Dating Boom (1990s): A cheesy dating show ad for "Glorb-al Attraction," promising to find love between humans and Glorbs (despite their vastly different physical forms).
Glorb-onomics (2000s): A slick corporate ad for "Glorb-Co," a company utilizing the Glorbs' natural slime production for a new line of eco-friendly cleaning products.
The Grunge Years (2010s): A gritty commercial for "Glorb-Fighter 5000," a high-powered cleaning robot designed for the truly dedicated Glorb-haters.
The Rise of the Glorb Lobby (2020s): A heartwarming PSA from the "Glorb Integration Initiative," advocating for equal rights for the messy extraterrestrials.
Interspersed between these ads are glimpses of news reports, political debates, and legal battles reflecting the changing social climate. A class-action lawsuit against Glorb-Co for "bio-hazard negligence" transitions into a documentary on the rise of a powerful Glorb lobby pushing for "Glorb-Positive" legislation.
The humor comes from the absurdity of the changing social dynamics, the exaggerated efficiency of the cleaning products, and the ever-present Glorb slime that seems to permeate every aspect of human life.
Themes:
The challenges of interspecies coexistence.
The power of advertising to shape public opinion.
The human capacity to adapt, even to the messiest situations.
Ending:
The final ad campaign depicts a utopian future where humans and Glorbs live in harmony. A new product called "Glorb-Tastic Slime" is marketed as a fun, gooey toy for children, showcasing how even the most disruptive change can be normalized over time.
Slogans is a humorous and thought-provoking exploration of social change and adaptation in the face of the unexpected. It's a reminder that sometimes, the most profound stories can be told through the most mundane means – even a commercial for slime remover.
#ai generated#stupid ideas#shitpost#imaginary movie#google gemini#movie poster#poster#genre: science fiction#genre: experimental#advertising#ad breaks#aliens#alien invasion#messy#slime#metafiction
2 notes
·
View notes
Text
A revolution in weight loss is apparently underway. It started in 2021, when the FDA approved the diabetes drug semaglutide for weight loss. The weekly injectable—sold under the brand name Wegovy—can help users lose 5 to 10 percent of their body weight, leading commentators to describe the drug as both a “medical breakthrough” and a “silver bullet” for obesity. Elon Musk says he’s taking it, Kim Kardashian is rumored to be using it, and everyone from Hollywood to the Hamptons reportedly wants a prescription.
Soon, there will be a new weight loss medication on the block—and it’s even more potent than its peers. Last fall, the FDA fast-tracked the review process for using tirzepatide as a weight loss drug after a clinical trial showed that people with BMIs labeled “overweight” or “obese” lost a staggering 22.5 percent of their body weight on the highest dose. If all goes according to plan, that will make Mounjaro the latest in a fast-growing biomedical sector—spanning everything from bariatric surgery to deep brain stimulation for binge-eating—that aims to combat, if not cure, the problem of “excess” weight.
For pharmaceutical companies, the race to market is financially motivated: Wegovy and Mounjaro cost more than $1,000 a month. Weight loss drugs are rarely covered by insurance, but people who can afford them have proven they’re willing to pay. And the market seems effectively limitless: Despite an ongoing “war on obesity,” more than 1.9 billion adults globally are considered overweight or obese, and the number of prospective users is growing every year. Now doctors—desperate to treat what is widely seen as an “obesity epidemic”—are coming on board. In January, the American Academy of Pediatrics recommended such medications for kids as young as 12.
The victorious narratives gilding drugs like Mounjaro are already being positioned as a direct challenge to fat activism. For decades, the movement has pushed for social and economic opportunity for people of all sizes through civil rights, fat pride and liberation, and biomedical evidence itself. Thanks to prominent voices like Audrey Gordon and Michael Hobbes, many people now know that “lifestyle changes” like calorie restriction and exercise fail to produce sustained weight loss for 97 percent of people and that many dieters end up gaining back more weight than they lost. But what happens to the strength of these arguments when a weight loss drug seems to work?
Like other purported weight loss solutions, Mounjaro promises “to fix weight stigma by making you thinner, instead of removing the stigma,” says Susanne Johnson, a fat activist and family nurse practitioner in Pennsylvania. In so doing, these drugs and surgeries further exacerbate anti-fat discrimination. Instead of criticizing people in larger bodies for their perceived lack of willpower—that old “calories in, calories out” adage—people can now blame those in bigger bodies for something more akin to a techno-pessimist, or even anti-science, stance: “Just take the miracle cure!”
The history of the weight loss industry is more akin to prospecting for gold or investing in crypto than transplanting organs and developing antibiotics; less a story of scientific progress than an endless cycle of wild speculation, where boom inevitably gives way to bust. Fen-Phen was a miracle until it was linked to heart valve damage. Intermittent fasting was going to fix what caloric restriction couldn’t until researchers showed the two produce exactly the same results. And then there’s the complicated case of bariatric surgery.
From their inception in the 1950s, operations like gastric bypass (which reroutes food away from the stomach, inducing malabsorption) and gastric sleeve (which involves partially amputating the stomach so it holds less food and produces fewer hunger hormones) have been sold as a potential panacea, says Lisa Du Breuil, a clinical social worker at Massachusetts General Hospital. While fewer than 1 percent of people who qualify actually undergo bariatric surgery, those who do can lose up to 70 percent of their “excess” weight (or the weight above a BMI of 24.9).
But Du Breuil, who specializes in eating disorders and substance abuse disorders, has seen some of the worst of bariatric’s side effects. People can develop dumping syndrome—wherein sugar-rich meals leave the stomach too quickly, causing sweating, dizziness, rapid heart rate, and vomiting. Gastric bypass in particular raises the risk of postoperative alcohol abuse. Rates of suicide and self-harming behaviors also rise in the years after bariatric surgery. And even when people follow strict post-operative diets, malnutrition, tooth loss, gout, and new or resurging eating disorders are possible. “It can be really challenging to get a full picture,” Du Breuil says. She learns about new side effects all the time.
Semaglutide and tirzepatide—both part of a larger family of GLP-1 receptor agonists—were developed for diabetes management at lower doses. When pharmaceutical companies noticed their trial participants were also losing weight, they realized “if we can turn the volume up to 11, we can really enhance this side effect,” says Johnson, the nurse. “That means you’re also turning up the other side effects.”
The primary complaints from users of Ozempic, Wegovy, and Mounjaro sound like the kind of thing you can fix with a bottle (or three) of Pepto Bismol: nausea, upset stomach, diarrhea, and what one patient called “power vomiting.” But these might be less like classic “side effects” of a drug than a mechanism of weight loss itself, as The Guardian recently reported. By making the feeling of eating (and, in some cases, even hydrating) actively disgusting to the user, the drug curbs their consumption—similar to the experience of bariatric patients, who can only fit a few ounces of food in their stomachs at a time.
The list of complications doesn’t end there. For example, both GLP-1 receptor agonists may increase the risk of thyroid cancer—one of the many BMI-linked diseases that supposedly makes weight loss absolutely imperative for people in larger bodies. And there’s good reason to believe that other side effects will reveal themselves in years to come, as the number of long-term users grows.
The biggest surprise for many prospective patients is that long-term weight loss isn’t guaranteed—a reflection, perhaps, of the faulty assumption that people are obese because they overeat. Current estimates suggest that the average bariatric surgery patient regains 30 percent of the weight they lost in the 10 years after surgery. One in four regain all of their weight in that time. And 20 percent of people don’t respond to surgery in the first place.
The same is true for GLP-1 receptor agonists: If you stop injecting, the weight returns.
In case it wasn’t clear by now, biomedical weight loss interventions often mimic the deadly logic of anorexia, bulimia, or other forms of disordered eating, says Erin Harrop, a clinical social worker and researcher. Harrop would know. At the height of their own eating disorder, Harrop wished they could fill their stomach with air instead of food, or cut their stomach out, or wire their jaw shut. Later, they learned these things exist—in the form of gastric balloons, gastric sleeves, and even a magnetic jaw trap.
It’s no surprise, then, that some people who undergo bariatric surgery experience a resurgence of a preexisting eating disorder, or develop a new one. Frequent vomiting, never knowing what foods will upset your stomach, and feeling pressure to maintain a post-surgical weight—“you can create an eating disorder that way,” Du Briel says.
But semaglutide and tirzepatide promise to fulfill an even stranger fantasy: eliminating appetite itself. While a drug like Mounjaro works on numerous fronts—including preventing the body from storing fat and “browning” existing adipose tissue—it’s the feeling of being untethered from desire that seems to fascinate patients and physicians alike. People for whom the drug works often say, “I forget to eat,” says Fatima Cody Stanford, an obesity medicine specialist at Massachusetts General Hospital’s Weight Center.
If doctors really believe that obesity is the greater of any two evils, then this approach makes sense. When it comes to bariatric surgery, for example, a review of the medical literature suggests it is, on balance, associated with a reduction in all-cause mortality—or death of any cause*—*compared to patients with high BMIs who don’t go under the knife (though such studies are profoundly limited, as they often do not control for social factors, like income or education). Many hope that semaglutide and tirzepatide will one day prove just as vitalizing.
But eating disorders kill too. In many contexts, sustained hunger is considered a travesty. And desire—for food, or anything else—is a great way to know you’re alive. “It’s wild to me that we see no appetite as a positive thing,” says Shira Rosenbluth, an eating disorder therapist who works with people of all sizes. Anna Toonk agrees: “I realized that there are worse things than being fat,” she told The Cut last fall. “The worst thing you can be is wanting to barf all the time.”
Ultimately, the proliferation of drugs like Mounjaro means medicine is not only in the business of dictating “normal” weights (a thing it still hasn’t quite figured out), but “normal” appetites. What was once an intuitive process, in which your body tells you what it needs, became a dictate under diet culture: You tell your body what it can have. Now medicine promises a radical reset: With the right drug, your body will hunger for nothing at all.
Weight loss technology can’t be stopped entirely—nor should it be. Everyone has the right to choose what they want to do with their bodies. But informed consent is built on information, and we may not have enough. Mounjaro was fast-tracked by the FDA based on studies designed to observe weight loss over just 72 weeks, a small fraction of the time real patients will be on the drug. At the very least, patients should be informed that in the first years after a drug hits the market, they are participants in an ongoing experiment.
As biomedicine’s war on obesity continues, people must work harder to combat anti-fat bias—not on a technicality, but as part of the expansive vision of justice fat activists began articulating more than 50 years ago. For semaglutide, tirzepatide, bariatric surgery, and their ilk are neither miracles nor cures. There have always been fat people, and there always will be, whether they’re “non-responders” to treatment, refuseniks, or languishing on the waitlist. Notably, even those who experience dramatic weight loss after surgery or on injectables may still be overweight or obese, depending where they started.
Perhaps most importantly, the American weight loss discourse must move away from a reflexive scientism, which has enabled biomedicine to subject the entirety of human experience to its single-minded scrutiny. Weight, like almost every aspect of embodiment, is not an exclusively biological phenomenon or a clear-cut medical “problem” to solve. It is shaped by countless factors, like power distribution in society, personal psychology, and that most frightening of forces: the desire for more.
If you or a loved one is struggling with an eating disorder, the National Eating Disorders Association Helpline is available at (800) 931-2237.
11 notes
·
View notes
Text
Clinical Development Solutions
In the rapidly evolving field of healthcare, clinical development plays a crucial role in bringing novel treatments and therapies to patients worldwide. Clinical Development Solutions (CDS) is at the forefront of this exciting journey, pioneering innovative approaches to accelerate the development and approval of life-saving drugs and medical devices. With a dedicated team of experts and cutting-edge technologies, CDS is committed to transforming the landscape of clinical research and improving patient outcomes.
At CDS, we understand the challenges and complexities of clinical development. Our comprehensive suite of solutions is designed to address these challenges head-on, providing tailored strategies and support throughout the entire drug development lifecycle. From early-phase clinical trials to post-marketing studies, we offer a wide range of services that enable pharmaceutical and biotech companies to navigate the regulatory landscape efficiently and effectively.
One of the key strengths of CDS lies in our expertise in clinical trial design and optimization. We work closely with our clients to design robust and scientifically rigorous trials that generate high-quality data while minimizing risks. By leveraging our extensive knowledge and experience, we can identify the most appropriate patient populations, endpoints, and study designs to maximize the chances of success. Our statistical and data management teams ensure that the collected data is accurate, reliable, and compliant with regulatory requirements.
In addition to trial design, CDS also excels in patient recruitment and retention strategies. We understand the importance of enrolling a diverse and representative patient population to ensure the generalizability of study results. Through our innovative patient-centric approaches, such as digital recruitment platforms and targeted engagement campaigns, we connect with potential study participants and enhance their overall trial experience. By fostering strong relationships with patients and investigators, we improve retention rates and reduce dropout rates, ultimately leading to faster and more reliable study results.
CDS is at the forefront of adopting emerging technologies to drive efficiency and innovation in clinical development. We harness the power of big data analytics, artificial intelligence, and machine learning to uncover valuable insights from complex datasets. These advanced analytics enable us to identify trends, predict outcomes, and optimize trial protocols, thus accelerating the development timeline and reducing costs. Our investment in digital health technologies and wearable devices further enhances data collection and remote monitoring capabilities, enabling more flexible and patient-friendly trial designs.
In the realm of regulatory affairs, CDS provides comprehensive support to ensure compliance with global regulations and standards. Our regulatory experts have in-depth knowledge of regional requirements, including those of the FDA, EMA, and other regulatory authorities worldwide. From preparing regulatory submissions to managing post-marketing safety surveillance, we guide our clients through every step of the regulatory process, ensuring timely approvals and post-approval compliance.
CDS is also committed to fostering collaboration and knowledge sharing within the clinical research community. We organize scientific symposia, webinars, and training programs to facilitate the exchange of ideas and best practices. By promoting interdisciplinary collaboration and staying up to date with the latest industry advancements, we continuously enhance our capabilities and stay at the forefront of clinical development.
In conclusion, Clinical Development Solutions is a leading provider of innovative solutions in clinical development. Through our expertise, technology-driven approaches, and commitment to patient-centricity, we strive to transform the drug development landscape and improve patient outcomes. By partnering with CDS, pharmaceutical and biotech companies can navigate the complexities of clinical research with confidence, bringing new therapies to patients faster and more efficiently. Together, let us shape the future of healthcare through innovation and collaboration.
#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
2 notes
·
View notes
Text
Dealing with challenges in Quality Evidence Generation with a Real-Time Analytical Framework that makes Clinical Sense for Innovators
Evidence linking interventions with health outcomes is vital for healthcare decision-making. Making sound choices about healthcare requires the best possible and quality evidence from clinical research. However, some of the decisions currently made during the drug development process are not supported by high-quality evidence. As such, making informed decisions for allocating adequate resources to guide clinical Research development becomes challenging. At mid-stage clinical development, the challenge entails in determining the specific indication, if there are multiple potential indications. Moreover, evidence that is complete for some individuals or groups may be incomplete for others, leading to inefficiencies in decision-making.
Evidence generation strategies are especially important at Phase III and Phase IV trials to allow for effective navigation through competitive and regulatory hurdles, while it may be difficult to effectively communicate potentially attractive product attributes to the stakeholders, especially when it has no clear advantage over comparators. Stakeholders also lack the evidence needed to make real-world decisions on approval, coverage and use of treatments as most current processes used in evidence generation focus narrowly on the safety and efficacy of treatment.
Datasets to inform real-time decision making
The traditional demarcation between pre- and post-approval phases does not fit many medical products, as regulatory decisions could be informed by the same evidence that informs the use and coverage decisions, though the criteria for decisions should not be the same for both cases. Validated tools, based on large datasets to help inform real-time decision making are invaluable, yet they are currently limited. When new treatments are approved, healthcare payers and those who participate in shared savings base coverage determination on their value which is calculated by the evidence of benefit and net costs. The incorporation of real-world data (RWD) and patient-reported outcomes (PRO) into the evidence generation process could assist in making coverage determinations by rendering clinical evidence and research more immediately translatable to the beneficiary population.

Real-world data (RWD) and real-world evidence (RWE)
Additionally, large differences usually exist between the evidence required for initial adopters, such as surveys and studies, and that required for most prospective randomized control trials (RCTs). While the healthcare community uses RWD and RWE to develop decision support tools for use in clinical practices, medical product developers use these data to support clinical trial designs and observational studies to generate innovative treatment approaches. FDA uses RWE and RWD to monitor adverse events, post-market safety of the drug, and to make regulatory decisions. While RWD can be collected from various sources such as electronic health records (EHRs) and product and disease registries, RWE can be generated by different study designs including observational studies and randomized trials.
Aligning stakeholders for evidence generation
Aligning stakeholders is another big challenge of evidence generation as different stakeholders will have their own perspectives on uncertainties throughout the drug development lifecycle. Regulators may have different views as to what is acceptable to that of the patient. As such, it remains an industry-wide challenge to provide credible evidence for clinical research to innovators and investigators. Challenges exist for healthcare innovators to keep up to date with compliance and regulations about evidence generation as regulatory space evolves fast.
Because pharmaceutical companies tend to delegate evidence generation to individual departments that are often siloes, the process occurs sequentially, resulting in delays in crucial milestones such as getting regulatory approval before initiating an outcomes-based study.
https://www.futuremedicine.com/doi/10.2217/cer-2017-0073

An analytical framework model that makes clinical sense
There is a pressing need for high-quality evidence generation as regulators and payers seek more long-term data on product safety and effectiveness. As such, more efficient methodologies for generating evidence are required for decision-making, and to enhance clinical evidence collection and interpretation. An analytical framework model makes clinical sense as an evidentiary pathway, however, the challenge for investigators in evidence gathering is to fill out the framework. If the study design is weak, then the link in the evidence chain is also weak. Studies need to be carefully and prospectively designed, and opportunities exist to add well-designed studies into current practices. Study teams and researchers should consider how to most effectively translate diagnostic tests into practice needs within clinical settings.
Quality clinical evidence of safety and efficacy
The Jeeva™ eClinical Cloud platform provides clinical decision-makers with a modular and integrated approach to evidence planning and generation. From a single dashboard, study leaders can monitor data in real time to track safety and efficacy in representative patient populations across vast distances. The Jeeva™ eClinical Cloud is designed for efficient, remote long-term follow-up, natural history and other observational studies as well as interventional clinical trials regardless of therapeutic area. Jeeva™ enables quality clinical evidence generation to evaluate treatment safety and efficacy and tracks patients’ adherence to medications, in compliance with regulatory agencies such as Institutional Review Boards, EMA, FDA, and GDPR.
Digital-first approach to evidence generation
Study teams, innovators, drug developers, biopharmaceutical sponsors, clinical researchers, hospital sites and contract research organizations (CROs) face challenges to overcome the “no evidence, no implementation—no implementation, no evidence” paradox. Jeeva™ provides a new, digital-first, patient-centric approach to evidence generation that considers patients as partners for clinical trials, not merely subjects.
The Jeeva™ eClinical Cloud is user-designed software-as-a-service (SaaS) platform that allows volunteers to conveniently complete clinical trials wherever they are. The flexible and modular bring-your-own-device (BYOD) solution works on any browser-enabled mobile device and cuts out 70% of logistical burdens for study teams and patients. The modular and flexible Software as a Service (SaaS) subscription-based model is enriched with many features such as automated enrollment workflows, electronic patient-reported outcomes, 2-way email and SMS communication, uploading of lab reports, and more that are designed to encourage innovators to undertake research activities, rather than be intimidated by the complexity, logistical burdens, duration and costs of the traditional evidence generation approaches.
Quickly setup clinical studies of any scale or duration
Jeeva™ applies an innovative approach to remote screening, eConsent, patient registries and natural history studies can enable the generation of higher-quality, low-cost and more timely evidence generation for clinical trials. Jeeva™ offers a cost-effective solution to quickly set up and conduct clinical studies, of any scale or duration, with or without patient travel involved (e.g. hybrid or fully decentralized clinical trial protocols). Jeeva™ provides a more effective clinical trial design in terms of evidence generation, accelerating patient recruitment, site feasibility and endpoints that bring unmatched efficiencies in terms of the quality of evidence, time, and costs.
#clinical trial protocol software#clinical trials recruitment and retention#patient retention in clinical trials#software used in clinical trials#clinical solutions research platforms#clinical research software#software for clinical trials#software used in clinical research#clinical trials software#clinical study software#decentralized clinical trials software#clinical trial recruitment challenges#mobile eclinical#clinical trial cloud software#eclinical platform#eclinical cloud
2 notes
·
View notes
Text
AI Transforming Drug Discovery: The Future of the Pharmaceutical Market
Artificial Intelligence in Pharmaceutical Market is projected to be valued at USD 3.05 billion in 2024 and is anticipated to reach USD 18.06 billion by 2029, with a CAGR of 42.68% during the forecast period (2024-2029).
Artificial Intelligence (AI) in Drug Discovery Market is gaining momentum as pharmaceutical companies increasingly adopt AI-driven solutions to streamline research and development (R&D) processes. According to Mordor Intelligence, the market is expected to grow significantly, driven by the rising need for faster, cost-effective drug discovery methods, combined with advances in AI technologies like machine learning, natural language processing (NLP), and deep learning.
AI’s Role in Transforming Drug Discovery
Accelerating Drug Discovery Process:
Traditionally, drug discovery is a time-consuming and costly process, often taking 10–15 years and billions of dollars to bring a new drug to market. AI is revolutionizing this by speeding up the identification of drug targets and optimizing lead compounds. Algorithms can rapidly analyze vast datasets, predicting drug behavior and outcomes more accurately than traditional methods.
Predictive Modeling and Simulation:
AI's ability to create predictive models is transforming how pharmaceutical companies approach drug discovery. AI can simulate how compounds will interact with biological targets, reducing the need for trial-and-error lab work. This not only accelerates the development process but also enhances the chances of finding successful drug candidates.
Data-Driven Research:
With the growing availability of biological and chemical data, AI can sift through and analyze enormous datasets, identifying patterns and insights that humans might miss. Machine learning models can analyze genomic data, protein structures, and chemical compounds, helping researchers understand complex biological mechanisms and predict potential therapeutic outcomes.
AI in Target Identification and Validation:
AI tools are being employed to identify novel biological targets for therapeutic intervention, as well as to validate their potential effectiveness. This is especially crucial in the development of drugs for complex diseases like cancer, neurodegenerative disorders, and infectious diseases.
AI for Drug Repurposing:
AI is also playing a pivotal role in drug repurposing, where existing drugs are investigated for new therapeutic uses. AI algorithms can identify new applications for existing compounds by analyzing data on drug interactions, mechanisms of action, and patient outcomes.
Reduction in Drug Development Costs:
By reducing the time and cost involved in early-stage drug discovery, AI is contributing to significant cost savings. Traditional methods often lead to high failure rates, especially in late-stage clinical trials. AI can help mitigate these risks by improving early predictions on a drug’s efficacy and safety profile.
Key Market Growth Drivers
Rising Demand for Personalized Medicine: AI’s ability to analyze genetic, environmental, and lifestyle factors is driving growth in personalized medicine. AI-powered platforms can predict how individuals will respond to treatments, leading to more targeted and effective therapies.
Increasing Partnerships between Pharma and AI Companies: Collaborations between pharmaceutical companies and AI technology firms are growing. These partnerships aim to combine pharma's clinical expertise with AI’s data-processing capabilities to revolutionize drug discovery and development.
AI in Clinical Trials: AI is optimizing the clinical trial process by identifying ideal patient cohorts, predicting trial outcomes, and improving trial design. This not only speeds up the trial process but also reduces costs, which is critical in the pharmaceutical market.
Challenges and Opportunities
Data Quality and Integration: While AI offers immense potential, one of the primary challenges remains the quality and integration of data. Pharmaceutical companies must ensure they have access to clean, structured data to fully leverage AI’s capabilities in drug discovery.
Regulatory Concerns: As AI becomes more integrated into drug discovery, regulatory agencies are working to establish frameworks to ensure the safety and efficacy of AI-driven drugs. Navigating these evolving regulatory landscapes will be crucial for pharmaceutical companies.
AI Talent Shortage: As the demand for AI in drug discovery grows, there is a shortage of skilled professionals who can build, implement, and manage these technologies. Addressing this talent gap is essential for sustained market growth.
Regional Insights
North America leads the market due to the presence of major pharmaceutical companies, strong healthcare infrastructure, and early adoption of AI technologies. The region’s advanced regulatory environment also supports the integration of AI in drug discovery.
Europe follows closely, driven by increased R&D funding and government support for AI initiatives in healthcare. Asia-Pacific is also expected to see rapid growth due to rising investments in AI and a growing pharmaceutical industry in countries like China and India.
Future Outlook
AI’s transformative impact on drug discovery is still in its early stages, but the potential is vast. As AI continues to evolve, it is expected to significantly reduce the cost and time associated with bringing new drugs to market, while also improving success rates in clinical trials. This will not only benefit pharmaceutical companies but also patients, who will gain faster access to innovative treatments.
In conclusion, AI is reshaping the future of the pharmaceutical industry by optimizing drug discovery processes, improving patient outcomes, and driving cost-efficiency. The next few years will be critical as AI’s role in drug discovery continues to expand, opening up new opportunities for innovation in the pharmaceutical market.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/artificial-intelligence-in-pharmaceutical-market
#Artificial Intelligence (AI) In Pharmaceutical Market#Artificial Intelligence (AI) In Pharmaceutical Market Size#Artificial Intelligence (AI) In Pharmaceutical Market Share#Artificial Intelligence (AI) In Pharmaceutical Market Analysis#Artificial Intelligence (AI) In Pharmaceutical Market Trends#Artificial Intelligence (AI) In Pharmaceutical Market Report#Artificial Intelligence (AI) In Pharmaceutical Market Research#Artificial Intelligence (AI) In Pharmaceutical Industry#Artificial Intelligence (AI) In Pharmaceutical Industry Report
0 notes
Text
Neonatal Ventilator Market: Analyzing Regional Developments

The neonatal ventilator market is witnessing dynamic growth across the globe, influenced by various regional developments that shape healthcare practices and technology adoption. As healthcare providers increasingly focus on improving outcomes for premature and critically ill infants, understanding the regional dynamics of the neonatal ventilator market becomes crucial. This article explores the developments in key regions and their impact on the market.
North America
1. Market Leadership
North America, particularly the United States, holds a dominant position in the neonatal ventilator market. The region benefits from advanced healthcare infrastructure, high levels of investment in medical technology, and a significant number of neonatal intensive care units (NICUs). The increasing prevalence of preterm births and respiratory disorders drives demand for advanced ventilatory support.
2. Innovation and Research
The region is a hub for innovation, with major players investing heavily in research and development. Companies are introducing cutting-edge technologies such as high-frequency oscillatory ventilation (HFOV) and smart ventilators equipped with real-time monitoring capabilities. These innovations are aimed at enhancing patient outcomes and optimizing care.
3. Government Support
Government initiatives to improve maternal and child health also contribute to market growth. Policies promoting neonatal care and funding for advanced medical equipment further support the adoption of neonatal ventilators in hospitals across the region.
Europe
1. Rising Demand for Advanced Care
Europe is experiencing a surge in demand for neonatal ventilators, driven by an increasing awareness of neonatal care standards and guidelines. Countries like Germany, France, and the UK are at the forefront of adopting advanced ventilation technologies to improve respiratory management in neonates.
2. Focus on Non-Invasive Ventilation
There is a notable trend towards non-invasive ventilation methods, such as Continuous Positive Airway Pressure (CPAP) and High-Flow Nasal Cannula (HFNC). European healthcare providers emphasize these methods to minimize complications associated with invasive ventilation. Manufacturers are responding by developing more effective non-invasive devices.
3. Collaborative Research Efforts
Collaborations between healthcare institutions and medical device manufacturers are common in Europe, leading to the development of tailored solutions for regional needs. Clinical trials and research initiatives are often conducted to validate the efficacy of new technologies, ensuring that advancements are aligned with clinical practice.
Asia-Pacific
1. Emerging Market Potential
The Asia-Pacific region represents a rapidly growing market for neonatal ventilators, driven by an increase in preterm births and improved healthcare infrastructure. Countries like China and India are expanding their neonatal care facilities, leading to higher demand for advanced ventilation solutions.
2. Government Initiatives
Governments in this region are investing in healthcare improvements and neonatal care programs. Initiatives aimed at reducing infant mortality rates are increasing access to neonatal ventilators, particularly in rural and underserved areas.
3. Local Manufacturers
A rise in local manufacturers offering cost-effective neonatal ventilators is enhancing competition in the market. These companies are catering to regional demands by developing affordable, reliable products that meet the specific needs of healthcare providers in developing countries.
Latin America
1. Growth in Healthcare Investment
Latin America is witnessing increased investment in healthcare infrastructure, particularly in neonatal care. Countries like Brazil and Mexico are focusing on improving their NICUs, which is driving the demand for neonatal ventilators.
2. Challenges in Access
Despite the growth potential, challenges such as economic disparities and limited access to advanced medical technologies persist in some areas. Efforts to address these issues through partnerships and funding from international organizations are crucial for improving neonatal care.
3. Awareness and Training
Increasing awareness of the importance of neonatal care is leading to more training programs for healthcare professionals. Enhanced education around the use of neonatal ventilators is essential for improving patient outcomes and ensuring the effective application of technology in clinical settings.
Request a sample PDF for the Neonatal Ventilator Market report
(The sample serves as a general overview and contents of the final report, without actual data. Accessing the facts and figures of the complete report will incur a cost.)
#Neonatal Ventilator Market#Regional Developments in Healthcare#NICU Innovations#High-Frequency Oscillatory Ventilation#Non-Invasive Ventilation Methods
0 notes
Text
Clinical Trial Imaging 2023 Industry Report Potential Growth, Share, Demand And Forecast to 2030
Clinical Trial Imaging Industry Overview
The global clinical trial imaging market size was estimated at USD 1.14 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 7.60% from 2024 to 2030. The market growth is anticipated to be fueled by the growing biotechnology and pharmaceutical sectors, coupled with rising investments in research and development for the creation of new drugs aimed at treating various diseases. Medical imaging plays a pivotal role in advancing the development of innovative life science products.
Gather more insights about the market drivers, restrains and growth of the Clinical Trial Imaging Market
Despite the ever-changing nature of the medical imaging industry, the biotechnology and pharmaceutical industries are showing sustained growth. This is primarily due to the increased investment in medical imaging companies, as well as the occurrence of mergers and acquisitions that involve the incorporation of cutting-edge imaging technologies to facilitate clinical trials for medical devices.
Advancements in technology are bringing substantial improvements to the collection, evaluation, and submission of clinical trial imaging data. Technology-enabled imaging, especially image analysis software, provides various benefits to clinical studies, such as consistency, data accuracy, adaptability, and compliance. For instance, image analysis software is used to direct and manage a reader by analyzing imaging time points. In addition, the increased use of imaging technology, along with the enhanced power of computing, is expected to drive the usage of imaging in clinical trials. The Quantitative Imaging Biomarkers Alliance (QIBA) protocol has come up with standardized methods and imaging procedures with uniform procedures to be implemented for attaining statistical and precise endpoints in clinical trials.
The Covid-19 pandemic has adversely impacted the healthcare system in most countries, leading to a disruption in medical studies, and research activities, and reduced sponsorship for research involving clinical trials. The pandemic hampered the clinical trial timeline as numerous ongoing studies were delayed and planned studies were cancelled. Unfavorable changes in regulations and guidelines, supply chain disruption, recruitment challenges for clinical trials, fear of viral spread, and shutting down of most manufacturers during lockdown have adversely impacted the market. However, introducing virtual imaging trials during the COVID-19 pandemic is expected to open new avenues for adopting these devices. The development of advanced computational models helps better assess CT and radiography images, which are expected to help in the early diagnosis of COVID-19 patients. The market has witnessed a bounce back by 2022 Q2 due to increased R&D activities and improvement in supply and distribution channels.
Many patents have been filed in the realm of enhancing image evaluation and capturing. In addition, imaging core lab provider’s offer patented technologies that are anticipated to assist pharmaceutical companies in reducing their development timelines. As an example, IXICO provides a diagnostic tool called Assessa, which enhances decision-making in clinical trials for conditions related to memory, including schizophrenia, Parkinson's, and Alzheimer's disease, as well as neurological disorders such as dementia and cognitive impairment.
However, the high cost of machinery and their installation, and the enormous cost of clinical trials may limit the market growth during the forecast periods. Advancements in technology are bringing substantial improvements to the collection, evaluation, and submit clinical trial imaging data. Technology-enabled imaging especially image analysis software provides various benefits to clinical studies such as consistency, data accuracy, adaptability as well as compliance. For instance, image analysis software is used to direct and manage a reader via analysis of imaging time points.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global mobile stroke unit market size was valued at USD 35.80 million in 2023 and is projected to grow at a CAGR of 5.2% from 2024 to 2030. Rising incidence of strokes increased focus on early treatment of stroke patients are driving the demand for efficient and timely stroke care services.
• The global brain tumor diagnosis and therapeutics market size was valued at USD 3.11 billion in 2023 and is projected to grow at a CAGR of 7.1% from 2024 to 2030. The growing launches of brain tumor therapeutics products and the rise in cancer awareness for brain tumor medications drive the market over the forecast period.
Clinical Trial Imaging Market Segmentation
Grand View Research has segmented the clinical trial imaging market on the basis of on service, modality, application, end-use and region:
Clinical Trial Imaging Service Outlook (Revenue, USD Million, 2018 - 2030)
• Clinical Trial Design and Consultation Services
• Reading and Analytical Services
• Operational Imaging Services
• System and Technology Support Services
• Project and Data Management
Clinical Trial Imaging Modality Outlook (Revenue, USD Million, 2018 - 2030)
• Computed Tomography
• Magnetic Resonance Imaging
• X-Ray
• Ultrasound
• Optical Coherence Tomography (OCT)
• Others
Clinical Trial Imaging Application Outlook (Revenue, USD Million, 2018 - 2030)
• NASH
• CKD
• Diabetes
• Cardiovascular Diseases
• Ophthalmology
• Musculoskeletal
• Oncology
• Gastroenterology
• Pediatrics
• Others
Clinical Trial Imaging End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Biotechnology and Pharmaceutical companies
• Medical Devices Manufacturers
• Academic and Government Research Institutes
• Contract Research Organizations (CROs)
• Others
Clinical Trial Imaging Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o India
o China
o Japan
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East & Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Clinical Trial Imaging Market Intelligence Study, published by Grand View Research.
Key Companies profiled:
• IXICO plc
• Navitas Life Sciences
• Resonance Health
• ProScan Imaging
• Radiant Sage LLC
• Medpace
• Biomedical Systems Corp
• Cardiovascular Imaging Technologies
• Intrinsic Imaging
• BioTelemetry
Recent Developments
• In March 2023, Clario launched a cloud-based image viewer specifically for clinical trials. This innovation aims to streamline medical image analysis and improve its accessibility within the clinical research context
• In May 2023, Cleerly has partnered with ProScan Imaging to provide personalized solutions for cardiac health, which involve analyzing and devising treatment strategies for cardiovascular issues. The partnership is expected to leverage Cleerly's AI-powered platform to examine coronary CT angiography (CCTA) images
0 notes
Text
Alzheimer's Therapeutics Market 2024 Size, Share, Business Overview, Trends and Forecast to 2032
The global Alzheimer's therapeutics market, valued at USD 3.78 billion in 2023, is projected to experience significant growth over the coming years, reaching a market size of USD 13.42 billion by 2032. This represents a compound annual growth rate (CAGR) of 15.14% from 2024 to 2032, driven by the increasing prevalence of Alzheimer’s disease, advancements in therapeutic treatments, and ongoing research into disease-modifying therapies.
Alzheimer's disease, a progressive neurodegenerative disorder, affects millions of people worldwide, posing a major health challenge as populations age. The Alzheimer’s therapeutics market includes drugs, therapies, and treatments aimed at managing symptoms, slowing disease progression, and improving patient quality of life.
Key Market Drivers
Growing Prevalence of Alzheimer’s Disease: The rising incidence of Alzheimer’s disease, particularly in aging populations, is a primary driver of market growth. According to the World Health Organization (WHO), the number of people living with dementia globally is expected to triple by 2050, with Alzheimer’s accounting for 60-70% of these cases. As the disease burden grows, so does the need for effective therapeutic solutions.
Advances in Disease-Modifying Therapies: Significant progress is being made in developing disease-modifying therapies (DMTs) aimed at slowing or halting the progression of Alzheimer’s. Traditional treatments primarily focused on symptom management, but new research is paving the way for therapies that target underlying mechanisms of the disease, such as beta-amyloid and tau protein buildup in the brain. These advancements have the potential to reshape the therapeutic landscape, offering hope for more effective long-term treatment options.
Increase in Government and Private Funding for Research: Governments and private organizations worldwide are investing heavily in Alzheimer’s research. Large-scale initiatives like the U.S. National Plan to Address Alzheimer's Disease and significant funding allocations from organizations like the Alzheimer’s Association are driving research efforts, leading to breakthroughs in therapeutics. This financial backing is crucial for advancing clinical trials and bringing new treatments to market.
Rising Demand for Innovative Treatment Options: With the growing number of patients diagnosed with Alzheimer’s, there is a strong demand for novel treatment options that go beyond traditional drug therapies. Innovations in biologics, gene therapy, and immunotherapy are emerging as promising approaches to treating the disease, providing new avenues for market growth.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4481
Challenges and Opportunities
Despite the rapid growth potential, the Alzheimer's therapeutics market faces challenges, including the high failure rate of drug trials and the complexity of the disease. Many late-stage clinical trials for Alzheimer’s treatments have been unsuccessful, presenting a major hurdle for pharmaceutical companies. However, advancements in understanding the biology of the disease and the development of biomarkers for early diagnosis are expected to mitigate these challenges in the long term.
The increasing use of artificial intelligence (AI) and machine learning (ML) in drug discovery and development is also opening new opportunities. AI-powered platforms can accelerate the identification of therapeutic targets and optimize clinical trial processes, potentially reducing the time and cost involved in bringing new Alzheimer’s therapies to market.
Regional Insights
North America dominates the Alzheimer’s therapeutics market, accounting for the largest market share due to its strong healthcare infrastructure, high awareness levels, and significant investment in research. The region is home to several leading pharmaceutical companies actively engaged in developing Alzheimer’s treatments, alongside a supportive regulatory environment.
Europe holds the second-largest market share, driven by rising healthcare spending and increased government initiatives for Alzheimer’s care. Meanwhile, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period. This growth is attributed to an aging population, growing healthcare infrastructure, and a rising awareness of neurodegenerative diseases across countries like China, Japan, and South Korea.
Future Outlook
The future of the Alzheimer’s therapeutics market looks promising, with the anticipated introduction of next-generation therapies and personalized treatment approaches. As research efforts intensify and more disease-modifying therapies progress through clinical trials, the market is set to expand at a significant rate. Additionally, the development of early diagnostic tools and combination therapies is expected to enhance treatment outcomes, further driving market growth.
In conclusion, the Alzheimer’s therapeutics market is on the cusp of a major breakthrough, with a projected growth from USD 3.78 billion in 2023 to USD 13.42 billion by 2032. The rising prevalence of Alzheimer’s, combined with advancements in therapeutic research and government initiatives, will propel the market forward, offering new hope for patients and their families affected by this debilitating disease.
0 notes
Text

How Can Blockchain Revolutionize the Future of Healthcare and Pharmaceuticals?
Blockchain technology is changing many domains, and health care is no different. In this sector, the data security issues, inefficiencies, and skyrocketing costs bring a decentralized, transparent platform with blockchain. This blog shall focus on how blockchain technology is changing healthcare-expecially in pharmaceuticals-and companies like Justtry Technologies, which are helping unlock the potential of blockchain in this sector.
Why Blockchain in Health Care?
Blockchain makes use of distributed data and is, therefore, decentralized in that it has no central entity controlling the data. This can be invaluable in the healthcare industry, with sensitive patient information and, indeed, clinical trial data that must be kept secure. Blockchain does this by keeping healthcare data tamper-proof, thus instilling trust and security into the whole system.
Some major benefits of blockchain in health care are as follows:
More Security: Blockchain guards against cyberattacks and unauthorized access to the patient's information; therefore, health data remains private and secure.
Transparency in Data Sharing: Blockchain provides a hassle-free and reliable sharing of a patient's records with hospitals, doctors, and insurance companies.
It Prevents Fraud: Blockchain does not allow alterations in clinical trial data, and hence the integrity of research is kept intact.
Operational Cost Efficiency: It spares the healthcare providers from operational costs mainly because it saves healthcare providers from the hassle of going through intermediaries and automates administrative tasks.
At Justtry Technologies, we help healthcare organizations integrate blockchain solutions that provide data security, transparency, and operational efficiency.
How Blockchain is Applied in Healthcare?
Blockchain is being used in various critical areas of healthcare:
Patient Records: Blockchain allows patients who are choosing to share their medical histories securely to choose who can access the documents. This increases data privacy because the patients' personal information is kept anonymous. It also facilitates easy transfer of information among different healthcare providers.
One of the biggest hurdles in pharma is drug authenticity. Blockchain allows the validation of the entire journey of the medicine - its creation to its delivery - to restrict entrance of counterfeits in the market.
Applications in Clinical Trials: Blockchain makes sure the clinical trial data is held immutable with active transparency. Trust in the process is built because no one can manipulate or alter the data as part of clinical trials.
Blockchain in Medicine: Solving the Key Pharma Challenges
The pharmaceutical company is presently being faced by quite a few challenges, including fake drugs, strict regulatory compliance, and a long drug development process. Blockchain offers solutions to such problems, catering to safe data management and real-time insights.
A number of key applications related to blockchain within the pharmaceuticals include:
Drug Traceability: Using blockchain, a pharmaceutical company can trace their drugs all along the supply chain, meaning that with blockchain assurance, they know that their drugs are not tampered with, meaning that they, indeed, meet regulatory standards.
Personalized Medication: Blockchain sees to it that data is conveyed between parties, hence allowing for more accurate and bespoke treatments considering patient information.
Clinical Trial Data Integrity: Blockchain means that clinical trial data is secure and transparent. This helps in ensuring regulation compliance as well as safeguarding research integrity.
Justtry Technologies is integrating blockchain technology to help pharmaceutical companies improve security levels, efficiency, and speed in the entire development of drugs.
Blockchain Health Data: Security and Compliance
Healthcare organizations still face threats regarding security over health data; blockchain offers the best solution by giving decentralized, transparent, and immutable data storage. This not only enhances security but also assists in compliance to regulations on data privacy.
Blockchain allows healthcare organizations to ensure:
Secure Data Storage: Patient records are stored in an immutable format that cannot be altered to ensure data integrity.
Regulatory Compliance: Blockchain provides an easy, auditable trail of how the data accessed is utilized, thus significantly aiding such regulations as HIPAA in terms of compliance.
Trust and Transparency: Patients, healthcare providers, and regulators will trust that the data being put in blockchain is accurate and not tampered.
Conclusion: Future of Healthcare with Blockchain
Blockchain is unlocking the future of healthcare, some of the very tough challenges that the industry faces. From safeguarding patient data to improving the speed of the pharmaceutical supply chain, blockchain stands as the game changer. At its core lies the exciting innovative measure of stand-alone leader Justtry Technologies in revolutionizing the use of blockchain solutions tackling innovation and improvement in health care and pharmaceutical.
#blockchain in health sector#blockchain development services#blockchain development#blockchaintechnology#blockchain technologies#blockchain in medicine#blockchain in healthcare#blockchain development companies#blockchain development company in US
0 notes
Text
The Growing Importance of Pharma Contract Manufacturing Services

In today’s highly competitive pharmaceutical landscape, companies are continuously seeking ways to improve efficiency, reduce costs, and accelerate time-to-market for their products. One increasingly popular solution is leveraging pharma contract manufacturing services (CMO), where a pharmaceutical company outsources some or all of its manufacturing processes to a specialized third party. These partnerships are transforming the industry, allowing both large and small pharmaceutical firms to focus on innovation while CMOs take care of the complexities of production.
In this blog, we’ll explore the growing role of pharma contract manufacturing services, their benefits, and the future of the CMO market.
What Are Pharma Contract Manufacturing Services?
Pharma contract manufacturing refers to the outsourcing of the production of pharmaceutical products to third-party manufacturers. CMOs offer a wide range of services, including the production of Active Pharmaceutical Ingredients (APIs), finished dosage forms, packaging, labeling, and even regulatory support. These companies are equipped with specialized facilities, cutting-edge technology, and in-depth regulatory knowledge to handle all aspects of drug production.
The Benefits of Pharma Contract Manufacturing
Cost Efficiency Developing and maintaining in-house manufacturing facilities is extremely costly. By outsourcing production, pharmaceutical companies can significantly reduce capital investment in equipment, infrastructure, and personnel. CMOs offer economies of scale that make production more cost-effective, particularly for smaller companies or those developing niche products.
Faster Time-to-Market Speed is critical in the pharmaceutical industry, especially with the pressure to introduce new drugs to the market quickly. CMOs offer expertise, established manufacturing processes, and regulatory knowledge that help companies avoid delays in production and approval processes. This allows pharmaceutical companies to get their products into the hands of patients faster.
Access to Expertise and Advanced Technology Many CMOs have specialized capabilities and cutting-edge technology that may be too expensive or complex for pharmaceutical companies to maintain in-house. For example, CMOs often have facilities dedicated to advanced techniques such as biologics manufacturing, high-potency API production, or sterile manufacturing. Pharmaceutical companies can benefit from this expertise without needing to invest in these resources themselves.
Scalability One of the key advantages of partnering with a CMO is scalability. Whether a company needs to produce a small batch for clinical trials or scale up for full-scale commercial production, CMOs offer flexible solutions that adapt to changing demands. This flexibility helps pharmaceutical firms manage production without over-committing resources.
Focus on Core Competencies By outsourcing manufacturing, pharmaceutical companies can concentrate on their core competencies such as research and development (R&D), marketing, and sales. This focus on innovation can lead to the development of new drugs, therapies, and treatments, without the distraction of managing complex production processes.
Challenges Faced by Pharma CMOs
While the benefits are clear, pharma contract manufacturing does come with some challenges:
Quality Control Ensuring that the CMO maintains the same quality standards as the pharmaceutical company is critical. Any deviation in manufacturing processes or contamination can lead to recalls, regulatory scrutiny, and damage to the company’s reputation.
Regulatory Compliance Pharmaceutical production is heavily regulated, and CMOs must adhere to the same strict regulatory requirements as the company they are manufacturing for. This includes meeting FDA, EMA, and other global standards. Any failure to comply with regulations can result in costly delays or product rejections.
Intellectual Property (IP) Concerns Some pharmaceutical companies may have concerns about sharing proprietary formulations and processes with a third party. While CMOs typically have strong IP protection measures in place, trust and legal safeguards are essential in these partnerships.
The Future of Pharma Contract Manufacturing
The global pharmaceutical contract manufacturing market is expected to grow significantly in the coming years. Several trends are driving this growth:
Biologics and Biosimilars: As biologics and biosimilars become more prevalent, specialized CMOs are becoming increasingly important. Manufacturing biologics is more complex than traditional pharmaceuticals, requiring advanced technology and expertise.
Specialty Drugs: The rise of personalized medicine and specialty drugs is creating opportunities for CMOs that can handle niche and small-batch manufacturing.
Increased Outsourcing in Emerging Markets: Many pharmaceutical companies are looking to CMOs in emerging markets such as India and China to take advantage of lower costs and faster turnaround times. These markets are also seeing investments in new facilities and advanced technologies to meet global standards.
Conclusion
Pharma contract manufacturing services have become a critical component of the pharmaceutical industry’s strategy to remain competitive, agile, and cost-effective. By outsourcing production to specialized CMOs, pharmaceutical companies can reduce costs, accelerate time-to-market, and focus on innovation. As the demand for complex biologics and specialty drugs continues to grow, so too will the role of CMOs in shaping the future of healthcare.
Pharma companies and CMOs must continue to work collaboratively to ensure that they meet regulatory standards and maintain the highest quality, delivering safe and effective drugs to the market swiftly and efficiently.
0 notes
Text
Shriram Pharmacy College: Top Pharmacy Degree Choice IN India

At Shriram Pharmacy College, students delve into the fascinating world of pharmacology, studying the effects of drugs on the human body. The curriculum covers drug mechanisms, therapeutic uses, and potential side effects, providing a comprehensive understanding of how medications interact with biological systems. This foundational knowledge is crucial for anyone pursuing a career in pharmacy, as it lays the groundwork for effective medication management and patient care.
## Focus on Pharmaceutical Science
Shriram Pharmacy College emphasizes pharmaceutical science, a core component of its curriculum. This includes studying drug formulation, chemistry, and the physical properties of drugs. Students gain insights into the scientific principles behind drug development and manufacturing processes, equipping them with the knowledge needed to contribute to advancements in pharmaceutical technology and innovation.
## Learn About Medication Development
Understanding medication development is a key aspect of the pharmacy program at Shriram. Students explore the entire lifecycle of drug development, from discovery and preclinical testing to clinical trials and regulatory approval. This comprehensive approach ensures that graduates are well-prepared to participate in or oversee the development of new medications, playing a crucial role in improving patient health outcomes.
## Explore Drug Interactions Deeply
Drug interactions can significantly impact patient safety and treatment efficacy. At Shriram Pharmacy College, students learn to analyze and manage potential drug interactions, including those between prescription medications, over-the-counter drugs, and dietary supplements. This knowledge is essential for ensuring that patients receive safe and effective treatment plans tailored to their individual needs.
## Understand Patient Care Principles
Patient care is at the heart of pharmacy practice. Shriram Pharmacy College emphasizes the importance of patient-centered care, teaching students how to communicate effectively with patients, understand their needs, and provide personalized medication management. The curriculum includes training in empathy, ethical considerations, and the role of pharmacists in supporting overall patient wellness.
## Engage in Practical Lab Work
Hands-on experience is a crucial part of the pharmacy education at Shriram. The college provides state-of-the-art laboratory facilities where students can practice their skills in drug formulation, analysis, and testing. Practical lab work reinforces theoretical knowledge and prepares students for real-world challenges in the pharmacy profession.
## Master Pharmacy Industry Essentials
Shriram Pharmacy College prepares students to excel in the pharmacy industry by covering essential topics such as pharmaceutical marketing, regulations, and management. Students learn about industry trends, business practices, and the regulatory environment, equipping them with the skills needed to thrive in various pharmacy settings, including retail, clinical, and research environments.
## Prepare for Diverse Career Paths
A degree from Shriram Pharmacy College opens doors to a wide range of career opportunities. Graduates are well-prepared for roles in community and hospital pharmacies, pharmaceutical industry positions, research, and academia. The college’s comprehensive curriculum ensures that students are equipped with the knowledge and skills needed to pursue diverse career paths within the pharmacy field.
/media/08c4d6262b56bfa12db40a0612a1baf6
## FAQ
**1. What makes Shriram Pharmacy College a top choice in India?**
Shriram Pharmacy College is renowned for its comprehensive curriculum, state-of-the-art facilities, and focus on practical experience. The college offers in-depth education in drug effects, pharmaceutical science, and patient care, preparing students for diverse career paths in the pharmacy field. Its emphasis on both theoretical knowledge and hands-on training ensures that graduates are well-equipped for success in their careers.
**2. How does Shriram Pharmacy College prepare students for real-world pharmacy practice?**
Shriram Pharmacy College prepares students for real-world practice through its extensive practical lab work and hands-on training. Students engage in drug formulation, analysis, and testing in state-of-the-art laboratories, reinforcing theoretical knowledge. The curriculum also includes patient care principles, drug interaction management, and industry essentials, ensuring that graduates are well-prepared for various pharmacy settings.
**3. What career opportunities are available for graduates of Shriram Pharmacy College?**
Graduates of Shriram Pharmacy College have a wide range of career opportunities available, including roles in community and hospital pharmacies, pharmaceutical industry positions, research, and academia. The college’s comprehensive curriculum and focus on practical experience equip students with the skills needed to pursue diverse career paths and excel in the pharmacy profession.
**4. What aspects of pharmaceutical science are covered in the program?**
The pharmacy program at Shriram Pharmacy College covers various aspects of pharmaceutical science, including drug effects, formulation, chemistry, and medication development. Students study the mechanisms of drug action, therapeutic uses, potential side effects, and the entire drug development process, providing a thorough understanding of the scientific principles behind pharmaceuticals.
**5. How does the college support students in developing patient care skills?**
Shriram Pharmacy College emphasizes patient-centered care by incorporating training in communication, empathy, and ethical considerations into its curriculum. Students learn to understand patient needs, provide personalized medication management, and support overall wellness. This focus on patient care principles ensures that graduates are well-prepared to offer high-quality, compassionate care in their professional roles.
## Conclusion
Shriram Pharmacy College stands out as a premier choice for pharmacy education in India, offering a robust curriculum that covers all aspects of pharmaceutical science. From drug effects and medication development to patient care and industry essentials, the college provides a well-rounded education that prepares students for successful careers in pharmacy. With its emphasis on practical experience and career preparation, Shriram Pharmacy College is an excellent choice for aspiring pharmacists seeking a top-tier education.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
At Shriram Pharmacy College, students delve into the fascinating world of pharmacology, studying the effects of drugs on the human body. The curriculum covers drug mechanisms, therapeutic uses, and potential side effects, providing a comprehensive understanding of how medications interact with biological systems. This foundational knowledge is crucial for anyone pursuing a career in pharmacy, as it lays the groundwork for effective medication management and patient care.
## Focus on Pharmaceutical Science
Shriram Pharmacy College emphasizes pharmaceutical science, a core component of its curriculum. This includes studying drug formulation, chemistry, and the physical properties of drugs. Students gain insights into the scientific principles behind drug development and manufacturing processes, equipping them with the knowledge needed to contribute to advancements in pharmaceutical technology and innovation.
## Learn About Medication Development
Understanding medication development is a key aspect of the pharmacy program at Shriram. Students explore the entire lifecycle of drug development, from discovery and preclinical testing to clinical trials and regulatory approval. This comprehensive approach ensures that graduates are well-prepared to participate in or oversee the development of new medications, playing a crucial role in improving patient health outcomes.
## Explore Drug Interactions Deeply
Drug interactions can significantly impact patient safety and treatment efficacy. At Shriram Pharmacy College, students learn to analyze and manage potential drug interactions, including those between prescription medications, over-the-counter drugs, and dietary supplements. This knowledge is essential for ensuring that patients receive safe and effective treatment plans tailored to their individual needs.
## Understand Patient Care Principles
Patient care is at the heart of pharmacy practice. Shriram Pharmacy College emphasizes the importance of patient-centered care, teaching students how to communicate effectively with patients, understand their needs, and provide personalized medication management. The curriculum includes training in empathy, ethical considerations, and the role of pharmacists in supporting overall patient wellness.
## Engage in Practical Lab Work
Hands-on experience is a crucial part of the pharmacy education at Shriram. The college provides state-of-the-art laboratory facilities where students can practice their skills in drug formulation, analysis, and testing. Practical lab work reinforces theoretical knowledge and prepares students for real-world challenges in the pharmacy profession.
## Master Pharmacy Industry Essentials
Shriram Pharmacy College prepares students to excel in the pharmacy industry by covering essential topics such as pharmaceutical marketing, regulations, and management. Students learn about industry trends, business practices, and the regulatory environment, equipping them with the skills needed to thrive in various pharmacy settings, including retail, clinical, and research environments.
## Prepare for Diverse Career Paths
A degree from Shriram Pharmacy College opens doors to a wide range of career opportunities. Graduates are well-prepared for roles in community and hospital pharmacies, pharmaceutical industry positions, research, and academia. The college’s comprehensive curriculum ensures that students are equipped with the knowledge and skills needed to pursue diverse career paths within the pharmacy field.
/media/08c4d6262b56bfa12db40a0612a1baf6
## FAQ
**1. What makes Shriram Pharmacy College a top choice in India?**
Shriram Pharmacy College is renowned for its comprehensive curriculum, state-of-the-art facilities, and focus on practical experience. The college offers in-depth education in drug effects, pharmaceutical science, and patient care, preparing students for diverse career paths in the pharmacy field. Its emphasis on both theoretical knowledge and hands-on training ensures that graduates are well-equipped for success in their careers.
**2. How does Shriram Pharmacy College prepare students for real-world pharmacy practice?**
Shriram Pharmacy College prepares students for real-world practice through its extensive practical lab work and hands-on training. Students engage in drug formulation, analysis, and testing in state-of-the-art laboratories, reinforcing theoretical knowledge. The curriculum also includes patient care principles, drug interaction management, and industry essentials, ensuring that graduates are well-prepared for various pharmacy settings.
**3. What career opportunities are available for graduates of Shriram Pharmacy College?**
Graduates of Shriram Pharmacy College have a wide range of career opportunities available, including roles in community and hospital pharmacies, pharmaceutical industry positions, research, and academia. The college’s comprehensive curriculum and focus on practical experience equip students with the skills needed to pursue diverse career paths and excel in the pharmacy profession.
**4. What aspects of pharmaceutical science are covered in the program?**
The pharmacy program at Shriram Pharmacy College covers various aspects of pharmaceutical science, including drug effects, formulation, chemistry, and medication development. Students study the mechanisms of drug action, therapeutic uses, potential side effects, and the entire drug development process, providing a thorough understanding of the scientific principles behind pharmaceuticals.
**5. How does the college support students in developing patient care skills?**
Shriram Pharmacy College emphasizes patient-centered care by incorporating training in communication, empathy, and ethical considerations into its curriculum. Students learn to understand patient needs, provide personalized medication management, and support overall wellness. This focus on patient care principles ensures that graduates are well-prepared to offer high-quality, compassionate care in their professional roles.
## Conclusion
Shriram Pharmacy College stands out as a premier choice for pharmacy education in India, offering a robust curriculum that covers all aspects of pharmaceutical science. From drug effects and medication development to patient care and industry essentials, the college provides a well-rounded education that prepares students for successful careers in pharmacy. With its emphasis on practical experience and career preparation, Shriram Pharmacy College is an excellent choice for aspiring pharmacists seeking a top-tier education.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
#pharmacist#pharmacy#hospital#youtube#public health#shriram medical college#medicine#online pharmacy#shriram nursing college#shriram pharmacy college
0 notes
Text
Beyond the Hype: Unveiling the Real Impact of Generative AI in Drug Discovery
New Post has been published on https://thedigitalinsider.com/beyond-the-hype-unveiling-the-real-impact-of-generative-ai-in-drug-discovery/
Beyond the Hype: Unveiling the Real Impact of Generative AI in Drug Discovery
Since Insilico Medicine developed a drug for idiopathic pulmonary fibrosis (IPF) using generative AI, there’s been a growing excitement about how this technology could change drug discovery. Traditional methods are slow and expensive, so the idea that AI could speed things up has caught the attention of the pharmaceutical industry. Startups are emerging, looking to make processes like predicting molecular structures and simulating biological systems more efficient. McKinsey Global Institute estimates that generative AI could add $60 billion to $110 billion annually to the sector. But while there’s a lot of enthusiasm, significant challenges remain. From technical limitations to data quality and ethical concerns, it’s clear that the journey ahead is still full of obstacles. This article takes a closer look at the balance between the excitement and the reality of generative AI in drug discovery.
The Hype Surrounding Generative AI in Drug Discovery
Generative AI has captivated the imagination of the pharmaceutical industry with its potential to drastically accelerate the traditionally slow and expensive drug discovery process. These AI platforms can simulate thousands of molecular combinations, predict their efficacy, and even anticipate adverse effects long before clinical trials begin. Some industry experts predict that drugs that once took a decade to develop will be created in a matter of years, or even months with the help of generative AI.
Startups and established companies are capitalizing on the potential of generative AI for drug discovery. Partnerships between pharmaceutical giants and AI startups have fueled dealmaking, with companies like Exscientia, Insilico Medicine, and BenevolentAI securing multi-million-dollar collaborations. The allure of AI-driven drug discovery lies in its promise of creating novel therapies faster and cheaper, providing a solution to one of the industry’s biggest challenges: the high cost and long timelines of bringing new drugs to market.
Early Successes
Generative AI is not just a hypothetical tool; it has already demonstrated its ability to deliver results. In 2020, Exscientia developed a drug candidate for obsessive-compulsive disorder, which entered clinical trials less than 12 months after the program started — a timeline far shorter than the industry standard. Insilico Medicine has made headlines for discovering novel compounds for fibrosis using AI-generated models, further showcasing the practical potential of AI in drug discovery.
Beyond developing individual drugs, AI is being employed to address other bottlenecks in the pharmaceutical pipeline. For instance, companies are using generative AI to optimize drug formulations and design, predict patient responses to specific treatments, and discover biomarkers for diseases that were previously difficult to target. These early applications indicate that AI can certainly help solve long-standing challenges in drug discovery.
Is Generative AI Overhyped?
Amid the excitement, there is growing skepticism regarding how much of generative AI’s hype is grounded versus inflated expectations. While success stories grab headlines, many AI-based drug discovery projects have failed to translate their early promise into real-world clinical results. The pharmaceutical industry is notoriously slow-moving, and translating computational predictions into effective, market-ready drugs remains a daunting task.
Critics point out that the complexity of biological systems far exceeds what current AI models can fully comprehend. Drug discovery involves understanding an array of intricate molecular interactions, biological pathways, and patient-specific factors. While generative AI is excellent at data-driven prediction, it struggles to navigate the uncertainties and nuances that arise in human biology. In some cases, the drugs AI helps discover may not pass regulatory scrutiny, or they may fail in the later stages of clinical trials — something we’ve seen before with traditional drug development methods.
Another challenge is the data itself. AI algorithms depend on massive datasets for training, and while the pharmaceutical industry has plenty of data, it’s often noisy, incomplete, or biased. Generative AI systems require high-quality, diverse data to make accurate predictions, and this need has exposed a gap in the industry’s data infrastructure. Moreover, when AI systems rely too heavily on historical data, they run the risk of reinforcing existing biases rather than innovating with truly novel solutions.
Why the Breakthrough Isn’t Easy
While generative AI shows promise, the process of transforming an AI-generated idea into a viable therapeutic solution is a challenging task. AI can predict potential drug candidates but validating those candidates through preclinical and clinical trials is where the real challenge begins.
One major hurdle is the ‘black box’ nature of AI algorithms. In traditional drug discovery, researchers can trace each step of the development process and understand why a particular drug is likely to be effective. In contrast, generative AI models often produce outcomes without offering insights into how they arrived at those predictions. This opacity creates trust issues, as regulators, healthcare professionals, and even scientists find it difficult to fully rely on AI-generated solutions without understanding the underlying mechanisms.
Moreover, the infrastructure required to integrate AI into drug discovery is still developing. AI companies are working with pharmaceutical giants, but their collaboration often reveals mismatched expectations. Pharma companies, known for their cautious, heavily regulated approach, are often reluctant to adopt AI tools at a pace that startup AI companies expect. For generative AI to reach its full potential, both parties need to align on data-sharing agreements, regulatory frameworks, and operational workflows.
The Real Impact of Generative AI
Generative AI has undeniably introduced a paradigm shift in the pharmaceutical industry, but its real impact lies in complementing, not replacing, traditional methods. AI can generate insights, predict potential outcomes, and optimize processes, but human expertise and clinical testing are still crucial for developing new drugs.
For now, generative AI’s most immediate value comes from optimizing the research process. It excels in narrowing down the vast pool of molecular candidates, allowing researchers to focus their attention on the most promising compounds. By saving time and resources during the early stages of discovery, AI enables pharmaceutical companies to pursue novel avenues that may have otherwise been deemed too costly or risky.
In the long term, the true potential of AI in drug discovery will likely depend on advancements in explainable AI, data infrastructure, and industry-wide collaboration. If AI models can become more transparent, making their decision-making processes clearer to regulators and researchers, it could lead to a broader adoption of AI across the pharmaceutical industry. Additionally, as data quality improves and companies develop more robust data-sharing practices, AI systems will become better equipped to make groundbreaking discoveries.
The Bottom Line
Generative AI has captured the imagination of scientists, investors, and pharmaceutical executives, and for good reason. It has the potential to transform how drugs are discovered, reducing both time and cost while delivering innovative therapies to patients. While the technology has demonstrated its value in the early phases of drug discovery, it is not yet prepared to transform the entire process.
The true impact of generative AI in drug discovery will unfold over the coming years as the technology evolves. However, this progress depends on overcoming challenges related to data quality, model transparency, and collaboration within the pharmaceutical ecosystem. Generative AI is undoubtedly a powerful tool, but its true value depends on how it’s applied. Although the current hype may be exaggerated, its potential is genuine — and we are only at the beginning of discovering what it can accomplish.
#ADD#adoption#ai#AI for molecular prediction#AI in biotechnology#AI in drug development#AI models#AI platforms#AI systems#ai tools#Algorithms#applications#approach#Article#Artificial Intelligence#attention#billion#Biology#biomarkers#biotechnology#black box#box#challenge#change#Collaboration#Companies#complexity#data#data quality#data-driven
0 notes