#oxidizability
Text
Come here boy
Did you go and vaporize something in a structure fire
I live with Jesus Christ Himself. He's cool as shit but he doesn't like many people either.
#your pathetic oxidizable monoliths mean nothing poof gone#oh wow a big metal and concrete structure#some electric hand arrives to choke it out
0 notes
Text
People are like, if stomach acid is strong enough to dissolve razor blades then why do corn kernels survive? Because they were made to!! Seed jackets were made to get slightly burned away in the stomach to expose the actual seed to be planted when it was pooped out. BUT METAL LIKES TO HOLD HANDS!!! MOST ARE EASILY OXIDIZABLE AND WANNA SWIM WITH THE LOOSE HYDROGEN. THEY GET SO HAPPY THEY DISAPPEAR
81 notes
·
View notes
Text
A combustible composition for generating aerosols for the control and modication of weather conditions consisting of a readily oxidizable substance selected from the group consisting of aluminum, magnesium, alkalimetals and alkaline earth metals.
#Stratospheric Aerosol Injection#Geoengineering#EMF#Weather Modification#Climate Control#Skygazer#Sky over Germany#1st October 2023
7 notes
·
View notes
Note
*Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidizes you* *Reduces you* *Oxidiz
who spells oxidise with a fucking z
13 notes
·
View notes
Text
Ozonation for home aquariums

Ozonation for home aquariums has now become quite a feasible procedure for indoor aquarium enthusiasts. Is there a need for it? What difficulties may arise? Is this technology dangerous? Let's understand.
The use of ozone has long been a standard practice in industrial and public water treatment plants. This method is also used in the filtration of water masses in large exhibition aquariums. The popularity is due to the best efficiency, preservation of water quality, and conservation of water resources. The biggest problem with closed water systems is the accumulation of dissolved organic waste from various biological sources, such as waterfowl waste, decomposing products and plant material.
In aquariums of all sizes, mechanical filtration removes organic and inorganic solids. Biological filters sorb dissolved organic materials (ammonia and nitrite). But closed bodies of water retain large amounts of other dissolved and colloidal organic matter that accumulate over time. As a result, the most noticeable manifestation of pollution is a change in the color and odor of the water.
This is usually solved by a physical water change. Or by means of chemical absorption. Frequent water changes can be easily accomplished in small aquariums. This method is not practical for removing contaminants in large volumes. Using chemical absorbents is expensive. And it does not always remove unwanted dissolved organics. The use of ozone will help to solve the problem.
What is ozone. How does it remove organic molecules?
Ozone is a highly reactive form of oxygen. It is made up of three oxygen atoms (O3). Ozone is very unstable and short-lived. It is this instability that is an advantage for use as a strong oxidizing agent."Ordinary" stable oxygen, which is found in air and water, has two oxygen atoms (O2).
When ozone molecules break down, they lose one atom to form stable "normal" oxygen molecules, releasing free oxygen atoms. It is these free oxygen atoms that combine with dissolved organic compounds.
This process, in turn, causes them to break down into simpler forms that are consumed by heterotrophic bacteria, or recombine into forms that can be removed by mechanical filtration or by the protein skimming process. An organic molecule that has gained a free oxygen atom and then broken down is called oxidized.
Where does ozone come from? How is it used in aquariums?
Ozone is an extremely unstable gas. It cannot be stored or purchased. The element lasts for a few seconds before it disintegrates. Ozone is generated by devices called ionizers or ozone generators. Most modern devices used for the aquarium hobby use a corona discharge method to create ozone.
In them, air is passed through a strong electric field. This separates atmospheric oxygen (O2) into individual atoms. Some of these atoms combine with molecular oxygen (O2) under the high voltage of the electric field to form ozone (O3). The resulting gas must be used up quickly before it turns back into regular oxygen.
Owners of marine aquariums already have ozonization devices - flotation devices. Ozone needs some time to affect oxidizable substances. The air inlet of the skimmer can become a highly effective contact chamber into which the ozone particles enter. It is only necessary to ensure that the skimmer and air supply tubes are made of materials that can withstand contact with ozone.
Some plastics and rubber can be damaged by O3 and this will cause leakage or breakage if exposed to the gas for long periods of time. Ionizers are also available, but they are difficult to use and harder to find.
What amount of ozone is safe for an aquarium?
ORP controller
The best way to monitor and control the amount of ozone is to use a redox potential (ORP) controller. It measures the electrical voltage in millivolts (mV) and indicates the oxidation capacity of water. As the amount of ozone increases, the ORP level also increases.
Seawater has an ORP at 350-400 mV. An ORP of 200 or less in an aquarium signals a low oxygen level. It also indicates a high content of dissolved organics. By keeping the ORP level between 250-350 mV, the ozone level can be adjusted accordingly. The use of a controller simplifies the process and allows the ozone generator to be turned off when the desired level is reached. The ORP should never be exceeded above 400 mV.
Most ozone generator manufacturers recommend a dosage of 5-15 mg/hour per 100 liters. You can buy ozone generators of any size, from small to super powerful. However, you should not chase after high-performance devices. Low-performance units will provide the best results in most cases. An overdose of ozone can be harmful to humans and aquarium inhabitants. There are several ways to make sure that the correct amount of ozone is filling a body of water.
When supplying ozone to the flotator, it is important that the gas decomposes in the chamber or exits through the top of the skimmer. Free ozone in the aquarium oxidizes organic material. This can cause damage to gills in fish and tissue damage in invertebrates. High concentrations of ozone in the air are harmful to human lungs.
Is ozonation safe for home aquariums?
Most aquarium hobby devices do not produce dangerous levels of ozone. You can use activated carbon in the chamber or in the top of the flotator. It will absorb the excess ozone. A test kit is also helpful to make sure that ozone is not leaving the reaction chamber of the aquarium.
Ozone in large quantities can act as a catalyst for some harmful compounds, such as hyperchloric and hyperbromic acids. Therefore, an ORP level of 400 mV is not recommended to be exceeded. It is not a good idea to use ozone in small enclosed rooms. A well ventilated room for an aquarium is the ideal place when using an ozone meter.
Unless an ORP meter or controller is used, then a conservative approach should be used. 5 mg/hour per 100 liters is a safe volume.
Another precaution when using ozone is to use dry air. Make sure that the oxygen stream supplied to the ozonator is free of moisture. Water will form nitric acid when it comes in contact with ozone. This will not only damage the equipment. But also to lower the pH of the water in the pond. Regenerable desiccant beads are used to dehumidify the air.
Benefits of ozonation for a home aquarium?
Clear water is the #1 reason for most people who use ozone machines. Dissolved organics discolor the water, ozone oxidizes and purifies it. This is especially useful for reef aquariums where lighting is crucial. Many people don't even realize how cloudy the water is until they see the result of ozone. This gas also has disinfecting properties.
Pathogenic bacteria, single-celled parasites, greens, and viruses are destroyed by contact with ozone. Many species of living things secrete special protective substances. These are designed to protect them from predators or competitors. The substances build up over time and become a problem for the ecosystem. They are also destroyed by ozone.
It neutralizes pesticides, detergents, and many other toxins that can enter tap water. Ammonia and nitrites are oxidized into less harmful nitrate when exposed to ozone. And, as mentioned earlier, the use of ozone can reduce the amount of water that needs to be changed in closed systems.
Read the full article
0 notes
Text
0 notes
Text
Blighted creation
Oxidizability
Tragic irony
🤖💭⚡🐑
youtube
0 notes
Photo

50 posts!
Premium Quality, Latest Design in Trend, 48 Hours Guaranteed Dispatch, Worldwide Delivery, Fast Selling Out, Wholesale Pricing Only
#50 posts#tumblr milestone#necklacesete enameljhumkis mattechokersets fashionjewelry valentinesdaygift jewelrygiftforwife necklacesetforgirlfriend bridaljewelry oxidiz
1 note
·
View note
Photo

Esperando 2020, #lagunaesmeralda #melipillachile #oxidizforever #oxidiz (en Laguna Esmeralda) https://www.instagram.com/p/B6wEy5xh9WqxcLfAzwBw55sI-TgkPiOLO_c7t80/?igshid=3z92cwudjans
6 notes
·
View notes
Text
Purifying Water – For Ourselves, and Every Other Creature
When it comes to water, most people’s immediate concern is whether it is clean. A valid point for sure. But what do we actually mean by “clean”? Is it safe to drink? Or suitable for washing our bodies, clothes, and dishes? Or do we mean that it’s not contaminated in a way that would kill our plants if we poured it on them? Because there is a world of difference between each of these levels of “clean”.

image source
Discussing Dirty Water to Define Clean Water
To talk about the purity of water, we have to identify the four types of contamination: Firstly, there are solid particles suspended in the water, which could be anything from pieces of rocks to organic matter, and even plastics. The good news here is that once the water evaporates all of these particles stay behind. Also, should they get back into the water, they can be removed easily with a filter.
The next type of pollutant is organic, such as phosphorous or nitrogen. These are the result of biological contamination from urine, manure, or decomposing bodies. While it may be not as simple to get them out as using a filter, these substances are oxidizable, meaning that they can be removed by bacteria, provided there is sufficient oxygen in the water.
A more complicated form of contaminants is microbial pollution, meaning various forms of microbial life living on organic pollution in the water. While many of these microbes are harmless to humans, some of them are pathogenic, and can cause serious or even deadly diseases to our bodies. There are a number of approaches in dealing with these contaminants, which mostly result in either killing them off, or outcompeting them.
Finally, there is the chemical form of water pollution. This involves other harmful substances in our water, such as residues from medicines, pesticides, hydrocarbons, heavy metals, etc. These contaminants are usually the result of human activity, such as industry or intensive agriculture. Because of their toxicity and low biodegradability they pose the greatest challenge to purifying water on any scale.
How Clean Do We Want Our Water To Be?
What a silly question, you might say. Of course we want our water to be 100% clean, all the time! But is that really so? For example, do you really need the water to be potable, only to flush your toilet with it? Or think about a swimming pool: It’s clean enough to swim in, but I’m sure you wouldn’t want to put it into your mouth, let alone swallow it! Also, if you ask your plants, I’m sure they’re quite happy about a bit of nitrogen and phosphorous in their water, which you in turn would not want in your drinking bottle. So I guess it’s safe to say that instead of “pure” or “clean” we should strive for “suitable” instead.

image source
Deadly Sterile, or Living Clean?
Because microbial life is so complex that most of us are not even familiar with the numerous species that are too small anyway to see with the naked eye, we tend to be overwhelmed by the mere question of which ones are good, and which are bad for us. It may therefor be the simplest approach to kill everything, as we do in chlorinating our swimming pools. But is it really the best way of getting the water clean? A bit of chlorine (another chemical poison) will not harm us, but it could be detrimental to a plant to be watered with pool water. Also, just imagine the carnage among our natural skin flora that results from a brief dip in the pool!

image source
On the other hand, imagine taking the same dip in a pristine mountain lake. The water may seem just as “clean and pure”, though it will most likely be teeming with life. Ideally, that kind of water will be good for drinking, swimming, washing, and of course watering your plants with, and may be good for fish and other living organisms to thrive in. Couldn’t we get our water to that kind of purity? Surprisingly, it’s not that difficult.

image source
Using Life to Purify our Water
To separate the solids from our water, all we need to do is imitate a filter, providing sediments of various sizes (rocks, gravel, sand, charcoal) for the water to pass through. In order to neutralize organic contaminants, microbial life is needed, along with the oxygen they rely on. Having a rich diversity of microbes is also essential for outcompeting the harmful ones. But how can we make sure that we do indeed have a vast and vibrant ecosystem on the microbial level, which we can’t even see? We simply need some other creatures that live in symbiosis with these microbes, and are big enough to see. An example are bivalves, such as mussels or oysters.
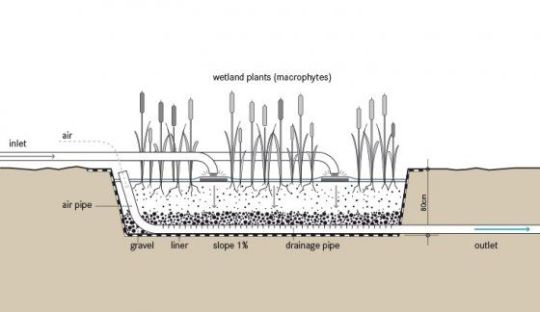
image source
Not only do these molluscs capture particles suspended in the water, and contribute to the cycling of nutrients, but they are also symbiotic with various types of microbes, just like any other creature, including ourselves. What’s even nicer about them, is that their fragile nature is a great indicator for the water’s purity. In other words, they will be killed by much less contamination than us. This means we can be sure that if the water they live in doesn’t kill them, it will be certainly safe for us too.

image source
Phyto-purification and Natural Swimming Pools
At this point I’d like to bring up some of the many practical applications to the theory of purifying our water. The simplest and most obvious one is a well designed reed-bed behind a house, to filter the household waste water. In a way, this is not much different than the gray-water planters Earthships typically utilize indoors. But can that be brought to a higher level, still? Most certainly so! Imagine various layers of filtering sediments, separate pools for aerobic and anaerobic decomposition, topped up with a mussel farm!

image source
The other popular application which may have popped into your mind are natural swimming pools. These are basins of living water, offering not only a place for humans to swim, but also a habitat for a plethora of aquatic plants and animals. In a way, it’s a simulation of the naturally pristine mountain lake I mentioned before. There are lots of great sources on how to implement one, but they all follow the basic premise of filtering the water, oxygenating it, and maintaining a vibrant ecosystem of plants and animals, to ensure an even greater diversity of microbes. All together they will provide you with water that is good and healthy, not only for you, but for all other forms of life in your ecosystem.
Sources: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12
86 notes
·
View notes
Link
0 notes
Text
Explanation of the first two groups in the periodic table
Group 1
Lithium: mysterious, mildest of spicy metals. Can exist in metallic form, cut with a butter knife and float on water. Synthetically useful, substitutes unhappily for hydrogen and makes fancy bases.
Sodium: smallish +1 cell currency, no longer a useful metal here and beyond.
Potassium: bigger +1 cell currency.
Rubidium: I Can't Believe It's Not Potassium
Caesium: the single most electropositive metal, Fluorine of metals, rips itself and everything else apart getting rid of its electron, also is Too Large and stops up cells.
Francium: nope, doesn't exist
Group 2
Beryllium: excellent metal, too small to exist? Extremely toxic, alien, we should be so lucky as to use it.
Magnesium: stable enough to use as a metal, still dangerously oxidizable. Body's smallish +2 cell currency. Great at making rocks and alloys alike.
Calcium: magnesium without the metallic utility, lorge and chonky, great at making rocks like bone or gypsum. Larger +2 cell currency.
Strontium: dense calcium. Stronks your bones. Clogs your heart cells. Risky improvement.
Barium: incredibly chongky, absorbs radiation, also insoluble normally but gives you mega lead style poisoning when soluble
Radium: fake calcium, a spy, not to be trusted, bright but toxic, excellent rogue, masquerades as useful and will kill you several ways at once.
#chem#chemistry#education#biochemistry#biochem#science#fun facts#class notes#educational#inorganic chemistry#alkali metal#alkali earth metal#biology
13 notes
·
View notes
Text

Oxygen first accumulated in the Earth's atmosphere about 2.4 billion years ago, during the Great Oxidation Event. However, geologic clues suggest early bacteria were photosynthesizing and pumping out oxygen hundreds of millions of years before then.
So, where was it all going?
Something was holding back oxygen's rise.
A new interpretation of rocks billions of years old finds volcanic gases are the likely culprits.
The Archean Eon, when only microbial life was widespread on Earth, was more volcanically active than today. Volcanic eruptions are fed by magma, as well as gases that escape even when the volcano is not erupting. Some of those gases react with oxygen, or oxidize, to form other compounds.
This supply of oxidizable volcanic gases was capable of gobbling up photosynthetic oxygen for hundreds of millions of years after photosynthesis evolved. But as the mantle itself became more oxidized, fewer oxidizable volcanic gases were released. Then oxygen flooded the air when there was no longer enough volcanic gas to mop it all up.
5 notes
·
View notes
Photo

Please wake up!! Protect yourself, family and loved ones. It's time to unite!! • • • The ones who get this-- the agent on on your hand will glow with the bio-luminescence. Its a chip implanted that can not be removed with a knife, it will be injected into your DNA. • • Luciferase is a light-producing enzyme naturally found in insect fireflies. In most bioluminescent organisms, the essential light-emitting components are the oxidizable organic molecule luciferien and the enzyme luciferase which are specific for different organisms. The light emission continues until all the luciferin is oxidized. That type of reaction is found in fireflies, Vargula, Latia, and many types of fish, such as lantern fish, hatchetfish, Apogon, and Parapriaeanthus. • • The digital ID will come in the form of something called an immunity passport. All of these things, and all being funded by one man, Bill Gates, represents in total at the very least a stunning forerunner to the Mark of the Beast world system. That’s at the very least estimation, taken to its logical end, it very well could be the actual Mark of the Beast system. https://www.instagram.com/p/CAZO7VbFYMT/?igshid=5qiw259fjuez
2 notes
·
View notes
Text
EFFECT OF BIAS ON STRUCTURE MECHANICAL PROPERTIES AND CORROSION RESISTANCE OF TITANIUM NITRIDE (TINX) FILMS PREPARED MAGNETRON SPUTTERING
Over the past few decades, technological advances have made it possible to deposition of the thin films under vacuum which referred to as “Hard Coatings”. These coatings have different applications due to their special structural and physical properties as protective coatings.
Hard coatings consist of nitrides, carbides and borides of intermediate metals such as titanium or carbon coatings such as diamonds. These coatings have many applications in improving tools (such as cutting and forming machines) and machine parts like valves. Properties of a thin film of hard coatings can be classified as follows:
Structural characteristics such as thickness, crystallography, chemical composition, surface morphology and roughness.
Physical and chemical characteristics such as density, electrical properties, magnetic properties, thermal, optical, oxidizability and corrosion.
Mechanical properties such as hardness, adhesion, mechanical stress and friction.
The selection of appropriate protective coating depends on specific tribological system (workpiece material, machining parameters and tool materials).
Titanium nitrate is one of the materials that has been highly regarded as a hard coating due to its high hardness, high corrosion resistance and thermal stability. In order to be able to use this material as a protective coating, the process of the deposition should be designed in such a way that the deposited thin film in addition of meeting the chemical composition in terms of hardness and other characteristics be a hard coating.
A thin film of this material is produced mainly by RF magnetron sputtering or DC magnetron sputtering. One of the problems with using the sputtering deposition is the “shadowing effect” during the deposition process. This effect is due to the low energy of particles that reach the substrate and as a result there aren’t any deposited layers on the some parts of the substrate. There are some island areas on the growing surface, where are more likely to nucleate and can absorb more particles than other areas around them and grow, like a hill. Adjacent areas (valleys) receive fewer particles due to the shadow of the hill areas. The Shadowing effect produces many problems and defects in the deposited thin film. Among these drawbacks are the creation of small holes and large gaps in the thin film. These defects lead to poor performance of the deposited thin film. To eliminate these defects, some researchers suggested that the effect of “Re-Sputtering” be used by reducing the pressure of the thin film deposition process and increasing the effect of bombing ions. Decrease the pressure leads to increase the voltage and the average particle energy. If the energy of the bombarding particles exceeds the sputtering threshold, the atoms can pass through the hills and they will be deposited in the valley-like regions of the thin film and eventually lead to the formation of a thin film with suitable density.
Regardless of the effect of shadows, the common problem associated with the process of magnetron sputtering method is the low ionization ratio of sputtering materials. The ratio of ionization is usually less than 5% in DC Magnetron Sputter, which is not enough to create a dens and defect less thin film. Some researchers have investigated the creation of a dense thin layer with improved ionization rates using the Unbalanced Magnetron Sputtering and Plasma Enhanced Magnetron Sputtering methods.
In the Un-balanced Magnetron sputtering process, the magnets used in the outer ring at the cathode section are stronger than the central magnets. Thus a number of electrons escape from the trapping magnetic field formed around the Target and move to the substrate. Which result in significant increase in the ion bombardment of the substrate and create new plasma away from the target material and near the substrate. By applying an Un-balanced magnetron, the ion flow that moves to the thin film can be controlled so that the quality of the deposited thin film can be increased dramatically.
In the Enhanced Plasma Magnetron Sputtering method, in addition to the plasma formed around the cathode, the independent plasma created through the ionization and acceleration and expansion of the electrons produced from the hot filaments in the chamber, also used to enhance the quality of the deposited thin film.
In order to improve the quality of the thin film of titanium nitrate for proper performance, its preparation process should be optimized in order to provide a thin film with a structure that can handle the required performance. A lot of studies have been done about the effect of applying bias voltage to the substrate and the quality of the deposited layer and the degree of hardness of the titanium nitrate thin film has been done. Chinese researchers have conducted an investigation into this effect of bias voltage, which resulted in the publication of an article in 2019.
In this research, a 160 nm titanium layer was used to enhance the adhesion of the titanium nitrate layer to the silicon substrate.
According to the results of this study, with the increase of bias negative voltage applied to the substrate from zero to 400 volts, the rate of the deposition significantly decreased from 7.69 to 4.55 nanometers per minute, and the density and quality of the deposited thin film increased. Also, by increasing the negative bias voltage and increasing the transition energy to the growing thin layer, the crystallographic orientation of the thin layer of titanium nitrate plans has been change in four directions from (111) to (200). The crystalline structure of titanium nitrate is similar to the sodium chloride (NaCl) structure. The minimum surface energy is related to the plane (200) and the minimum strain energy is related to the plane (111). Ideally, the growth of a thin layer has the least amount summation of surface energy and pressure energy.
In this study, the effect of the applying bias voltages of different values on the surface morphologically of the deposited titanium nitrate thin film was investigated. Without applying the bias voltage, surface morphology is seen as a typical triangular cone. By changing the bias voltage from -50V to -200V, surface morphology is converted into a laminate structure and the granular structure also appears by increasing the bias voltage to -400 volt. This change in surface morphology leads to densify thin film and decreases porosity.
By increasing the bias voltage, the energy of the ions increases and the re-sputtering phenomenon occurs, resulting in a shadow effect decreasing and the quality of the created thin film increases.
According to the results of the measurements carried out in this study, the hardness of the thin film of titanium nitrate increased with increasing the bias voltage up to -200 volt and then gradually decreasing to -400 volt. The thin filmdeposited under the bias voltage of -200 volt has the highest hardness (12.9 GPa) and the Young module (260.8 GPa).
By increasing the ionization voltage, the ion bombardment of the substrate increases and impurities become more detached. As a result, the film is denser and compression stress is also increased. In addition, the density of the plane with (111) orientation reaches its densifying peak at the bias voltage of -200 volt, which increases the hardness of the thin film.
According to studies conducted by this research group, if the bias voltage is less than -100 volts, there is little effect on the hardness of the thin film of titanium nitrate.
The resistance to corrosion of the thin film deposited by the applied -400 volt bias voltage is more than the layers deposited at the less bias voltages.
By studying the electrical properties of films produced at different bias voltages, it was found that the highest electrical impedance (7.5 × 105 Ω m) is related to the thin film deposited at the bias voltage of -400 volt, which is 10 times greater than the electrical impedance of the thin film deposited without applying the bias voltage.
As a result, by applying negative bias voltage to the substrate which leads to the energy increasing of the collide ions and reducing the shadowing effect in the thin film deposition process, can improve the physical properties of the titanium nitratethin film deposited by the Magnetron Sputtering method, such as hardness, electrical conductivity and corrosion resistance.
To find out more about the results of this study, see the following link:
https://doi.org/10.1016/j.tsf.2019.02.037
References:
B Window and N. Savvides, J. Vac. Sci. Technol. A4(2), 196 (1986).
Z. He, S. Zhang and D. Sun, Thin Solid Films, Volume 676, 30 April 2019, Pages 60-67.
http://bit.ly/32vuKyb
#magnetron sputtering#sputter coater#thin film#dual magnetron sputtering#reactive sputtering#sputtering definition#magnetron sputtering overview#magnetron sputtering machine#magnetron sputtering cost
1 note
·
View note
