#subshells of electron levels
Explore tagged Tumblr posts
Text

16.10.2024
.
Currently studying: energy level diagrams, electron subshell configurations, and valence electrons
I remember learning about this when I first started at uni and being so lost but now I’m speeding through it as if it’s a second language to me and that alone is motivating me to keep going :)
Today isn’t meant to be a study day but I didn’t finish all the work I wanted to do yesterday so I’m catching up on it.
68 notes
·
View notes
Note
Hey so you said you were doing physical chemistry- my school teacher ochem and inorganic at the same time so I am just gonna ask:
What the hell is up with the d orbitals? Like how they let transition metals change colour and act as catalysts? If you can, thank you!
Hey there!
Ohhhh the d-block elements are really cool! The d-orbitals are funky, what you're asking about here is pretty interesting. I wish I could help you with both of these concepts, but unfortunately we haven't covered catalysis yet, so I really don't feel qualified enough to talk about it. I can explain the part about changing colors though. It's all about the electrons, as usual <3
[Btw I'm actually studying this sort of thing in inorganic chem :D pchem is a whole different bag of bananas.]
So, the d-orbitals. I'm going to assume you're familiar with orbital diagrams - they'll come in handy here. With that in mind, please consider the following ions: Ti(4+), Cu(+), both of which are colorless, and then: Ti(3+) (purple) and Cu(2+) (blue). Sc(3+), Y(3+), and Zn(2+) are also colorless. See a pattern here? If not, let me help you out:


As you can see, all the colorless ions have either full or totally empty outer d-orbitals, while the colorful ions have unpaired electrons on their outer d-subshell.
Quick and necessary digression: why are things colorful anyway? You probably know what the general mechanism here is, but just in case you don't, the simplest explanation is that when a photon hits an electron in an atom, the electron can then jump up to a higher shell. Electrons don't like staying up there though, so an excited electron will quickly jump back down to its original shell and release a photon in the process - which we see as colors .
[Yes, it is more complicated than that. Yes, I did greatly simplify it. Please don't throw anything at me, dear physicist friends.]
Now, back to our orbitals.
If an ion's outer d-orbital is full, it means the electrons there have no space to "move". They can't jump from one square (or rather from one orbital) to another because as per Pauli's exclusion principle, there can only be two electrons in a single orbital. Similarly, if there are no electrons whatsoever in the d-orbitals, there's nothing to "jump". Duh. The substance is colorless.
However, unpaired electrons mean there's at least one square (orbital) that can still accommodate one extra electron without breaking Mr Pauli's heart. As photons hit the d-orbital electrons, they keep "moving up and down" and releasing photons in turn - which gives them their color.
This, too, is a huge simplification. In fact, the d-orbitals can also split into levels of different energy and it's those levels that the electrons actually jump between. It's called the crystal field theory and you can look it up if you really want to start crying violently and having nightmares every night. If you aren't in uni yet though, it really isn't necessary. I think my explanation should be enough. I sure do hope it can be helpful :)
#i need you to know i looked up catalysis too#but none of the textbooks i checked out had any satisfying explanations#and it's better to leave someone with an unanswered question than with a false answer#so i hope you won't be upset!#inbox#chemistry asks
32 notes
·
View notes
Text

Electron Configuration
Electron configuration is the arrangement of electrons in an atom's electron shells and subshells, following specific principles derived from quantum mechanics. This configuration dictates how electrons fill orbitals, starting from the lowest energy levels to the highest. The Aufbau principle guides this filling order, while the Pauli exclusion principle ensures that no two electrons within an atom share the same set of quantum numbers. Additionally, Hund's rule states that electrons will fill degenerate orbitals singly before pairing up, maximizing the atom's stability. The electron configuration of an atom not only defines its chemical properties and reactivity but also determines its position in the periodic table. For example, the electron configuration of carbon (C) is 1s² 2s² 2p², meaning two electrons fill the 1s orbital, two electrons fill the 2s orbital, and two electrons occupy the 2p orbital. Understanding electron configurations is crucial for predicting an element’s behavior in chemical reactions and understanding trends across the periodic table.
International Chemistry Scientist Awards
Website: chemistryscientists.org
Contact us: [email protected]
Nominate now: https://chemistryscientists.org/award-nomination/?ecategory=Awards&rcategory=Awardee
#sciencefather#researchawards#Professor,#Lecturer,#Scientist,#Scholar,#Researcher#ElectronConfiguration #Chemistry#AtomicStructure#QuantumMechanics #PeriodicTable#AufbauPrinciple#PauliExclusionPrincple#HundsRule#ElementProperties#ChemicalReactivity #ElectronShells #Subshells #ElectronDistribution #ChemistryConcepts #AtomsAndMolecules
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Blogger : https://www.blogger.com/blog/post/edit/6961521080043227535/467226973388921229
Twitter : https://x.com/chemistryS79687
Pinterest : https://in.pinterest.com/chemistryaward/
Instagram: https://www.instagram.com/alishaaishu01/
Youtube : https://www.youtube.com/channel/UCAD_pDvz3ZHqv_3hf-N0taQ
Blaze
0 notes
Text
two moods:
-thinkin bout homestuck
-"so due to the electrons of superheavy atoms moving near the speed of light, relativistic effects cause them to have a larger mass than electrons in most other atoms! this relativistic effect leads to shifts in the energy levels of electrons, causing the subshell splitting of the 7p subshell. since two of the six 7p electrons are subject to the inert pair effect in addition to the two 7s electrons, flerovium, which has 4 valence electrons, acts like it has a full shell. similarly, oganesson might not act like it has a full shell, despite having 8 valence electrons, so it's predicted to be both solid at stp and more reactive than what you'd expect from a noble gas! so basically, in our attempts to make cursed elements with unstable compound nuclei, we've found where periodic trends fucking break. cool right?"
and then the poor person i'm yapping at has a mental breakdown.
#i've been doing some research lately.#if someone with actual experience with this stuff can fact check that would be great lmao#my ramblings#no clue what to tag this as.#physicsposting on main?!
1 note
·
View note
Text
3.3 Ronald McDonald dimension.
Another example is Ronald McDonald, one of the human-like masks of dramaturgy.

Pure dramaturgy took the form of the timeless and bodiless magical being, Ronald McDonald, with the intention of making friendly connections. However, instead of being received as intended, people were afraid of the perceived "dead valley" that exists between a real human and Ronald. Some, like Stephen King, imagined IT as a spooky alien clown that absorbs and consumes human hopes, desires, and bodies.

Modern dramaturgy, in this case, discarded the Ronald McDonald persona, delighting in the Marvel Universe's creations. Same as epos of ancient Greek gods. Notice how all the Avengers aim to increase entropy to its maximum? The villains seek to destroy everything, and the heroes travel in a large, extravagant aircraft with a football field-sized underground garage just for six people. These elements consume vast amounts of energy and contribute to the spectacle.
Ronald McDonald and the Avengers are higher dramaturgical beings who wear "face masks" to engage directly in our world's affairs and play their own games. They do not age, and although they have no connection to space-time, we often perceive them as human-like. They are a tribute to absolute dramaturgy! The strongest, the fastest, the wisest and so on. This are an idealistic qualities, symbols of certain non-existent absolutes of different dramaturgy types.
I picked Ronald as a sample of this type of dramaturgical entities because he is outstanding! He has an identity, face, some business with people, their kids. And he manages to exist as a real human being in a real body! Real people who played Ronald during different events where sort of golems controlled by Him and his will that temporary got into person’s body. Ronald is out there. IT is out there.
So, pure dramaturgy can manifest as a human if it desires, and it certainly does. Not only dressing you as Ronald McDonald. Every time a regular person goes to work and puts on the uniform of a police officer, then proceeds to hit their neighbours during a protest in the town square, only to return to being friendly with them afterward, when off duty, it is not necessarily their personal desire to harm others and hit his neighbour with a rubber stick. Their job and the laws dictate their actions. This is how dramaturgy effortlessly dresses us in various roles such as cops, carpenters, mothers, bad boys, geeks, ship captains and plays out these roles through us according to its own will. A good ship captain always stays on the ship even if it’s drowning. That’s what “they” say.
Stepping into the "Ronald McDonald dimension" allows us to realize that time does not exist. Imagine our 3D world with all its energy and matter as a large Turing machine that performs new computations at each Planck's time. It would remain completely stable and motionless if it weren't for the driving force of dramaturgy! Matter and energy seek satisfaction of their primal desires.
For example, electrons in an atom cannot stay one the same shell with electrons that have same spin.

Atomic orbitals of the electron in a hydrogen atom at different energy levels. The probability of finding the electron is given by the color, as shown in the key at upper right.
What is spin if not pure dramaturgy? Why electrons tend to fill the lowest energy orbitals of atom first? Why does your spin-up (+1/2) while mine is spin-down (-1/2)? Am I not good enough? Let’s go out to the subshell and discuss it one on one like a real electrons! Why don't you allow me to be on the same energetic level?
Dramaturgy is so fundamental that it is impossible to describe or understand any other fundamental entities of this world, especially quantum, without its inclusion. Nothing can be precisely described or stated without accounting for dramaturgy.
At its core, dramaturgy involves entities changing their characteristics in space, chaos, and correlation with predicted results.
Mascots, Avengers, and Ronald McDonald all have their origins in the primitive and colourful dancers of ancient cultures. People would dress up in dramaturgical costumes and perform these dances around a fire. Fire, which was brought to the world by the will of ancient humans, served as a on demand source of light, heat and power. At that time, people believed they had tamed the gods and felt a sense of superiority and knowledge about the world. They believed there was little left to discover.

During these dances, people would enter trance-like states and invite different spirits to communicate through them. Some individuals may have experienced the presence of special spirits through the use of substances like marijuana or mushrooms. However, many others, both in ancient times and today, did not experience any spirits but instead used these practices to manipulate and deceive others for personal gain.
However, the miracle of fundamental dramaturgy is that it can illuminate even the ugliest truths. Both true shamans and scammers alike have tapped into dramaturgy. Whether they listened to the wind, vibrations of a multiverse, or their own selfish thoughts, they were all engaging in the process of creating dramaturgy.
Dramaturgy exists to end the cycle of entropy. From a broader perspective, reality does not distinguish between those who tell the truth and those who lie. What matters is that they tell their stories.

The particularities of the stories themselves are not important, only thing they need to be interesting and fun; but eventually this stories are merely fluctuations within the larger field of random fluctuations. "Yes" and "no" happen everywhere, whether on a grand scale or a minuscule one. Rocks are lying on the ground everywhere in the universe. Ultimately, it is all the same.
“Yes” rock is on the ground, “No” it is not going to change any time soon. This is a boring dramaturgy that happens everywhere.
However, conscious beings have the ability to "read" these fluctuations of “yes” and “No” and predict potential outcomes. For dramaturgy itself, there is no concept of "good" and "evil." It is the dramaturgical potential holders who create these distinctions and assign meaning to them as part of their personal dramaturgy. That's simply the nature of how it works. Actions lead to consequences.

Humans bring much more fun and tragic dramaturgy into the world. Lucky rock can turn into an Art, or brake the window. Rock will be honoured to play that role. Because “he” will stand out of billions of ordinary grey rocks. If a human simply picks it up.
#dramaturgy#computationalcreativity#quantum physics#innovation#dramaturgical potential#quantum dramaturgy#physics of important things#science#philosophy#psychology#psychic#psychonauts#fate#god#soul#wolfram#Donald Hoffman#game theory#weird#bizarre#creepy#odd#weird stuff#strange new worlds#life is strange#Ronald#ronald mcdonald#clown#horror
0 notes
Photo


Patreon | Ko-fi
#studyblr#notes#chemistry#chemistry notes#chem#chem notes#physics#physics notes#subshells#subshells notes#electron#electrons#electrons notes#subshells of electron levels#subshells of electron levels notes#electron levels#electron levels notes
1 note
·
View note
Text
Periodic table structure

arranging periodic table into blocks, we see that as you go left to right, top to bottom, it follows the increasing energies of subshells w/c occur due to increasing amounts of electron shielding (e.g. 4s → 3d)
n = row number
columns are grouped by l (b/c it indicates s, p, d, or f subshell)
s block elements have outermost electrons in s subshell
p block elements have outermost electrons in p subshell
d block elements have outermost electrons in d subshell
f block elements have outermost electrons in f subshell; f-block elements = lanthanides and actinides and they actually insert themselves here:

rows aka periods = principal quantum number n
Periodic table trends = based on Coulomb's law:

We can't calculate r exactly because of quantum mechanics, so we need to use a mean r value to calculate force of attraction between nucleus and an electron.
Core charge = change experienced by outermost electrons of an atom, assuming the inner electrons shield them 100% from the nucleus and outermost electrons don't shield each other at all.
Ignoring transition metals, core charge = group number (+1 to +8).
In reality, orbitals of outer electrons somewhat penetrate inner orbitals so effective shielding isn't 100% and electrons in the same shell can partially shield each other somewhat. So the actual amount of shielding and effective nuclear charge depends on interactions between different orbitals (e.g. s orbitals have more electron density near or on the nucleus than a p orbital with the same n, so s orbitals shield the nucleus better and is less affected by shielding from other orbitals than p). The actual amount of shielding isn't the same as the amount of shielding assumed in core charge calculations, and thus, the effective nuclear charge does not exactly equal core charge.
Atomic radii
Core charge increases as you go left to right w/c means 1 of the q's in Coulomb's law is increasing, assuming everything else is the same; core charge = one's place of group #. This means force of attraction between outer electron and nucleus increases as you go left to right because the higher the core charge, the more strongly the nucleus pulls the outer electrons towards itself. This means atomic radii decrease from left to right.
As you go down the periodic table, atomic radii increase because you increase in number of energy levels with each period (n ↑).
So largest atomic radii are at the bottom-left of the table.
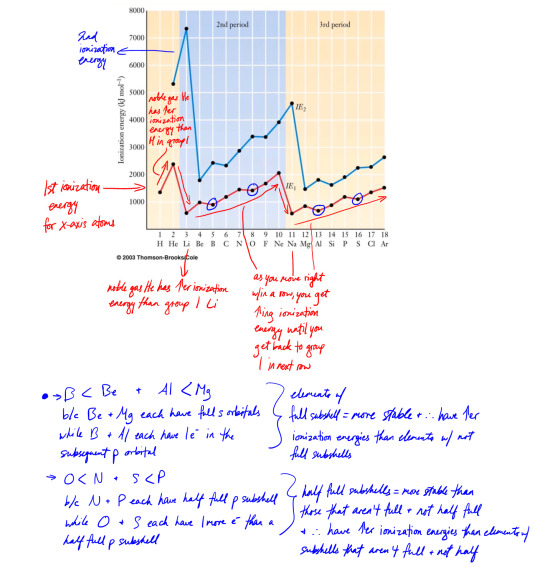
Ionization energy
Ionization energy = amount of energy (ΔH) that must be absorbed to remove an electron from an atom, to turn the atom into an ion (M → M+ + e-)
We tend to measure energy in enthalpy, so energy = ΔH
The more loosely an atom holds its electrons, i.e. the more electron shielding there is, and therefore the larger the atomic radii, the lower the ionization energy because it's easier for an electron to be removed.
The more tightly an atom holds it's electrons, i.e. the less shielding their is, the smaller the atomic radii, the higher the ionization energy.
So the highest ionization energies are at the top-right of the table (except noble gases because they have full valence shells).
First ionization energy - an outermost electron is removed first
Second ionization energy - a 2nd electron is removed after the first, w/c is always higher than the first because there's greater attraction to nucleus with fewer electrons in the way
You can also have third, 4th, etc. ionization energies, it just depends how many electrons in the atom you have that you want to remove
Sometimes the difference b/t 1st and subsequent ionization energies is small, sometimes it's a really big difference. Difference is really big when you go from outer electrons to core electrons. Difference is small when you're just dealing with outer electrons
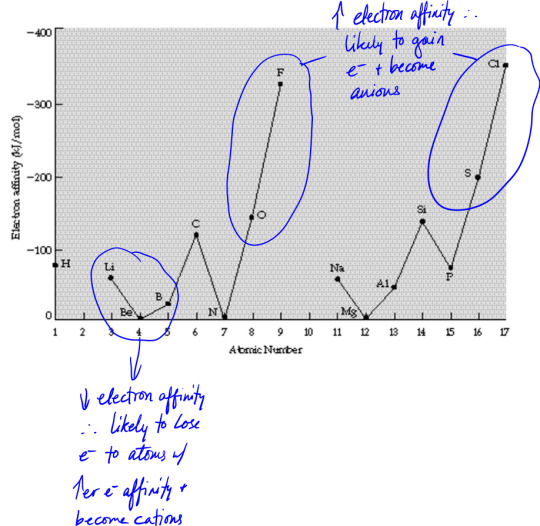
Electron affinity
Electron affinity is the amount of energy (ΔH) released when adding an electron to an atom (M + e- → M-)
All atoms have negative electron affinity except noble gases, so they all can take more electrons except for noble gases, but elements with the highest electron affinity are in the top-right of the periodic table (they tend to form anions)
Metals tend to have low ionization energy and low electron affinity, becoming cations
Non-metals tend to have high ionization energy and high electron affinity, becoming anions
So metals + non-metals → ionic compounds
Metalloids/semimetals can act metallic or non-metallic depending what they're paired with
Electronegativity
Electronegativity is how likely is an atom that shares an electron pair w/ another atom going to attract that pair to itself vs attracted away from it to the other atom and where the electrons spend most of their time? When high electron affinity elements form a covalent bond, they are more likely to hog electrons aka have high electronegativity.
Reactivity

group 1A / alkali metals → lose 1 electron, often form salts (react w/ halogens), reactivity increases as you go down the group
group 2A / alkali earth metals → lose 2 electrons, tends to react with atoms that gain 1 or 2 electrons
group 3A → tend to form 3+ ions except B b/c that would make it very unstable (instead, B forms covalent bonds)
group 4A → C and Si both form tetrahedral structures but Si can have more complex molecule geometries b/c it has d orbitals and tends to lose electrons like metals (hence, it's a metalloid), while C can lose or gain electrons
group 5A → N and P are non-metals forming 3- ions, As and Sb are metalloids so can go either way, Bi is a metal that tends to lose electrons b/c it's a large atom so low ionization energy
group 6A / chalcogens → O, S, and Se are non-metals that can form 2- anions, Te is a metalloid, Po is a metal that tends to lose electrons b/c it's a large atom so low ionization energy
group 7A / halogens → easily form 1- anions b/c they just need 1 more electron to fill their outer shell; F = the most electronegative and has the highest electron affinity of any atom in the table
group 8A / noble gases → least reactive b/c all their outer shells are filled
H can be classified as part of 1A or 7A because it can lose an electron → 1+ or gain an electron → 1-
2 notes
·
View notes
Note
Can you PLEASE talk more about expanded octets I just learned what an octet was in Chem and I NEED to know more. Also I want my Chem teacher to wonder why I know things I haven't been taught
*Excited chemistry major shaking*
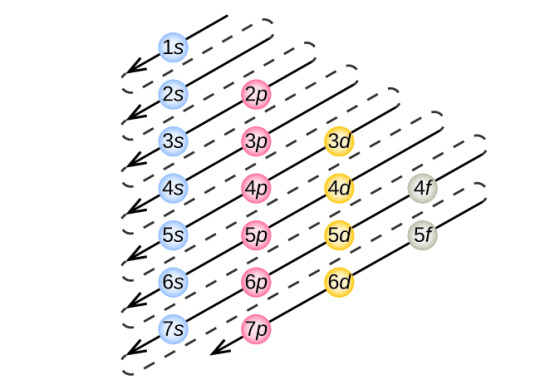
Ok so, the elements in the first 2 periods of the periodic table (nitrogen, oxygen, carbon, etc) obey the octet rule without exception because they don't have d orbitals. The 3rd period of the periodic table is the first one to have d orbitals, and they start with the 3rd subshell. The higher up in subshells you go, the lower the difference in energy between them. So, say the 1st and the 2nd electron sublevels are MUCH further to each other than the 3rd and the 4th. This is shown in this diagram:

The d levels essentially have more electrons, but because they only start in the 3rd sublevel, they are really close to other shells energetically. See how close 3d and 4s are in these diagrams!
This means that some nonmetals like sulfur and phosphorus can use their d sublevel to accommodate more electrons, aka- the expanded octet!
This is why the only elements to have expanded octets come AFTER the first row of the transition metals! If you look at the periodic table, sulfur and phosphorus come after zinc, which is the last 1st row d block element. The elements that come BEFORE the d block like oxygen and nitrogen obey the octet rule without exceptions because they don't have d orbitals to accommodate more than the usual 8 valence electrons!
23 notes
·
View notes
Text
Electron Configurations
The quantum mechanics theory
This is including subshell and orbitals. Every shell has subshell(s) in it. When we can consider that shell and subshells as a house and its rooms.
After that we got to know about this Aufbau’s diagram of subshells’ energy levels. The horizontal lines show the shells (e.g. 1s is s subshell in 1st shell, 2s and 2p is s an p subshells in 2nd shell) and the vertical lines show the subshell (e.g. the left side line is for s subshells in every shell).
The rule of filling the subshells:

Take a look. There are four subshells in the configuration. They are s, p, d, and f. The “squares” there are the orbitals and those arrows are the electron. Every orbital can filled by 2 electrons.
If you see clearly the electrons of the subshells, how many the electrons can fill the, each orbital? The answer is:
s=2 p=6 d=10 f=14
Each electron has different quantum numbers. According to Pauli’s Exclusion Principle, rule of filling the subshells, states that no 2 electrons in the same atom can have identical values for all four of their quantum numbers. Later we’ll hit that explanation.
If there’s a question
”Determine the electron configuration of Na and Br (again but different section)”
1. See the energy level diagram. Which is the weakest? How to sort them is follow the arrows

Let’s sort. 1s2, 2s2, 2p6, 3s2, 3p6…
UNDER CONSTRUCTION!!!
2 notes
·
View notes
Text
Exchange Energy in d-Block Elements
Spin-exchange interaction
Mutual electrostatic repulsion leads to two types of interaction among molecule.
The first is called charge correction. When two electron particles separated by distance r then the Coulombic energy of each given by e2/r where e = electrostatic charge. Therefore two electrons get stabilizes when r increases.
The second interaction is known as spin correction and it is more important than charge correction. According to the spin correlation of electrons, the same spin tends to keep away from each other while electrons of opposite spin tend to come closer.
What is exchange-correlation energy?
Electrons of similar spin develop an exchange interaction which leads to stabilizes the system. Thus the electrons of similar spin repulsion less by an amount called exchange energy. Therefore greater the number of electrons with parallel spins the greater is the exchange energy and greater is the stability. This theory is the basis of the Hunds rule of maximum spin multiplicity in chemistry or physics.
For the d quantum level, maximum stability will results when five d electrons are with parallel spins in five d orbitals where each orbital contains one electron. When K = exchange energy per pair of parallel spins and n = number of electrons in parallel spins then the total exchange energy of the electron, Eexchange = n(n-1)K/2.
Stability of half-filled shells
Thus for five unpaired electrons with parallel spins the exchange energy = 10K. When one electron has withdrawn we have a d4 system with exchange energy = 6K. If we consider d-block chemical elements in the periodic table and introduce six electrons in the d quantum level, the exchange energy still 10K but significant Coulombic repulsion because of two electrons beings the same orbitals.
We see that half-filled subshell has greater stability than less than half-filled or more than half-filled subshell. Thus the electron configuration of chromium with atomic number 24, [Ar]18 4S1 3d5
3 notes
·
View notes
Text
OCD AT AN ATOMIC LEVEL
Just as we are concerned about what ingredients go in the food we eat, what cement or rods or bricks goes in the houses we build, the content in a story book similarly we should also be concerned of what constitutes us and every single thing around us. Well, everything around us is composed of atoms. Life exists only because of gases that under very high pressures and temperatures combined to form compounds billions of years ago, that led to the existence of life forms. Atoms are composed of protons (+ve charge), neutrons (neutral) and electrons (negative charge). Protons and neutrons compose the nucleus. Nucleus constitutes the maximum mass of the atoms. Electrons revolve around the nucleus in specific permissible orbitals and can get excited to form ions. The permissible orbitals are decided by quantum numbers.

Principle quantum number: The principal quantum number is symbolized by the letter n. The principal quantum number tells which shell the electron is in and can take on integral values starting with 1.

Azimuthal (angular momentum) quantum number: The azimuthal quantum number is symbolized by the letter l. The azimuthal quantum numbers give the shape of an orbital. Orbitals have shapes that are best described as spherical (l = 0), polar (l = 1), or cloverleaf (l = 2). Values for l depend on the principal quantum number and can range from 0 to n–1.

Magnetic quantum number (2l+1): The magnetic quantum number associated with the quantum state is designated as m. The quantum number m refers, loosely, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud. Given a particular ℓ, m is entitled to be any integer from -ℓ up to ℓ.
s ----- 0; p------0, +1, -1; d---------0, +1, -1, +2, -2; f------0, +1, -1, +2, -2, +3, -3 etc
Spin quantum number: Spin quantum number (s) characterizes the revolutions of electrons about themselves. Magnetic moments due to spin can have only two orientations in space; either up or down; + ½ or – ½; clockwise or anti-clockwise.
The electronic configurations of atoms depend on three rules:
Aufbau’s Principle: E=n+l Electrons are filled first in orbitals having lower energy followed by orbitals with higher energy.
Pauli exclusion principle: No two electrons can have the same four quantum numbers.
Hund’s rule of maximum multiplicity: Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied. The two electrons in an orbital have opposite spins.
Example: N=7 1s22s22p3

BONDING
“The beauty of a living thing is not the atoms that go into it, but the way those atoms are put together.” ― Carl Sagan
Just like humans, atoms too like to indulge in bonds. Humans tend to marry, have children, grandchildren to form the bond of family in an ultimate motive to gain stability amidst the ups and downs of life. Similarly, atoms bond with other atoms of the same type or different type in order to gain stable electronic configurations.

Types of chemical bonds:
Types of chemical bonds:
Ionic Bond: Bonds made by the actual transfer of electrons amongst oppositely charged atoms.
Covalent Bond: Bonds made by the sharing of electrons amongst atoms.
Coordinate Bond: A coordinate covalent bond, also known as a dative bond or coordinate bond is a kind of covalent bond in which the two atoms share the same set of electrons.

VSEPR Theory (Valence Shell electron pair repulsion theory): The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
Steric Number = Number of bonded atoms + Number of lone pair electrons
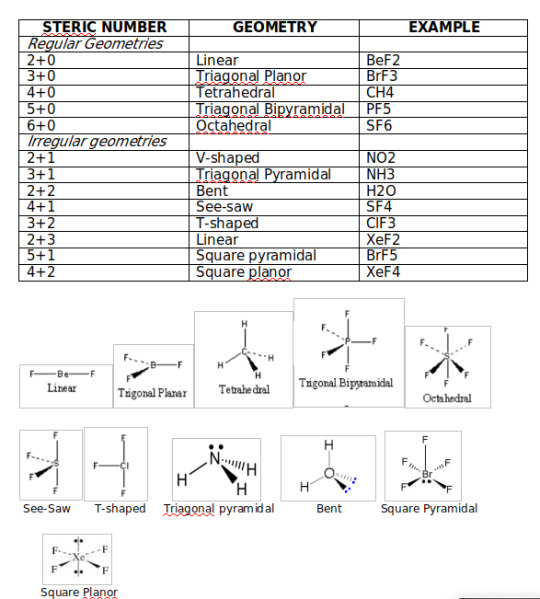
Theories explaining chemical bonding:
Valence Bond Theory:
This theory states that bonds are formed by the overlapping of orbitals. The strength of a bond depends on the degree of overlap. A sigma bond is formed by the head on overlap of orbitals while a pie bond is formed by the sideways overlap of orbitals.

VBT couldn’t explain tetravalent carbon atom.
Hybridization theory:
The intermixing of two atomic orbitals with the same energy levels to give a new type of hybrid orbitals with same energy is called hybridization. The atomic orbitals having same energy can only take part in hybridization. Moreover, both full filled and half-filled orbitals can take part in the formation of hybrid orbitals provided they have equal energy.

Hybridization theory doesn’t explain the biradical nature of Oxygen atom.
Molecular orbital theory:
According to the Molecular Orbital Theory, individual atoms combine to form molecular orbitals. The formation of orbitals is because of Linear Combination of atomic orbitals which combine to form the molecule. The combining atomic orbitals have their own wave functions which on linear combination give us the wave function of the molecular orbital. Bonding orbitals: When the addition of wave function takes place, the type of molecular orbitals formed are Bonding Molecular Orbitals. We can represent them by ΨMO = ΨA + ΨB. They have lower energy than atomic orbitals involved.
Anti-bonding orbitals: When molecular orbital forms by the subtraction of wave function, the type of molecular orbitals formed are antibonding Molecular Orbitals. We can represent them as ΨMO = ΨA – ΨB. They have higher energy than atomic orbitals.
Bond order: BO = ½ (No. of electrons in bonding orbitals – No. of electrons in Anti-bonding orbitals). Bond order must be calculated separately for sigma and pi bonds.
Example: O2

Bond Order 2s orbital Sigma bonds = 1/2 (2-2) = 0
Bond Order 2p orbital Sigma bonds = 1/2 (2-0) = 1
Bond Order 2p orbital pi bonds = 1/2 (4-2) = 1
Therefore, O2 has 1 sigma bond and 1 pi bond. Moreover, the biradical nature of oxygen or paramagnetic nature of oxygen is explained by the presence of two unpaired electrons in the pi* anti-bonding orbital.
1 note
·
View note
Text
Top 55+ Chemistry Topics Majorly Asked in Competitive Examination.
Bohr Atomic Model:
The Bohr atomic model, also known as the Bohr model or the planetary model, is a simple model of the hydrogen atom and its energy levels. This model was developed by Danish physicist Niels Bohr in 1913. In this model, electrons orbit the nucleus of the atom in defined shells or orbits and only certain orbits are allowed for electrons. The energy levels of these orbits are quantized, meaning that electrons can only occupy specific energy levels, not any value in between. Read More About Bohr Atomic Model
De Broglie Hypothesis:
The De Broglie hypothesis is a theory in quantum mechanics that states that all matter exhibits wave-like behavior. This hypothesis was proposed by French physicist Louis de Broglie in 1924 and was later confirmed by experiments. According to this hypothesis, particles of matter have a wavelength associated with them, which is inversely proportional to their momentum. Read More About De Broglie Hypothesis
De Broglie Equation:
The De Broglie equation is a mathematical expression that relates the wavelength of a matter wave to its momentum. The equation is given as λ = h/p, where λ is the wavelength, h is Planck's constant, and p is the momentum of the particle. This equation is a fundamental result of the De Broglie hypothesis. Read More About De Broglie Equation
Heisenberg Uncertainty Principle:
The Heisenberg uncertainty principle is a fundamental principle of quantum mechanics that states that the more precisely the position of a particle is known, the less precisely its momentum can be known, and vice versa. This principle was proposed by German physicist Werner Heisenberg in 1927. Read More About Heisenberg Uncertainty Principle
Schrodinger Wave Equation:
The Schrödinger wave equation is a partial differential equation that describes the behavior of a wave function in quantum mechanics. This equation was proposed by Austrian physicist Erwin Schrödinger in 1926 and is a fundamental equation in quantum mechanics. The wave function, described by the Schrödinger equation, represents the probability distribution of the position and momentum of a particle. Read More About Schrodinger Wave Equation
Shape of Orbital:
Orbitals are the three-dimensional regions around the nucleus of an atom where electrons are most likely to be found. The shape of an orbital is determined by the distribution of electron density around the nucleus and is described by mathematical functions called wave functions. Orbitals can be classified into different shapes, including s, p, d, and f orbitals. Read More About Shape of Orbital
Quantum Numbers:
Quantum numbers are a set of numbers used to describe the properties of electrons in an atom. There are four quantum numbers, including the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). These quantum numbers determine the energy level, shape, orientation, and spin of an electron in an atom. Read More About Quantum Numbers
Hund's Rule:
Hund's rule is a rule in quantum mechanics that states that when electrons are added to a subshell, they occupy the available orbitals in the subshell with parallel spins. This rule is named after German physicist Friedrich Hund. Read More About Hund's Rule
Pauli Exclusion Principle:
The Pauli exclusion principle is a fundamental principle of quantum mechanics that states that no two electrons in an atom can have the same set of four quantum numbers. This principle was proposed by Austrian physicist Wolfgang Pauli in 1925 and is a cornerstone of the theory of atoms and molecules. Read More About Pauli Exclusion Principle
Aufbau Principle:
The Aufbau principle is a rule in quantum mechanics that states that electrons are added to an atom in the order of increasing energy levels, starting with the lowest energy level first. This principle is used to predict the electron configuration of atoms. Read More About Aufbau Principle
Energy Level Diagram:
A diagram that represents the energies of electrons in an atom or ion. It shows the number of electrons in each energy level, or shell, and helps to understand the arrangement of electrons in an atom. Read More About Energy Level Diagram
Screening Effect or Shielding Effect Slater’s Rule:
This refers to the reduction in the effective nuclear charge experienced by an electron due to the presence of other electrons in the atom. Slater’s rule is a formula used to calculate the shielding effect. Read More About Screening Effect
s-block Elements:
The elements in the first two columns of the periodic table are called s-block elements. These elements have a single electron in their outermost energy level, and their properties are mainly those of metals. Read More About s-block Elements
p-block Elements:
The elements in columns three through eight of the periodic table are known as p-block elements. These elements have two or more electrons in their outermost energy level and are mainly composed of non-metals. Read More About p-block Elements
d-block Elements:
The elements in the transition metal section of the periodic table are known as d-block elements. These elements have partially filled d-orbitals in their outermost energy level, and are known for their ability to form complex compounds. Read More About d-block Elements
Atomic Radii:
The size of an atom is measured by its atomic radius. It is defined as the distance between the center of the nucleus and the outermost energy level. Read More About Atomic Radii
Ionic Radii:
The size of an ion is measured by its ionic radius. It is defined as the distance between the center of the nucleus and the outermost energy level. Read More About Ionic Radii
Ionization Energy:
The energy required to remove an electron from an atom or ion is known as its ionization energy. This value is different for different elements and is an important factor in determining the chemical properties of an element. Read More About Ionization Energy
Electron Affinity:
The electron affinity of an element is defined as the energy change that occurs when an electron is added to a neutral atom to form a negative ion. Read More About Electron Affinity
Electronegativity:
The electronegativity of an element is a measure of its ability to attract electrons to itself in a chemical bond. Read More About Electronegativity
Chemical Bonding:
Chemical bonding refers to the force that holds two or more atoms together to form a molecule. There are several types of chemical bonds including covalent bonds, ionic bonds, and metallic bonds. Read More About Chemical Bonding
Inert Pair Effect:
The inert pair effect is a phenomenon that occurs when the outermost electrons of certain elements are not participating in chemical reactions. This is because the electrons are so strongly attracted to the nucleus that they are not able to participate in chemical reactions. Read More About Inert Pair Effect
Lattice Energy:
It refers to the energy required to separate a solid crystal into its individual ions in gaseous form. The lattice energy is directly proportional to the charge on the ions and inversely proportional to the distance between them. Read More About Lattice Energy
Fajans Rule:
It is a rule that states that a large ionic charge combined with a small ionic radius will result in a highly polar covalent bond. This rule helps to predict the properties of complex ionic compounds. Read More About Fajans Rule
Covalent Bond:
It is a type of chemical bond formed between two non-metallic atoms by sharing electrons. Covalent bonds are characterized by their high stability, low reactivity, and low conductivity of electricity and heat. Read More About Covalent Bond
Coordinate Bond:
It is a covalent bond that is formed when one of the atoms donates both electrons to form the bond. The resulting bond is a shared pair of electrons between two atoms with a charge separation. Read More About Coordinate Bond
Metallic Bond:
It is a type of chemical bond formed between metallic ions. This bond is characterized by the sharing of electrons between metal ions, resulting in a lattice-like structure with high strength and conductivity of electricity and heat. Read More About Metallic Bond
Molecular Orbital Theory:
It is a theory that explains the behavior of molecules in terms of their constituent atoms and the interactions between electrons in these atoms. The theory is based on the idea that electrons in a molecule are not localized to specific atoms, but instead occupy molecular orbitals that span the entire molecule. Read More About Molecular Orbital Theory
Hydrogen Bond:
It is a type of chemical bond that is formed between a hydrogen atom and another atom with a high electronegativity, such as oxygen, nitrogen, or fluorine. Hydrogen bonds are weaker than covalent bonds but are important in determining the properties and behavior of molecules, such as water and DNA. Read More About Hydrogen Bond
Reagent:
It is a substance used in chemical reactions to produce a desired product. Reagents can be either reactants or catalysts and are an essential part of chemical synthesis and analysis. Read More About Reagent
VSEPR theory:
It stands for Valence Shell Electron Pair Repulsion theory, which explains the geometry of molecules based on the repulsion between the electron pairs surrounding the central atom. The theory predicts the shape of molecules based on the number and arrangement of electron pairs in the valence shell. Read More About VSEPR theory
Alkali Metals:
They are a group of elements in the periodic table, including lithium, sodium, potassium, rubidium, cesium, and francium. Alkali metals are characterized by their high reactivity, low ionization energy, and high electron affinity. They are highly reactive with water and form hydroxides and alkalis, which are strong bases. Read More About Alkali Metals
Alkaline Earth Metals:
Alkaline Earth Metals are a group of elements in the periodic table that include beryllium, magnesium, calcium, strontium, barium, and radium. They have a similar chemical behavior, and they are reactive, but less so than the alkali metals. Read More About Alkaline Earth Metals
SILICATES:
Silicates are minerals that contain silica, which is the most abundant mineral in the Earth's crust. They make up a large portion of rocks and soils and are used in various industries, including construction, ceramics, and glass production. Read More About Silicates
CARBIDES:
Carbides are chemical compounds that contain carbon and one other element, typically a metal. They are known for their high hardness and high melting points, and they are used in cutting tools, abrasives, and other industrial applications. Read More About Carbides
Noble gases:
Noble gases are a group of elements in the periodic table that are extremely unreactive and do not readily form compounds. This makes them useful in various industrial and scientific applications, such as lighting, welding, and cryogenics. Read More About Noble gases
Beryllium:
Beryllium is a lightweight, strong metal that is used in various applications, including aerospace, nuclear reactors, and electronic components. It has a high melting point and high thermal conductivity. Read More About Beryllium
Fluorine:
Fluorine is a highly reactive chemical element that is used in the production of fluorine compounds, including refrigerants, cleaning agents, and pharmaceuticals. It is also added to drinking water to help prevent tooth decay. Read More About Fluorine
Lithium:
Lithium is a soft, silvery metal that is highly reactive. It is used in various applications, including batteries, ceramics, and pharmaceuticals. Lithium is also used in the treatment of bipolar disorder and depression. Read More About Lithium
Molecular structure:
Molecular structure refers to the arrangement of atoms in a molecule and the bonds between them. Understanding molecular structure is important in determining a molecule's physical and chemical properties. Read More About Molecular structure
Hybridization:
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that are better suited for bonding. It helps to explain the shape and bond angles in a molecule. Read More About Hybridization
Van der Waals Forces:
Van der Waals Forces are weak, attractive forces between non-bonded atoms and molecules. These forces are important in various applications, including the design of adhesives, the behavior of liquids, and the stability of biological molecules. Read More About Van der Waals Forces
Inductive Effect:
Inductive Effect refers to the transfer of charge or electron density through a molecule due to the presence of electronegative atoms. It helps in predicting the polarity of a molecule. Read More About Inductive Effect
Resonance:
Resonance is a concept in chemistry that describes the distribution of electron density in a molecule or ion that cannot be adequately represented by a single Lewis structure. It occurs when multiple Lewis structures are used to represent the same species. Read More About Resonance
Hyperconjugation:
Hyperconjugation is a type of chemical bonding that involves the transfer of electron density from a carbon-hydrogen (C-H) bond to an adjacent carbon-carbon (C-C) bond. This results in an increased stability of the molecule. Read More About Hyperconjugation
Tautomerism:
Tautomerism is the phenomenon where a single molecule exists in two different forms in a rapid equilibrium. It involves a structural isomerization in which a chemical bond is broken and rearranged. Read More About Tautomerism
Alkane:
Alkanes are organic compounds made of only carbon and hydrogen atoms. They are also known as “saturated hydrocarbons” as all the bonds between the carbon atoms are single bonds and they are not capable of forming multiple bonds. Read More About Alkane
Cycloalkane or Alicyclic Compounds:
Cycloalkanes are organic compounds composed of a ring of carbon atoms with only single bonds between them. They are also known as Alicyclic Compounds and are considered as saturated hydrocarbons like alkanes. Read More About Cycloalkane
ALKENE:
Alkenes are organic compounds composed of carbon and hydrogen atoms with at least one double bond between two carbon atoms. They are unsaturated hydrocarbons. Read More About ALKENE
ALKYNE:
Alkynes are organic compounds composed of carbon and hydrogen atoms with at least one triple bond between two carbon atoms. They are considered as the most unsaturated hydrocarbons. Read More About ALKYNE
STEREOCHEMISTRY:
Stereochemistry is a branch of chemistry that deals with the study of the three-dimensional arrangement of atoms and molecules in space. It is concerned with the spatial arrangement of atoms in a molecule and how this affects the physical and chemical properties of the molecule. Read More About STEREOCHEMISTRY
Racemic mixture:
A racemic mixture is a mixture of two enantiomers that are mirror images of each other but are not superimposable. The two enantiomers have the same physical and chemical properties, but they rotate plane-polarized light in opposite directions. Read More About Racemic mixture
Geometrical Isomerism:
Geometrical isomerism refers to the presence of different spatial arrangements of atoms in a molecule, resulting in two or more compounds with the same molecular formula but different arrangements of their atoms. Read More About Geometrical Isomerism
Alkyl Halides:
Alkyl halides are organic compounds that contain an alkyl group (a hydrocarbon chain) and a halogen atom. These compounds play an important role in many chemical reactions and industrial applications. Read More About Alkyl Halides
Sn1 and Sn2 Reactions:
Sn1 and Sn2 are two types of nucleophilic substitution reactions that occur in organic chemistry. The difference between these two reactions lies in the mechanism, rate of reaction, and stereochemistry of the product. Read More About Sn1 and Sn2 Reactions
Grignard Reagents:
Grignard reagents are organometallic compounds that are widely used in organic synthesis as highly reactive nucleophilic reagents. These reagents are formed by the reaction of alkyl or aryl halides with magnesium metal, and they have been instrumental in the development of many new organic compounds Read More About Grignard reagents
0 notes
Text
16.11 Service - Tutoring
I started tutoring a friend of my sister, Mirek, as he’s in his freshman year of high school and he’s struggling with chemistry, which I take at Higher Level in IB2. During this session, we went over topics related to periodicity, and the structure of an atom, complete with drawing electron shells, subshells, and orbitals. I hope his chemistry test goes well next week. It’s nice to be able to help younger people, like my sister and her friends, with subjects that they’re not the best in, because it makes me feel like a good teacher to see them score high on tests, and, additionally, I also review the easier topics myself, which helps me study for my finals.
0 notes
Text
Valence electrons periodic table

#Valence electrons periodic table how to#
These ions and the atom of argon are known as isoelectronic. Notice that the three ions have electronic configurations identical to that of inert argon.
#Valence electrons periodic table how to#
The charges on the chlorine, potassium, and calcium ions result from a strong tendency of valence electrons to adopt the stable configuration of the inert gases, with completely filled electronic shells. Valence Electron BasicsLearn how to use the periodic table in order to determine the number of valence electrons.The valence electrons are the electrons foun. Table 2 compares three ions and a neutral atom. In Table, the common oxidation numbers in the last column are interpreted as the result of either losing the valence electrons (leaving a positive ion) or gaining enough electrons to fill that valence subshell. For example, in the H 2O molecule, each H has an oxidation number of +1, and the O is –2. In molecules, the various atoms are assigned chargelike values so the sum of the oxidation numbers equals the charge on the molecule. These are the valence electrons.įor ions, the valence equals the electrical charge. These electrons are most distant from the positive nucleus and, therefore, are most easily transferred between atoms in chemical reactions. Valence electrons, which comprise the valence shell of the atom.įor brevity, many chemists record the electron configuration of an atom by giving only its outermost subshell, like 4 s 1 for potassium or 4 s 2 for calcium. The electrons in the highest numbered subshells are the Study the third column of complete electronic configurations carefully so you understand how electrons are added to the subshell of lowest energy until it reaches its capacity then the subshell of the next energy level begins to be filled. Valence electrons govern many chemical properties, reactivity, and bonding The group numbers (columns) of the periodic table indicate the total number of outer electrons in the valence shell The periods (rows) of the periodic table indicate the number of. This module explains the arrangement of elements in the period table. The electronic configuration of an atom is given by listing its subshells with the number of electrons in each subshell, as shown in Tableġ. Valence electrons are the outermost electron in an electron configuration. The modern periodic table is based on Dmitri Mendeleev’s 1896 observations that chemical elements can be grouped according to chemical properties they exhibit. Quiz: Introduction to Oxidation-Reduction Reactions.Introduction to Oxidation-Reduction Reactions.Quiz: Heat Capacities and Transformations.Quiz: Introduction to Organic Compounds.Quiz: Compounds with Additional Elements.

0 notes
Text
Electron configuration periodic table

#Electron configuration periodic table how to
The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. The number of sub-shells will be 5 but 4s, 4p, 4d, and 4f in these four subshells it is possible to arrange the electrons of all the elements of the periodic table. So, the sub-energy levels are 4s, 4p, 4d, and 4f. So, the sub-energy levels are 3s, 3p, and 3d. So, the sub-energy levels are 2s, and 2p. The sub-energy levels are known as s, p, d, and f. The sub-energy levels depend on the azimuthal quantum number. The most probable region of electron rotation around the nucleus is called the orbital. These sub-energy levels are also called orbital. Electron configuration of lead through orbitalĪtomic energy shells are subdivided into sub-energy levels. The electron configuration of all the elements can be done through the orbital diagram. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. Therefore, the order of the number of electrons in each shell of the lead(Pb) atom is 2, 8, 18, 32, 18, 4.Įlectrons can be arranged correctly through orbits from elements 1 to 18. Therefore, a lead atom will have two electrons in the first shell, eight in the 2nd orbit, eighteen electrons in the 3rd shell, and thirty-two in the 4th shell.Īccording to Bohr’s formula, the fifth shell will have twenty-two electrons but the fifth shell of lead will have eighteen electrons and the remaining four electrons will be in the sixth shell. That is, the number of electrons in lead is eighty-two. The atomic number is the number of electrons in that element. Lead atom electron configuration (Bohr model) Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. The maximum electrons holding capacity in N orbit is 2n 2 = 2 × 4 2 = 32. The maximum electrons holding capacity in M orbit is 2n 2 = 2 × 3 2 = 18. The maximum electron holding capacity in L orbit is 2n 2 = 2 × 2 2 = 8. The maximum electron holding capacity in K orbit is 2n 2 = 2 × 1 2 = 2. The electron holding capacity of each orbit is 2n 2. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. These circular paths are called orbit(shell). The electrons of the atom revolve around the nucleus in a certain circular path. The complete idea of the orbit is given there. Scientist Niels Bohr was the first to give an idea of the atom’s orbit. Lead atom electron configuration through orbit Lead ion(Pb2+, Pb4+) electron configuration.Lead excited state electron configuration.
#Electron configuration periodic table how to
How to write the orbital diagram for lead?.Electron configuration of lead through orbital.Lead atom electron configuration through orbit.

0 notes
Text
Periodic table chemistry class

#Periodic table chemistry class series
In what respect electron affinity differ from electro negativity.Īns: Electron affinity of an element is the energy released when an electron is added to an isolated gaseous atom to form a gaseous anion or negative ion. This results in the gradual increase in ionization energy across the period.ġ4. Due to the gradual increase in nuclear charge and simultaneously decrease in atomic size the electrons are more and more tightly bound to the nucleus. How does ionization energy of the element vary across the period?Īns: The value of ionization energy increases with increase in atomic number across the period.
#Periodic table chemistry class series
As, In both series of elements, the outermost shell and penultimate shell are incompletely filled but the filling of only f - orbitals of ante-penultimate shell occurs.ġ3. Why lanthanides and actinides are placed in separate rows at the bottom of the periodic table?Īns: Lanthanides and actinides are placed in separate rows at the bottom of the periodic table to avoid unnecessarily sidewise expansion of the periodic table. Their general valence shell configuration is (n-1)d 1 - 10, ns 1 - 2, where 'n' represents the outermost energy level.ġ2. They exhibit variable valency.Īns: The elements in which the last electron enters the d-subshell of the penultimate energy level are called d- block elements. They are called transition elements because they are placed between the most reactive metals on the left and non-metals on the right. They resemble each other in several physical and chemical properties. What are typical elements and transition elements? Why are they called so?Īns: Elements of the second period are known as typical elements because each element is placed in a group whose number matches with the number of valence electrons.Īll the elements belonging to 3 to 12 groups are called transition elements. They are highly reactive and form ionic compounds e.g. They are highly electropositive in nature. Some characteristics of alkali metals are: Give the characteristics of alkali metals?Īns: Alkali Metals contains elements of group IA. Size of such ions depends upon nuclear charge.Įxample: Sulphide (S 2 -), chloride(Cl -) and potassium (K +) ions are iso-electronic ions because each has 18 electrons but have different nuclear charges i.e. What are iso-electronic species with examples.Īns: Neutral species or ions of different elements having same number of electrons but different magnitude of nuclear charge are called iso-electronic species or ions. The basis of classification of elements in s-, p-, d- and f-blocks is by identifying to which orbital the differentiating electron of the atom enters.Ĩ. What is the basis of classification of elements in s-, p-, d- and f-blocks?Īns: When electrons are filled in the orbitals according to Aufbau principle, to get the electronic configuration of atom, the electrons which differentiates it from previous atom is called differentiating electron. Define the term differentiating electron. Hence, Cause of periodicity is the recurrence of similar electronic configuration.ħ. Thus, the recurrence of similar properties is due to the recurrence of similar electronic configurations or atomic structure. Justify it.įrom the electronic configuration of these elements it is clear that all elements having same number of electrons in the valence shell have similar properties. Periodicity of elements is according to electronic configuration or depends on atomic structure. What is meant by periodicity? Explain with example.Īns: The recurrence of elements with similar properties after certain regular intervals when these elements are arranged in the increasing order of their atomic numbers is called periodicity.ģ7Rb = 1S 2, 2S 22P 6, 3S 23P 63d 10, 4S 24P 6, 5S 1ĥ5Cs = 1S 2, 2S 22P 6, 3S 23P 63d 10, 4S 24P 64d 10, 5S 25P 6,6S 1Īll these elements have one electron in their valence shell and have the similar properties. periodic repetition of properties) is the basis of classification of elements in the long form of periodic table.ĥ. What is the basis of classification of elements in the long form of periodic table?Īns: The order of increasing atomic numbers or Periodicity (i.e. What are horizontal rows and vertical columns of periodic table?Īns: Horizontal rows are called Periods and vertical columns are called Groups of periodic table.Īns: Mendeleev's periodic law states that, "The physical and chemical properties of the elements are periodic function of their atomic weights. Mention the necessity for the classification of the elements.Īns: The necessity for the classification of the elements is to arrange all the known elements according to their properties so that similar elements fall within the same groups and dissimilar elements are separated so as to simplify their study.Ģ.

0 notes