#CACO3
Text
Been writing some romance lately and it's really difficult because Ive given the main couple this awful awful dynamic that's like...halfway between 100 gecs in the Doritos and Fritos video and late stage Jenna and Julien
Which is great for their chemistry but it also makes them absurdly fucking annoying together lMAO
2 notes
·
View notes
Text
Supplier of Monolayer Blown Film Machine in Telangana

Adroit Extrusion is a Manufacturer, Exporter, and Supplier of Monolayer Blown Film Machine in Telangana.
Our ISO 9001:2015 certified manufacturing unit demonstrates our dedication to offering personalized solutions globally, assuring high-quality in all aspects of our operations.
We are well-known for our commitment to quality and specialize in co-extruded blown film lines with diverse configurations such as Monolayer, ABA, two-layer, three-layer, five-layer, and seven-layer setups.
We design and produce the high quality Monolayer Blown Films with constant perfection and assurance.
Our Monolayer Blown films have best configured as well as professionalized outcomes according to our client’s need and satisfaction.
Features:
Compact size results in a minimum operational space requirement.
Increased production with lower power usage
Cold Start Preventer with Alarm for Machine Failure
Lower product costs while maintaining great film quality
Universal gusseting system.
Bubble calibrating basket and iris ring, along with an embossing roller attachment from Rimzim.
Specially constructed spiral die for uniform flow and improved thickness control.
Rotary die head, vacuum loaders, and hopper air driers
Technical Specification:
Model Name: Monolayer Blown Film Plant
Material Used: LDPE, LLDPE, HDPE, CACO3, Virgin Raw Materials etc.
Output Range: 30-200 kg/hr
Screw Diameter: 35/45/55/65/75 (Customized)
Layflat Film Width: 500-3000 mm
Thickness Range: 20 micron to 150 micron
Applications:
General purpose film, HDPE pick-up bag, Shade-net film, Lamination grade film, HD twist wrap film, Stretch and cling film, Shrink film, Anti-rust film and Paper-like film
Adroit Extrusion is the trusted Supplier of Monolayer Blown Film Machine in Telangana, serving locations such as Hyderabad, Warangal, Nizamabad, Khammam, Karimnagar, Ramagundam, Mahbubnagar, Nalgonda, Siddipet, Miryalaguda, Suryapet, Jagtial, Bhongir, Kamareddy, Mancherial, Wanaparthy, Jangaon.
For further details and inquiries, please feel free to contact us.
Read the full article
#ABA#AdroitExtrusion#andseven-layersetups#Bhongir#CACO3#ColdStartPreventerwithAlarm#Exporter#five-layer#HDPE#highqualityMonolayerBlownFilms#hopperairdriers#Hyderabad#Jagtial#Jangaon#Kamareddy#Karimnagar#Khammam#LDPE#LLDPE#Lowerproductcosts#Mahbubnagar#Mancherial#Manufacturer#minimumoperationalspacerequirement#Miryalaguda#monolayer#MonolayerBlownFilmMachineinTelangana#Nalgonda#Nizamabad#Ramagundam
0 notes
Text
Bột đá là gì? 6 ứng dụng bất ngờ của bột đá
Rất nhiều sản phẩm, vật dụng chúng ta sử dụng hằng ngày đều có thành phần của bột đá. Trong bài viết này chúng ta sẽ cùng khám phá xem bột đá là gì và bột đá dùng để làm gì nhé!
Định nghĩa bột đá là gì? Có những loại bột đá nào?
Nếu bạn chưa biết bột đá là gì thì ngay bây giờ chúng ta sẽ cùng tìm hiểu ngay định nghĩa về nó. Bột đá hay còn có tên gọi hóa học là Canxi Cacbonat, nó vốn dĩ là một hợp chất hóa học có công thức là CaCO3. Và một nghiên cứu rất thú vị cho thấy rằng ước tính vỏ trái đất của chúng ta có 5% là một dạng nào đó của canxi cacbonat tức là bột đá.
1 note
·
View note
Text
BIOZYME FOR FISH
ENZYME TIÊU HÓA GIÚP CÁ KHỎE MẠNH, MAU LỚN – PHÒNG BỆNH ĐƯỜNG RUỘT CHO CÁ
THÀNH PHẦN:
Bacillus Subtilis
Saccharomyces Cerevisiae
Amylase
Protease
Lipase
ß-Glucanase
Cellulase
CaCO3 vừa đủ
CÔNG DỤNG: Bổ sung enzyme tiêu hoá cho cá giúp cá tiêu hoá tốt thức ăn, khoẻ mạnh, mau lớn, phòng bệnh đường ruột trên cá.
LIỀU LƯỢNG VÀ CÁCH DÙNG: Trộn đều vào thức ăn, cho ăn liên tục đến khi thu…

View On WordPress
#Amylase#Bacillus Subtilis#bGlucanase#CaCO3#cá#Lipase#Men Tiêu Hóa & Chế Phẩm Sinh Học#Men Tiêu Hóa & Chế Phẩm Sinh Học Bio#Men Tiêu Hóa Cho Cá#Protease#Saccharomyces Cerevisiae#thủy sản
0 notes
Text
writing chemical equations feels like magical mystical divination type shit i have no idea how youre supposed to predict what chemicals can combine into because i never do it right
#txt#i just had to do caco3+hcl and i was like... oh ok hco3 and cacl idk idk. and its. cacl2 h2o and co2. whered h2o and co2 come from man.
5 notes
·
View notes
Text
Unveiling the Advantages of CaCO3 Filler Masterbatches in the Plastic Industry
Introduction:
One such innovation that has been gaining traction in recent years is the utilization of CaCO3 filler masterbatches. These masterbatches, also known as calcium carbonate filler masterbatches, offer a myriad of benefits to the plastic industry, ranging from cost-effectiveness to enhanced mechanical properties. Let's delve deeper into the unique advantages that CaCO3 filler masterbatches bring to the table.

Cost Efficiency:
One of the most notable advantages of CaCO3 filler masterbatches is their cost efficiency. Calcium carbonate, the primary filler used in these masterbatches, is a widely available and inexpensive mineral. By incorporating CaCO3 filler masterbatches into plastic production processes, manufacturers can significantly reduce material costs without compromising on quality. This cost-saving advantage makes CaCO3 filler masterbatches an attractive option for companies striving to optimize their production expenses while maintaining competitiveness in the market.
Improved Mechanical Properties:
In addition to cost efficiency, CaCO3 filler masterbatches offer enhancements in mechanical properties when added to plastic formulations. Calcium carbonate acts as a reinforcing agent, effectively increasing the stiffness and strength of the plastic material. This results in improved dimensional stability, impact resistance, and overall durability of the final products. Whether used in packaging, automotive parts, or construction materials, the incorporation of CaCO3 filler masterbatches can lead to plastics with superior performance characteristics, meeting the diverse requirements of various applications.
Reduced Environmental Impact:
Another significant advantage of CaCO3 filler masterbatches is their potential to contribute to a more sustainable plastic industry. Calcium carbonate is a naturally occurring mineral abundant in nature, making it an environmentally friendly alternative to synthetic fillers derived from fossil fuels. By utilizing CaCO3 filler masterbatches, manufacturers can reduce their reliance on non-renewable resources and minimize carbon footprint. Furthermore, the lightweight nature of calcium carbonate helps in decreasing the overall weight of plastic products, leading to lower transportation emissions and energy consumption throughout the product lifecycle.
Enhanced Processability:
CaCO3 filler masterbatches also offer improved processability during plastic extrusion and molding processes. The addition of calcium carbonate filler facilitates better dispersion within the polymer matrix, resulting in smoother processing and reduced machine downtime. This enhanced processability translates into increased production efficiency and cost savings for manufacturers. Moreover, the compatibility of CaCO3 filler masterbatches with various polymer resins enables flexibility in formulation adjustments to meet specific performance requirements, further enhancing their utility across different industries.
Versatility and Customization:
Furthermore, CaCO3 filler masterbatches provide versatility and customization options to plastic manufacturers. With the ability to adjust filler loading levels and particle sizes, as well as incorporate additives for desired functionalities, such as UV stabilization or flame retardancy, CaCO3 filler masterbatches can be tailored to meet specific application needs. Whether it's achieving a specific color, texture, or performance attribute, these masterbatches offer a versatile platform for customization, empowering manufacturers to create innovative solutions that address market demands effectively.
Conclusion:
In conclusion, CaCO3 filler masterbatches represent a compelling solution for enhancing the cost efficiency, mechanical properties, and sustainability of plastic products. From reducing material costs to improving product performance and minimizing environmental impact, the advantages offered by CaCO3 filler masterbatches are undeniable. As the plastic industry continues to evolve, the adoption of innovative technologies like CaCO3 filler masterbatches will play a pivotal role in driving progress towards a more sustainable and competitive future.
0 notes
Note
herb?
🌿 Herb: What is a scent you find relaxing?
Hmm. If we're going with candle scents, pine and sandalwood. But irl, I really really love damp earth and petrichor. When you walk into a wet cave (or even a dry cave) there's this indescribable scent of earthworms, clay, and wet chalk that overtakes your whole body. I went to Kartchner Caverns as a kid and I think that scent fundamentally changed my universe.
#ptxt#thanks for the ask! :3#wet chalk is probably the most accurate descriptor because that's processed CaCO3.
1 note
·
View note
Video
youtube
Caco3 Bột
Canxi Cacbonat CaCO3 99%- Quy cách 25kg/bao. Canxi Cacbonat được dùng trong công nghiệp như phụ gia nhựa ,cao su ,sơn,bột bả tường... trong nông nghiệp làm phân bón, thức ăn chăn nuôi Mọi chi tiết xin liên hệ Mr Đường/0946546655
1 note
·
View note
Text
Canxi Cacbonat, Canxi Cacbonat CaCO3 -25-50kg/bao-Tel 0946546655
0 notes
Note
I LOVE YOU’RE FANFICS SO MUCH YOU DONT UNDERSTAND 💕 no one write Dottore x reader anymore 😭 could I request child reader who’s getting tutored by a segment and they yell at them for not understanding so the reader gets upset and doesn’t come out of there room not even to eat so prime goes looking for them female please 😊
Aww thank you anon<33

"Iota, it's time for you to tutor (Y/n)." Theta stated as he watched his fellow segment file through the documents handed to him.
"I'm busy. You can tutor her instead."
"Ah, I'm afraid not. Prime has given a schedule on which of us segments will be tutoring our dear (Y/n). And for today's tutor.. is you." Theta replied as he approaches Iota with you in his arms before placing you down on Iota's lap and dashing out the office.
"Theta you piece of s--!"
Iota had to cut himself off as he glanced down at you and stared into your curious eyes, you were wondering what he's about to say. Prime has initiated a rule that no segment should curse within your hearing range or else they would be neutralized. What's worse is that you can repeat the swear words that the segments would yell, making Prime immediately know who cursed.
Iota could only sigh before grabbing a piece of paper and wrote down a few equations for you. He's already stressed out from having to sort the files Prime and the other segments gave him. Him having to tutor you right now is adding an additional strain on his back that one more bad thing could make him snap.
Hopefully, he'll be able to finish sorting the documents while you solve the equation he gave you.
"Alright, brat. Solve this simple question and then you can go away and do what you want."
Iota handed you a pencil to use before placing you down on the ground and going back to his work. You were happy to do the task your tutor segments would give you. Each time you got a right answer, the segments would give you a sweet in return.
You looked down at the paper and tried to read the question. You were a slow reader but with the help of Omega, you were able to understand what the context of a sentence means. You narrowed your eyes in confusion as you stared at the piece of paper.
What volume of 0.125M HCl is required to react with 0.500g of CaCO3?
'Wait.. volume means sound and react is how the other things would respond..?'
You let out a hum and wrote down your answer. The sound of a pencil scribbling across paper can be heard. You stood up from the floor and went over to Iota, waving the paper in your hand.
Iota switched his attention to you as he grabbed the paper before looking at you as if you had grown two heads. You stood proudly in front of him, happy to be able to finish a task so quick. Your happiness was quickly cut off when Iota pinched and pulled at your cheek, making you whine in pain.
"You idiot, what do you mean 'a very loud volume'? That's not even the right answer!"
"But that was what I understand-!"
You whimpered when Iota grabbed your face, his fingers were digging into your cheeks that bruises were starting to form. You tried to tell Iota that he's hurting you but the way he was glaring at you was enough to make you shut up.
"Dumbass! Did the others not teach you this yet, huh? All of us can easily solve this thing! Even Kappa can solve this and he's seven! It could be all those sweets you've been eating that's making your brain dumb!"
You sucked in a breath before chomping down on his hand. Iota yelped and quickly let you go, he let out a small growl before slapping you across your face.
Iota could only blink in surprise as he watched you hold your cheek in pain. He reached out his hand towards you but you only moved back and ran out of the office, the sound of your cries can be heard throughout the palace.
"Fuck.."
Iota gripped and pulled at his hair as he leaned forward his chair and trying to process what had just happened. He glanced at his desk that was still full of reports and tiredly sighed. He has to finish this first before he can go to you. You might even forget about what happened just now due to your forgetfulness.
"(Y/n)?"
Beta called out as Delta knocked on your door. They heard the door to your room slammed shut, the loud slam causing the test tubes to rattle a bit from the lab. They were worried since you would never slam your door shut so they decided to know what happened.
"(Y/n) come on, open the door. What's wrong?"
When they still didn't receive a response, they decided that you might probably went to take a nap. Beta decided to come check up on you later when lunch time comes.
You still hadn't come out of your room and it was already evening. The segments, except Iota, kept calling out for you and asking you to come eat but you wouldn't respond to them.
"(Y/n), it's me Kappa! Are you not going to eat dinner with us?"
Omega could only sigh in disappointment as he told the others to head to the dining room and eat. Once the others have left, Omega glanced back at your door before heading to where the others are.
"So where is (Y/n)?"
Prime asked as he sat by the end of the long table, staring at the segments that came in. Iota was sitting away from him with his arms crossed, trying to remember what he forgot to do.
"She still doesn't want to come out.." is what Delta answered.
"She skipped lunch and might probably skip dinner too. She's going to starve herself." Theta added.
Prime narrowed his eyes before standing up from his seat and heading towards your room, leaving the segments to themselves. Theta watched as Prime left the dining room before glancing at Iota.
"Iota, weren't you tutoring her this morning?"
Iota froze in place, which the segments took note of, as he remembered what he had forgot.
"It didn't go well."
"Do explain what you mean by 'it didn't go well'."
"Let's hypothetically say that she may have gotten slapped.."
The segments could only stare at Iota in shock before they glared at him.
"She's just five!"
"Go apologize to her!"
"The reason why she hasn't left her room to eat is because of you!"
At the same moment, you were sniffling under your blanket, cuddling a small plushie of Prime that was gifted to you by Columbina.
You heard another knock coming from your door as you wiped your tears away. You winced when you accidentally touched your cheek, the one where Iota slapped you earlier.
"(Y/n), it's me. I hope you don't mind me coming in.
Just from the voice alone, you could immediately tell that it was Prime. You didn't expect him to be outside your room. You didn't expect him to be able to open your bedroom door when you were sure you locked it. You mentally facepalmed at yourself for forgetting that Prime has a key to your room in case of emergencies.
You squeaked in surprise when the blanket has been removed over your form, you held the Prime plushie close to your chest as you looked up to see Prime himself staring down at you.
"(Y/n), what ever happened to your cheek?"
Prime gently held your face, inspecting the reddened and bruised spot on your cheek. You whimpered when he accidentally grazed his finger against the area, making you flinch back. He noticed your reaction before narrowing his eyes.
"Tell me who did this. Now."
You were always scared when Prime's tone would be cold and demanding when it comes to you. You know he would never hurt you but it's always rare for him to use his commanding voice. With a small gulp, you told Prime what happened. Prime listened to your rambles, he heard the way your voice started to crack and tears making its way to your eyes. His hand gently stroked your hair as to calm you down, his other hand went to cup your cheek and gently wipe away your tears using his thumb.
Once you finished telling Prime everything, you were a sniffling mess. You didn't come out of your room for the entire day because you were scared that Iota might yell and hurt you again. You held onto your plushie as Prime picked you up before walking out of your room.
"You could have went and told me instead if events like this happen, my dear. Even if the segments are me from different ages, I would be upset if I find out they hurt my little flower."
You and Prime walked in the dining room to see the other segments glaring at Iota from the other side of the room.
"Iota, we need to talk now. I have been informed about what you did to (Y/n) earlier and how you yelled and hurt her."
"It's not my fault that she's a fucking dumbass!"
"She is literally five years old! Cognitive thinking takes time to develop especially with children of her age!"
"It's a simple problem, Prime!"
"Answering stoichiometry questions that advanced does not count as a simple problem for her to solve! She can barely read and understand it!"
The banter between Prime and Iota continued on. You were feeling a bit light headed from the lack of food in your system so you held onto Prime's clothes to steady yourself.
"Did you ever think about her feelings as well? After you yelled at her, even slapped her, she hid in her room all because she was afraid of seeing you. She was afraid you might hurt her again."
Iota's eyes widened in shock as he glanced at your tiny form. You looked up and saw Iota's gaze before hiding yourself further in Prime's chest, your head leaning against his shoulder.
"Starting now, you are banned from tutoring (Y/n). And if you even try to touch her... I suppose I'll have to put a shock collar on you to keep your hands in check."
Prime heard your stomach growl as he went to get a plate of food before walking back to your room. Before he left the room, he glanced back at the other segments.
"Let this be a lesson for you lot if you try to hurt (Y/n). I won't be merciful. Iota, I'll deal with you later."
Once back in your room, Prime sat you down on your bed before helping you eat. He watched as you hungrily ate the stew, making him relieved to know you're not going to be hungry anymore. Prime took the empty plate from you before standing up to leave, only to stop when he felt you tug on his lab coat.
"Um.. is what Iota said true? Am I a dumbass?"
Prime's eye twitched in annoyance when you said dumbass, he already had a plan in mind on how to deal with of Iota when he's done.
"Not at all, don't listen to what Iota said.. don't even repeat that word. You're already intellectual for your age, my dear. Don't let others' opinions bring you down. After all, you are my prodigy."
Prime ruffled your hair before tucking you in bed. He watched as you held the plushie of himself and cuddled it.
"Nighty night, Prime.."
"Good night, my sweet. May your dreams be kind tonight."
#genshin impact#genshin impact x reader#dottore x reader#dottore#zandik x reader#il dottore x reader#female reader
265 notes
·
View notes
Text
Science Saturday: How to make Limestone
Limestone is a type of sedimentary rock made by chemical processes (dare I say, a chemical sedimentary rock) rather than physical processes (what we call a clastic sedimentary rock).

Limestone in Big Cottonwood Canyon, Utah with my hammer for scale.
Limestone is primarily composed of the mineral calcite or aragonite which precipitate out of water containing dissolved calcium ions.
(CaCO3) Calcite and aragonite chemical formula


This can happen through both biologic and non-biologic processes. About 20-25% of all sedimentary rocks are carbonates and most of those carbonates are limestone. The remaining carbonates are mostly dolomite (or dolostone to avoid confusion with the mineral dolomite) which differs from limestone due to it's high magnesium content.

Dolomite Peaks, Italy
In fact, physically, you can't tell dolomite and limestone apart (at least not to my knowledge, I work in clastic sedimentary rocks so if you're reading this and you work in carbonates, feel free to chip in), usually just lump all carbonates I see as limestone. Technically, there are few things you can test physically but you absolutely need the right tools. Dolomite is slightly harder than limestone and does not as readily dissolve in HCl (fizzes less). Sometimes you can see a change but often they are just too similar to tell in my experience.

Limestone is commonly gray to white though iron or managnese can make it yellow, or red and high organic content can make it almost black.

Gray limestone in Provo Canyon, Utah

Red stained yellow limestone in Timpanogos Canyon, Utah
But how is limestone formed? One way is through biochemical processes. Many marine organisms have learned to precipitate a calcium carbonate shell. When these organisms die, they fall to the sea floor. Eventually, they are turned into bioclastic limestone with chemically precipitated calcite cement between them.

bioclastic limestone, Provo Canyon. Brachiopod shells with my hand for comparison.
One type of bioclastic limestone is finely-grained chalk like the White Cliffs of Dover which formed from coccoliths. Chalk can be formed from algae, foraminifera and plankton. It is very soft, porous version of limestone.

Limestone can also form chemically, precipitating straight out of the water as such: H2O + 3CO2 -> CaCO3.
And now you know the basic ways to make limestone.
47 notes
·
View notes
Text
Bột đá vôi trắng tự nhiên siêu mịn không tráng phủ axit stearic. Nguyên liêu được khai thác từ mỏ đá vôi trắng của Công ty với chất lượng tốt nhất, độ tinh khiết cao và được chế biến trên dây chuyền công nghệ tiên tiến hàng đầu thế giới – dây chuyền đồng bộ của hãng Hosokawa Alpine – CHLB Đức.
1 note
·
View note
Text
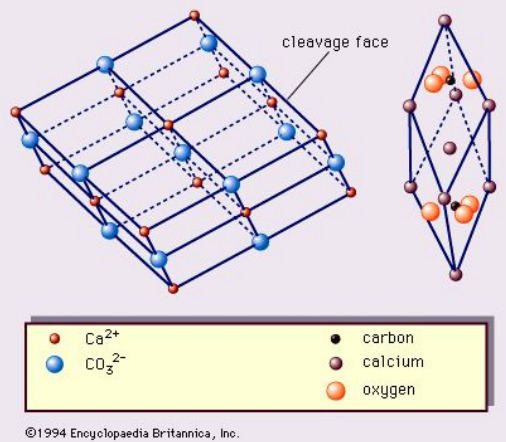
Calcite (CaCO3)


Calcite (CaCO3) is the most common carbonatic mineral on the Earth's surface. Carbonatic minerals are known for containing the CO3 compound in their structure, which then binds to an ion that is, in the calcite's case, calcium!
Carbonatic minerals are known primarily for forming in acquatic environments, be they in the ocean (carbonatic platforms) or on a continent (caves with stalagtites and stalagmites, lakes).


Calcite can take many different forms and shapes: the most well known variety is spatite, a completely transparent and flat crystal that is common in Iceland.


Sometimes it appears as an aggregate of long crystals that seem to all emerge from the same spot.

Often calcite will look colorless, white or a very dull light grey, but commonly it can take other colors: pink, yellow, green and blue





One of calcite's most remarkable properties is the double refraction effect, if you look at an image through a calcite crystal (most noticeable with spatite) the image will be shown twice!

Another remarkable thing about calcite is that by looking at a raw crystal's shape, you can assess if the water that it was formed in is rich or low in magnesium.
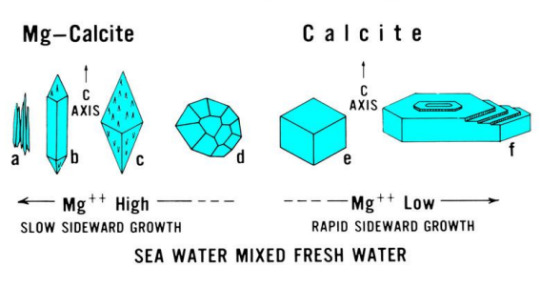
In general, the structure of a calcite crystal looks more or less like this, for those who are interested.
---
I wanted to talk about aragonite and dolomite too but I thought this was too long so maybe tomorrow I'll make a separate post about it👍
205 notes
·
View notes
Text
Marigold’s Witchcraft | Tip of the Day

If you plan on sprinkling any mixtures (like blended herbs, dried flowers, dried fruits etc.) into or onto soil (for a spell or ritual) or you plan to dispose of your spells outside, and the spell requires salt, replace the salt with crushed egg shells. Doing this ensures you don’t damage the soil because salt will basically poison the dirt so that plant life will not be able to grow.
Salts in the soil can absorb water. This results in less water being available for uptake by the plants, increasing water stress and root dehydration. This is referred to as physiological drought, which, if not corrected, can lead to reduced plant growth. XX
However, [chicken] egg shells are comprised of about 96% calcium carbonate (CaCO3). Calcium is an essential plant nutrient and moderates soil acidity.
Egg shells, good 👍🏻
Salt, bad 👎🏻
Magically speaking, egg shells and salt share very similar properties. So substituting one for the other shouldn’t be an issue. Unless however, you plan to ingest your mixture or blend. This substitution is really only necessary if you plan to bury your mixture or you plan to dispose of it in soil or outside.
#witchcraft#witch#spells#rituals#hexes#curses#witchy#witchlife#witch tips#witch community#witchcraft tips#salt#egg shells
107 notes
·
View notes
Text

Most of Carlsbad Caverns' speleothems (cave deposits such as these stalagmites) consist of the mineral calcite (CaCO3) that precipitates from groundwater that enters a cavern. Some speleothems are also composed of aragonite and gypsum. (USGS)
#archive#archiving#web archive#cave#caves#cavern#carlsbad caverns#carlsbad#speleology#speleothem#speleothems#stalagmites#calcite#usgs
56 notes
·
View notes
Text
Unveiling the Power of CaCO3 Filler Masterbatches in Plastic Production
In the realm of plastic manufacturing, innovation is the cornerstone of progress. One such groundbreaking innovation comes in the form of Calcium Carbonate (CaCO3) filler masterbatches. These masterbatches, laden with the mineral's properties, have revolutionized the plastic industry by offering a plethora of benefits ranging from cost-effectiveness to enhanced product performance. Let's delve into the depths of CaCO3 filler masterbatches and explore why they are becoming the go-to choice for manufacturers worldwide.

Understanding CaCO3 Filler Masterbatches
CaCO3 filler masterbatches are composite materials comprised of a polymeric carrier resin, typically polyethylene (PE) or polypropylene (PP), and high concentrations of calcium carbonate. Advantages of CaCO3 Filler Masterbatches:
1. Cost-Effectiveness:
Incorporating CaCO3 filler masterbatches into plastic production significantly reduces material costs. Calcium carbonate is abundant and relatively inexpensive compared to traditional polymer resins, thus offering a cost-effective solution without compromising quality.
The high loading capacity of calcium carbonate allows for the production of more volume with less polymer, resulting in substantial cost savings in large-scale manufacturing operations.
2. Improved Mechanical Properties:
Despite being a filler material, CaCO3 enhances the mechanical properties of plastics. It improves stiffness, tensile strength, and impact resistance, thereby enhancing the overall durability of plastic products.
This enhancement in mechanical properties enables the creation of lightweight yet robust products, ideal for applications requiring structural integrity such as automotive parts, pipes, and packaging materials.
3. Environmental Sustainability:
CaCO3 filler masterbatches contribute to environmental sustainability by reducing the carbon footprint of plastic products. The use of calcium carbonate, a naturally occurring mineral, decreases reliance on petrochemical-based polymers, thus lowering greenhouse gas emissions.
Furthermore, the lightweight nature of CaCO3-filled plastics translates to reduced transportation costs and energy consumption during product distribution, further aligning with eco-friendly practices.
4. Enhanced Processing Characteristics:
Incorporating CaCO3 filler masterbatches into plastic formulations improves processing characteristics such as melt viscosity and flowability. This facilitates easier processing on conventional manufacturing equipment, leading to increased production efficiency and reduced energy consumption.
Manufacturers can achieve faster cycle times and higher throughput rates, resulting in improved operational efficiency and reduced production costs.
Applications of CaCO3 Filler Masterbatches:
CaCO3 filler masterbatches find extensive applications across various industries, including:
Film and sheet extrusion for packaging materials
Injection molding for automotive components, household appliances, and consumer goods
Pipe and profile extrusion for construction and infrastructure
Wire and cable coating for electrical insulation
Conclusion:
In conclusion, CaCO3 filler masterbatches represent a paradigm shift in the plastic manufacturing landscape, offering a synergistic blend of cost-effectiveness, enhanced performance, and environmental sustainability. By harnessing the inherent properties of calcium carbonate, manufacturers can achieve remarkable advancements in product quality, process efficiency, and sustainability metrics. As the demand for eco-friendly and cost-efficient solutions continues to rise, CaCO3 filler masterbatches stand at the forefront, poised to shape the future of plastic production for generations to come.
0 notes