#ATP Synthesis
Explore tagged Tumblr posts
Text
Mitochondrial Dysfunction in mtARS Disorders
Introduction
Mitochondria are indispensable organelles that facilitate cellular bioenergetics, predominantly through oxidative phosphorylation (OXPHOS). Mitochondrial aminoacyl-tRNA synthetases (mtARS) are essential for the fidelity of mitochondrial translation, catalyzing the ligation of amino acids to their cognate tRNAs. Mutations in mtARS genes precipitate a spectrum of mitochondrial disorders, culminating in dysfunctional protein synthesis and aberrant mitochondrial bioenergetics. This review delves into the molecular pathogenesis of mitochondrial dysfunction in mtARS disorders, elucidating their biochemical perturbations, clinical phenotypes, and emerging therapeutic paradigms.
Molecular Pathophysiology of mtARS Disorders
MtARS enzymes ensure translational accuracy by charging mitochondrial tRNAs with their respective amino acids, a prerequisite for mitochondrial protein biosynthesis. Pathogenic variants in mtARS genes result in defective aminoacylation, perturbing mitochondrial translation and compromising the integrity of the electron transport chain (ETC). These perturbations induce bioenergetic deficits, increased reactive oxygen species (ROS) production, and secondary mitochondrial stress responses, leading to cellular demise.
Genetic Etiology of mtARS Mutations
Dysfunctional mtARS genes such as DARS2, AARS2, RARS2, and YARS2 have been implicated in autosomal recessive mitochondrial disorders. These mutations exhibit tissue-specific phenotypic heterogeneity, with neurological, muscular, and systemic manifestations. For instance, DARS2 mutations drive leukoencephalopathy with brainstem and spinal cord involvement, whereas AARS2 defects result in a constellation of neurodegenerative and ovarian pathologies.
Biochemical and Cellular Consequences
Dysfunctional mtARS enzymes manifest in multifaceted mitochondrial deficits, including impaired translation, defective OXPHOS, and dysregulated mitochondrial proteostasis.
Disruption of Mitochondrial Translation
Impaired aminoacylation abrogates the synthesis of mitochondrially encoded proteins, undermining the assembly of ETC complexes. This translational arrest culminates in defective ATP synthesis and precipitates a systemic energy deficit.
Electron Transport Chain Dysfunction and Bioenergetic Failure
Pathogenic mtARS mutations lead to OXPHOS inefficiencies, reducing mitochondrial membrane potential (Δψm) and ATP output. Perturbed electron flux exacerbates ROS accumulation, instigating oxidative damage and apoptotic cascades.
Mitochondrial Unfolded Protein Response (UPRmt) Activation
Cellular compensatory mechanisms, including UPRmt, are upregulated in response to mitochondrial translation failure. UPRmt mitigates proteotoxic stress via chaperone-mediated protein refolding and degradation pathways. However, chronic UPRmt activation fosters maladaptive stress responses, contributing to progressive cellular degeneration.
Clinical Manifestations
mtARS disorders exhibit phenotypic variability, spanning from mild neuromuscular impairment to severe multisystemic involvement. The pathophysiological hallmark includes disrupted neurological, muscular, and cardiac function.
Neurological Dysfunction
Neurodegeneration is a predominant feature of mtARS disorders, manifesting as ataxia, seizures, intellectual disability, and progressive leukoencephalopathy. Magnetic resonance imaging (MRI) frequently reveals white matter abnormalities, indicative of compromised oligodendrocyte function.
Myopathy and Metabolic Dysregulation
Muscle tissue, with its high ATP demand, is particularly susceptible to mitochondrial dysfunction. Clinical hallmarks include hypotonia, muscle weakness, and exercise intolerance, often concomitant with metabolic anomalies such as lactic acidosis and elevated pyruvate-to-lactate ratios.
Cardiomyopathy and Mitochondrial Energetics
Hypertrophic cardiomyopathy has been observed in YARS2-associated mitochondrial disorders, wherein compromised ATP synthesis in cardiomyocytes disrupts contractile function and electrophysiological stability.
Diagnostic and Functional Evaluation
A combination of genomic, biochemical, and imaging modalities facilitates the diagnosis of mtARS disorders.
Genomic and Transcriptomic Analysis
Whole-exome sequencing (WES) and whole-genome sequencing (WGS) are pivotal for identifying pathogenic mtARS variants. Transcriptomic profiling elucidates perturbations in mitochondrial gene expression networks, further refining diagnostic accuracy.
Functional Mitochondrial Assays
Biochemical assays, including high-resolution respirometry, ATP quantification, and ETC enzymatic profiling, provide insights into mitochondrial bioenergetics. Patient-derived fibroblasts and induced pluripotent stem cells (iPSCs) serve as valuable models for functional interrogation.
Neuroimaging and Biomarker Identification
Advanced imaging modalities such as MR spectroscopy (MRS) detect metabolic derangements, including lactate accumulation in affected brain regions. Circulating mitochondrial-derived peptides and metabolomic signatures are emerging as potential diagnostic biomarkers.
Emerging Therapeutic Strategies
Despite the absence of curative therapies, multiple avenues are under investigation to ameliorate mitochondrial dysfunction in mtARS disorders.
Mitochondria-Directed Antioxidants
Therapeutic compounds such as MitoQ, idebenone, and edaravone aim to attenuate oxidative stress and preserve mitochondrial integrity.
Genetic and RNA-Based Interventions
Gene therapy strategies utilizing adeno-associated virus (AAV)-mediated delivery and CRISPR-based genome editing are being explored for genetic correction of mtARS mutations. Additionally, RNA-based approaches, including antisense oligonucleotides (ASOs) and mRNA replacement therapy, hold promise in restoring mtARS functionality.
Metabolic Modulation and Supportive Therapies
Ketogenic diets, NAD+ precursors (e.g., nicotinamide riboside), and mitochondrial biogenesis activators (e.g., PGC-1α modulators) are under investigation to enhance cellular energy metabolism. Supportive interventions, including physical therapy and neuromuscular rehabilitation, remain integral to patient management.
Conclusion and Future Directions
Mitochondrial dysfunction in mtARS disorders arises from defective mitochondrial translation, OXPHOS perturbation, and maladaptive stress responses. Advances in genomic medicine, mitochondrial therapeutics, and precision medicine approaches are poised to transform the diagnostic and therapeutic landscape. Continued research into mtARS pathobiology, coupled with translational innovations, will be instrumental in developing targeted interventions for affected individuals.

#Mitochondrial dysfunction#Aminoacyl-tRNA synthetases (mtARS)#Oxidative phosphorylation (OXPHOS)#Electron transport chain (ETC)#Reactive oxygen species (ROS)#Mitochondrial translation#Mitochondrial unfolded protein response (UPRmt)#Bioenergetic failure#Neurodegeneration#Leukoencephalopathy#Hypertrophic cardiomyopathy#Myopathy#Whole-exome sequencing (WES)#Whole-genome sequencing (WGS)#ATP synthesis#Gene therapy#CRISPR-based genome editing#RNA-based interventions#Metabolomic biomarkers#Mitochondrial biogenesis
0 notes
Text
Your Energy Levels Could Be Tied To Your Mother’s Mitochondria – Find Out Why
A study from the University of Colorado Boulder emphasizes the importance of maternal mitochondrial DNA (mtDNA) in energy production and health. Published in Science Advances, it highlights that mtDNA, inherited from mothers, is vital for ATP synthesis, while paternal mtDNA is eliminated post-fertilization. Researchers demonstrated that delaying this elimination in a model organism affected energy levels and later health outcomes. The findings suggest implications for mitochondrial disorders and propose vitamin K2 (MK-4) supplementation as a potential treatment to restore ATP levels, offering hope for families with mitochondrial issues.
#maternal mitochondrial DNA (mtDNA)#energy production#ATP synthesis#mitochondrial inheritance#mitochondrial disorders#University of Colorado Boulder#vitamin K2 (MK-4) supplementation#health outcomes#Science Advances study#paternal mtDNA elimination
0 notes
Text
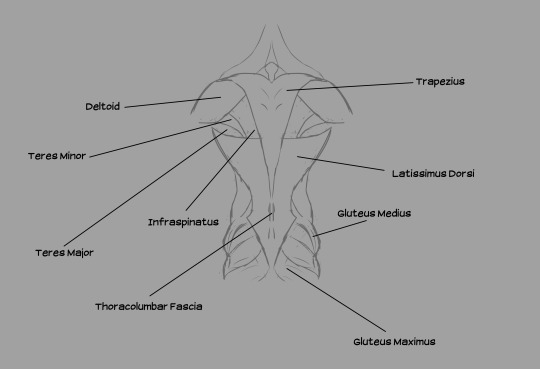
After referencing numerous diagrams, avian necropsy records, and my own prior knowledge of human musculoskeletal structure, I have devised speculative anatomical structure for Chozo.
We'll start small with my first attempts to properly chart basic skeletal structure from last year (seeing as how that's what I used as a jumping off point), then move on to the research-based stuff. I wanted to walk through the process of solving problems presented by the skeletal structure.
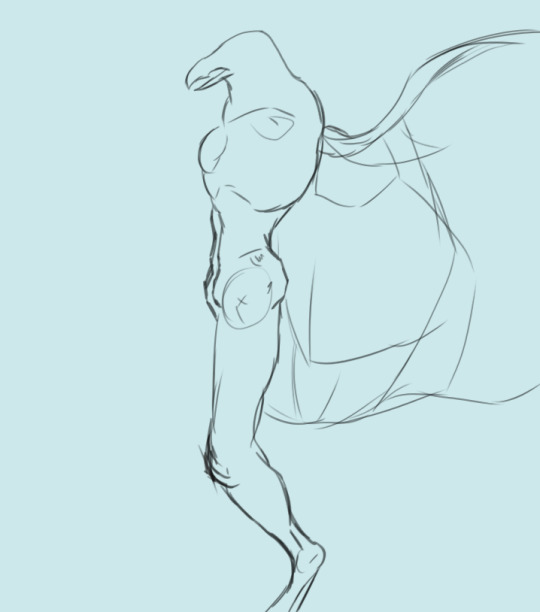
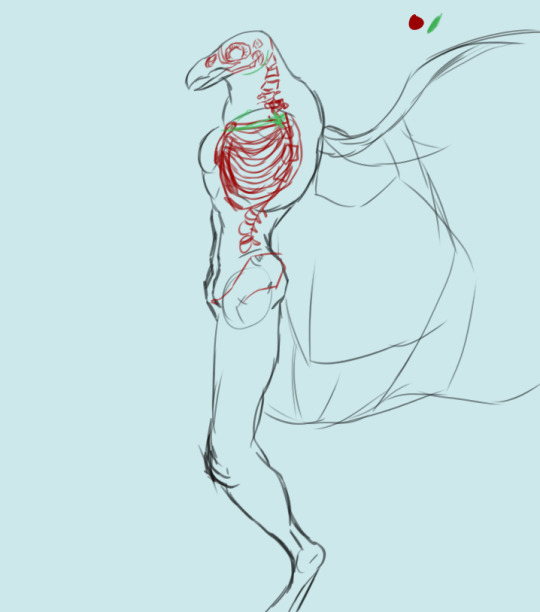
First we have a cursory look at the ribcage. Drafted June 7, 2023. Leaning into the more humanoid appearance.
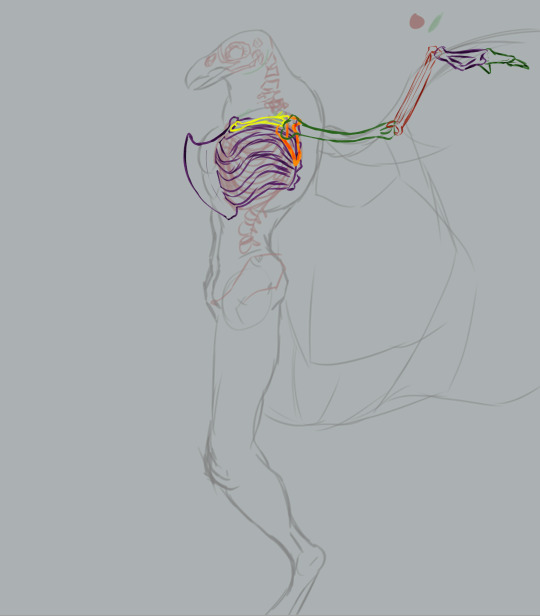
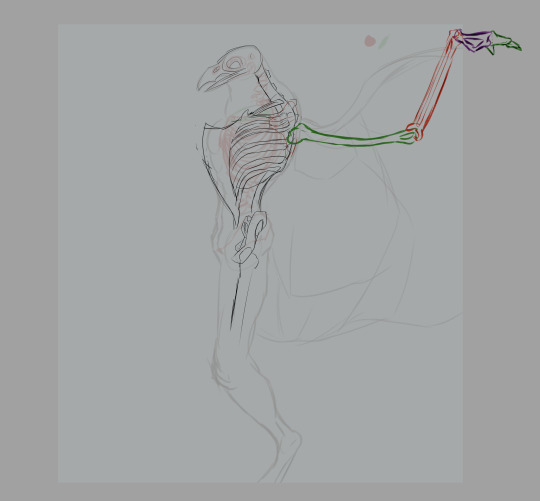
New addition, May 2024: a first shot at sussing out wing bones. These bones need to be much, much larger to accommodate the full breadth of the limb: this is just a rough outline. The skeleton also needs to bear muscles that are strong enough to carry the Chozo in flight, hence the new protrusion on the chest: a keel. Two variants of this new breast came out of this drawing session: one with a large keel that extends below the sternum and one with a normal keel.
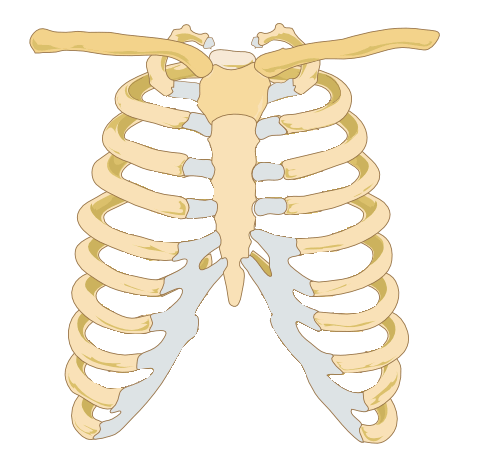
Image credit: Wikipedia
Human ribcages have this ridge along the bottom that the last six ribs are attached to (noted in grey on the image above). We're not doing that with the Chozo ribcage.
The sternum is the structure in the middle, which the ribs are attached to. See those two bones attached to the top of each side of the sternum, stretching away from the center of the ribs and forming sort of a capital "T" silhouette? Those are the clavicles. When you're drawing any humanoid form, the clavicles are an excellent landmark (and as I've been taught, the first place you should start on anatomy after you've laid out your pose, armature, etc).
It's also part of why wings are so difficult to suss out on Chozo skeletons. In birds, a bone that consists of a fusion of the two clavicles is a crucial part of flight: the clavicle bridges the gap between the ribs and the arms, and for birds, the wings are their arms. That's problem number one: effectively consolidating two pairs of arms on one torso.
We have a few bones to add onto the human skeleton in order to make flight possible for Chozo. First, we'll assume all bones are hollow. This makes them lighter, demanding considerably less energy to lift them off the ground in the first place.
I've modified the sternum to add a keel, which the base flight muscles are going to be anchored to.
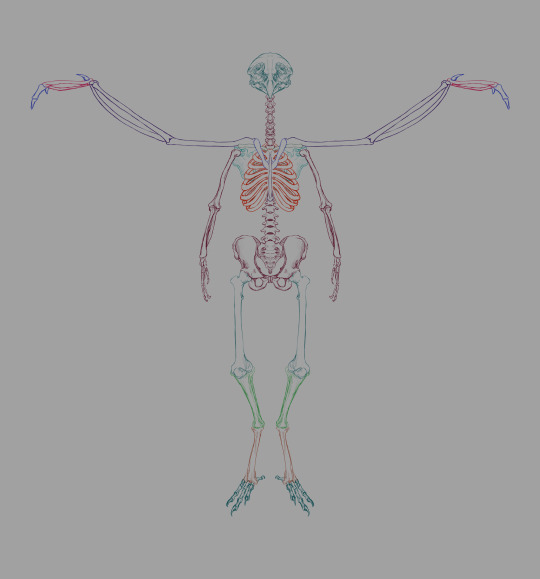
First pass at revising the skeletal structure. I made a few modifications unrelated to the wings. The pelvis is similar to that of a human, though a little wider to accommodate egg-laying. I may end up reworking the pelvis entirely to make it more bird-like, but I'm more interested in making those wings fit at the moment. Chozo have a human femur/patella, and avian lower legs.
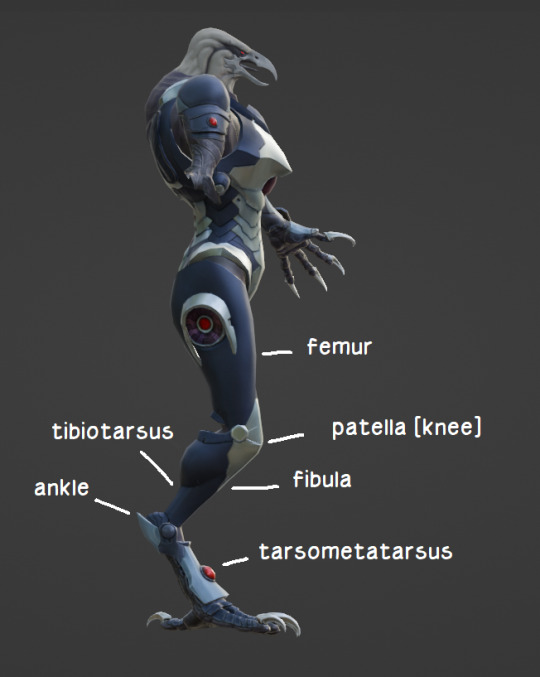
Here's a thing I slapped together in 3 minutes in January of last year to illustrate which bones are where for the layman. Onto different matters.
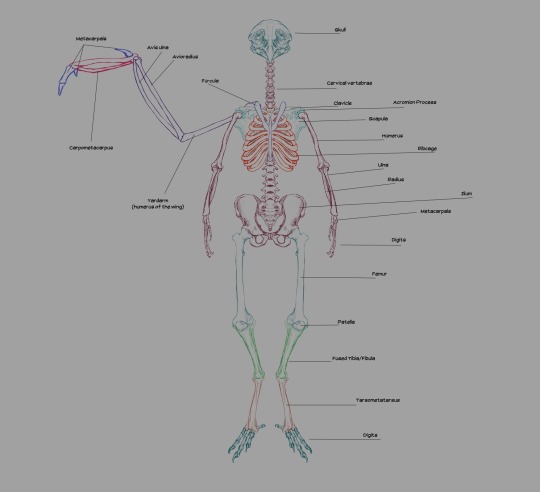
Skeleton, labeled.
Generally, the wings' humerus is attached to the scapulacoracoid, a bone attached to the keel that's sort of Y-shaped. That's how a real bird's shoulders are structured. Humans posses a scapula (shoulderblade), which has two protrusions: the acromion process and the coracoid process. The acromion process is where our humerus joins the shoulder. The coracoid process in humans is not exactly big enough nor ideally shaped to anchor flight muscles to.
At first, I had three ideas:
Invent a new bone attached to the keel that serves the function of the coracoid.
Modify the scapula to fit a new bone that anchors the flight muscles (the scapulacoracoid is analogous to the human scapula, after all).
Forego the keel and invent a bone on the spine that does the same thing.
To start, I added the furcula, a Y-shaped bone on the sternum, flanking the keel. Fun fact: not all birds have a furcula (better known as the "wishbone" in some parts of the world).
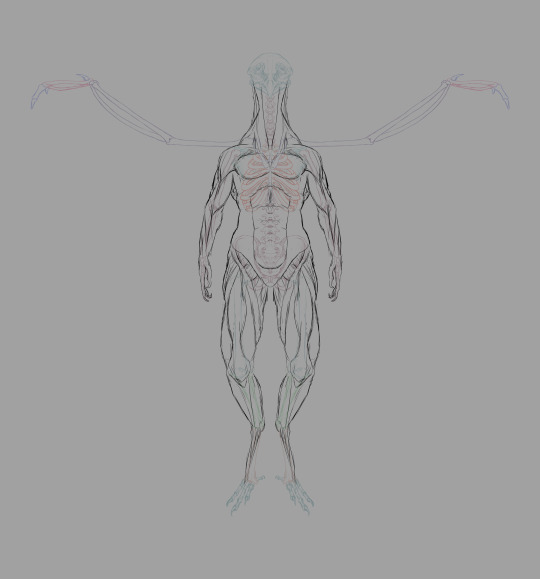
Real quick muscular structure layout sans flight muscles.
First pass at the flight muscles. Not the most accurate wing muscles in the world (neglected to depict the muscles near the tip of the wing, for one).
In this model of the musculature, a good deal of the flight muscles around the breast and torso are hidden by the pectoralis major, much like several non-flying human muscles.
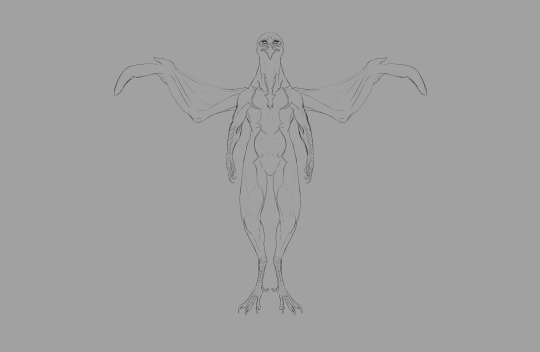
Flesh applied over muscle.

Feathers applied over flesh.
That was my first attempt at constructing the Chozo skeleton. You'll notice the wing bone solution is inelegant. See, wings are analogous to arms. Their metacarpals are finger bones. In order to give Chozo both arms and wings, we'll need to deviate from both avian and human skeletal structures to make the pieces fit together.
I can't make the flight muscles stretch comfortably over the clavicle: that has the potential to impede motion in the arms. My first idea in the second round of flight bone shenanigans was to invent a second bone that fit between the spine and the scapula, like shoulder-bound plate tectonics (working name "scapula trellis"). I wasn't wholly confident that I could configure flight muscles in a logical manner even with this setup.

At one point I consulted Raven Beak's model. Note the patches on the back of the torso on the powersuit: that's where the wings emerge in phase 2. It looks like they're anchored to the scapula or an adjacent structure.


Barring the fact that his wings are absolutely ridiculous, I wasn't sure I could work with this. Gorgeous structures, but the feathers don't seem big enough to handle flight.
So I was left to brainstorm, and drafted up a few sketches for a second scapula to anchor the wings' shoulder joints to. I was more confident in this than I was the previous design, but I wanted to fish for ideas from other parts of nature.
Enter dinosaurs. Specifically, the Pteranodon with its shoulder girdle.

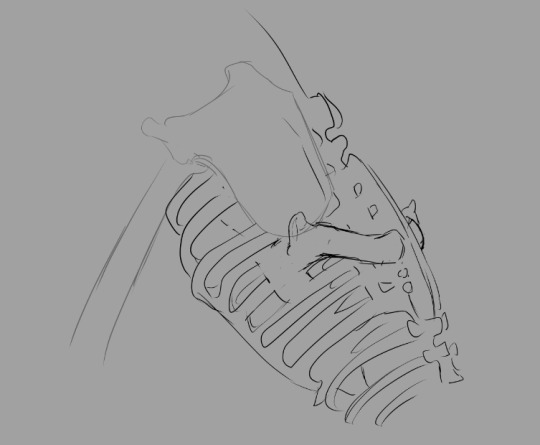
The addition of a shoulder girdle as an anchor for the yardarm (the term I'm using for the humerus of the wing, applicable only to creatures that have both wings and arms) seemed like a better solution. Positioning it below the scapula allows me to place the wings a little lower on the back, providing minimal interference between the two sets of limbs.
Whether we're rolling with the shoulder girdle or a second scapula, the intended result is the same: the wings have moved down on the back of the torso (personally, I'm digging the girdle, but the second scapula is on the table if anyone else wants to try their hand at this).
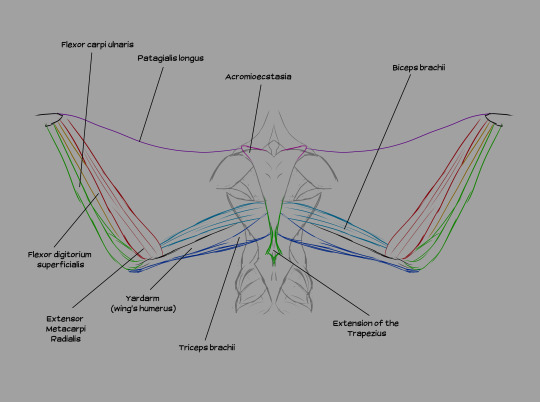
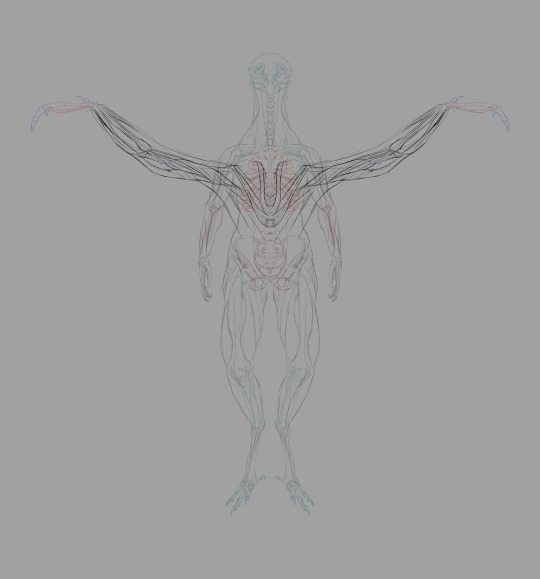
Muscles from the back, illustrated. Note the distance between the deltoid (shoulder muscle) and the wings. The shoulder girdle is situated in the lower-middle of the back of the ribcage.
A few notes: the acromioecstasia exists because the muscle that usually connects between the body and the patagialis longus on real birds is located on the pectoralis major. If I emulated that, we'd have flesh crossing over the deltoid to reach the front of the body, which would obstruct movement of the arms. We don't want that, so I moved that section of the wing to the back. We're compensating by adding additional musculature up front.
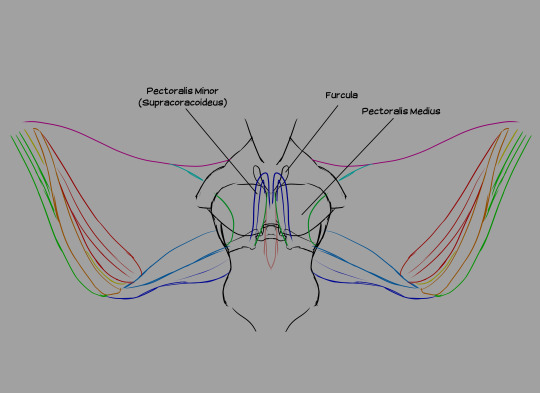
Wing muscles from the front. All three pectoral muscles are attached to the keel. The pectoralis medius is an extension of the pectoralis major, running beneath it and several other muscles. The pectoralis minor (also known as the supracoracoideus) lies beneath both the major and the medius. The pectoralis medius and major are responsible for the downstroke, while the supracoracoideus raises the wing between flaps.
Flight is very taxing on the individual. Power suit wearers actually have an easier time flying than non-wearers because the suit passively offsets the metabolic demands of flight with its own Energy.
It's important to note that these sizes are not necessarily "to scale". Chozo wings should actually be much bigger than my canvas permitted me to show. I had to keep increasing the size of the canvas on one of my files to accommodate a reasonable wingspan, but even that's not broad enough! I had to stop expanding the canvas for the sake of my CPU. If any muscles look too dinky or the scale seems off on some bones, that's why: I just needed to swiftly illustrate where things are.
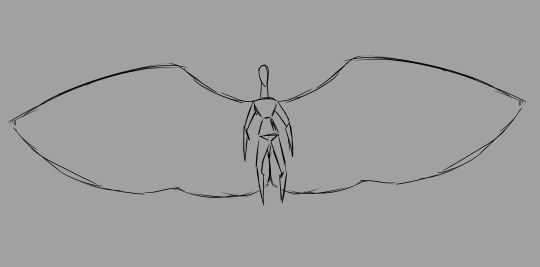
A Chozo's total wingspan should at least be twice the individual's standing height. Any smaller and there's no lift.
I still don't necessarily consider the wings "solved": if any speculative biology enthusiasts want to weigh in further on the subject, feel free!
After laying out the bulk of the skeleton (and before solving the wing problem), I decided to go a little further in my studies. Thus, we have organs.
First, the digestive system.
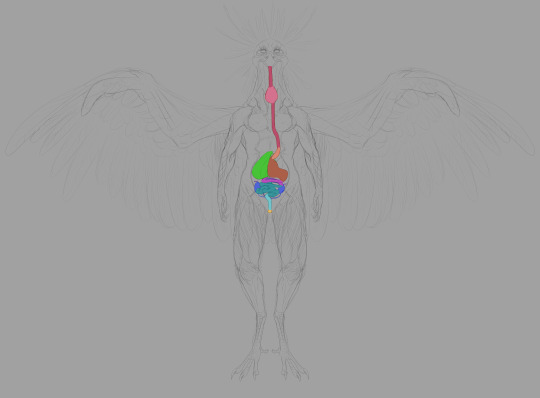
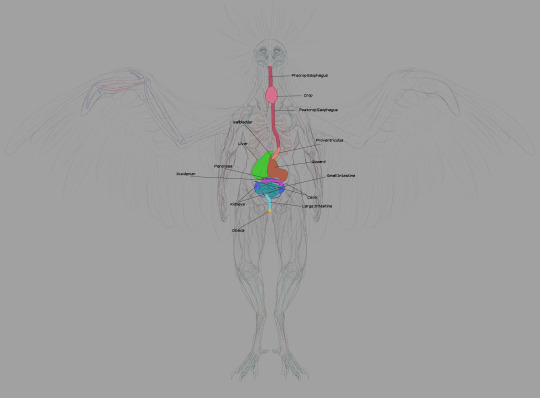
The esophagus is self-explanatory. Food goes in through the beak, traveling into the body through the esophagus.
The crop is used to store food: this is where digestion begins. Many parents regurgitate mushy, enzymatic food for their children from the crop. Very nutritious and promotes growth.
The proventriculus is the first half of the stomach: protein bonds begin to break down here. Gastric fluid produced here aids the gizzard in mushing things.
The gizzard is where the bulk of food-crushing occurs. Breaks larger matter down through transfer between areas within the organ.
The liver and gallbladder are crucial in digesting fats. Real bird livers have two lobes: the left is smaller than the right. Two bile ducts from the liver connect to the distal duodenum: the right duct is connected to the gallbladder. Chozo only need one.
The duodenum is the start of the small intestine, running in tandem with the pancreas. Pancreatic enzymes created by the latter assist in completing digestion, processing sugars, etc.
Digestion is finished in the other sections of the small intestine, where nutrients are absorbed.
Chozo kidneys largely resemble their human counterparts. Connected to the lower half of the gastrointestinal system. Urate is disposed of through the cloaca, transferred from point A to point B by the thin ureters bridging the kidneys to the large intestine.
Bacterial fermentation in the ceca extracts nutrients from plant material that can't be digested through enzymatic breakdown. The ceca and large intestine also reabsorb moisture, forming the solid portion of indigestible waste. The ceca are larger in tribes that eat more fruit and other plant products. Mawkin ceca are fairly small: they live quite an active lifestyle, and plant matter supplements their all-rounder diet with meat as the foremost staple.
The large intestine is the end of the line. Renal and intestinal waste is ejected here. The end of the reproductive tract forks to the distal segment to facilitate egg laying. Mammals have considerably larger large intestines than Chozo to dry out waste before expulsion.
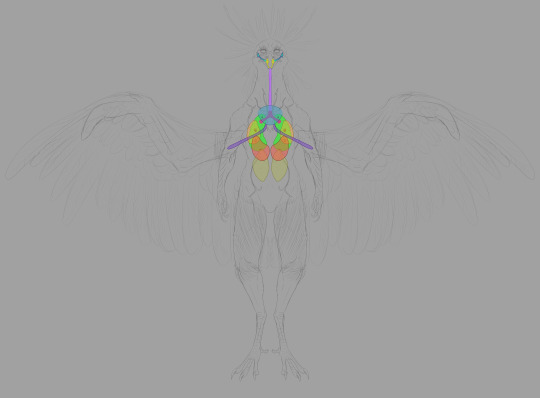
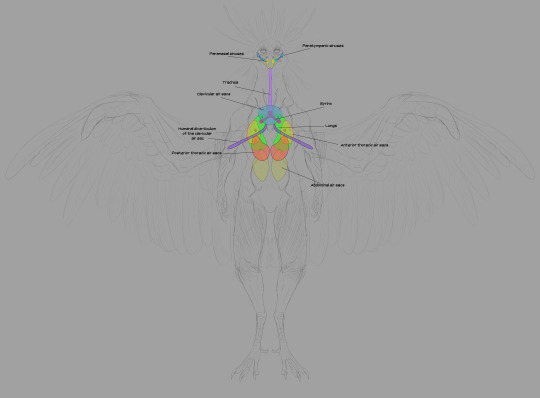
Next we have the respiratory system. The trachea takes in air and delivers it to the lungs. Unlike mammalian lungs, Chozo lungs are inelastic: they don't expand and contract. The air sacs do all the expanding and contracting: they're connected to the lungs through a network of bronchii.
The high metabolic rate required for flight demands a ton of oxygen, and Chozo respiratory organs are designed to do just that. The mechanics are fascinating but I won't take up too much of your time explaining the finer points. Wikipedia's write-up on the circulatory system of birds is a good place to start if you want to dive deeper.
The short version is thus: air enters through the nostrils, traveling into the bronchi through the trachea and syrinx (the syrinx helps Chozo vocalize). The bronchi deliver air to the lungs. When Chozo inhale, the posterior and anterior sacs expand: the posterior sacs take in fresh air while the anterior sacs fill with air that has already passed through the lungs. Air is constantly circulating through the lungs, and it's a one-way flow.
Parabronchii are microscopic tubes that run perpendicular to the blood capillaries. Parabronchii efficiently diffuse oxygen from the air into the blood.
The next image set deals with a few extraneous vital organs. I'm not going to illustrate the nervous system nor arterial network, just as I neglected to illustrate all the bronchi in the respiratory system. That's a lot of tubes!
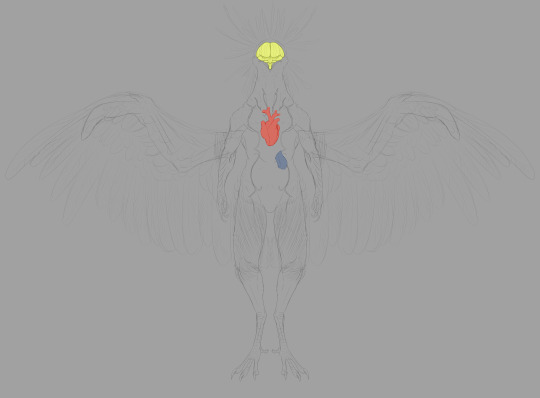
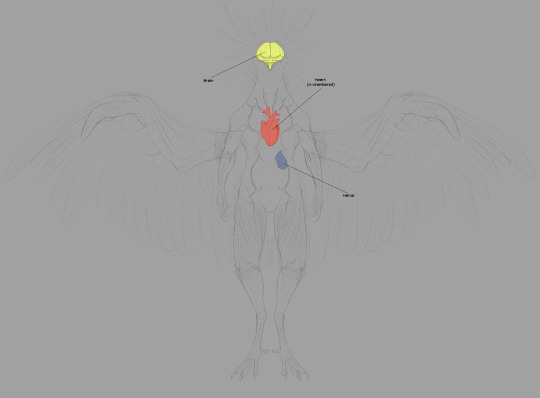
The circulatory system is pretty standard, but it pulls largely from Dread. Here's the thing: in the pre-boss fight cutscene for Raven Beak (aptly named 0086_comanderorbital_video_artwork_01.webm in the files), we see him contributing to Samus' biological makeup. His heart is set firmly in the center of his chest.

This is anatomically accurate to real birds! Bird hearts are placed similarly in the center of the chest, flanked by the left and right lobes of the liver (linked image is a labeled black and white illustration of a dissected pigeon, showing most of the major organs).
The brain is exactly what you think it is. No, the most interesting part of this last image set is the harus.
The harus is an accessory to the lymphatic system. You'll notice its proximity to the respiratory system. Lymphatic capillaries accompanying the parabronchi network filter more harmful atmospheric molecules into the harus, which makes use of specialized cells to recycle these molecules in a process that synthesizes ATP. In addition to this, the harus helps maintain the body's proper pH levels in hostile environments. This organ is what allows the Thoha to breathe in Zebes' toxic atmosphere.
Headcanon time: the majority of non-powersuit related genetic alteration done to Samus Aran in her youth is related to this organ: her respiratory system was altered with the proper instructions to produce specialized harus cells on their own without needing to transplant the organ. Samus can breathe on Zebes because her lungs can perform the function of the harus while she breathes.
Full-size pngs for everything are available on Ko-fi and Patreon. The canvas for this project was pretty big because I wanted to be able to capture the scale of the wings somewhat.
ADDENDUM, May 16, 2024: Chozo should have a modified pelvic bone that more closely resembles a synsacrum, not a humanoid ilium: I am a fool and completely forgot to make alterations in that department.
#headcanons#chozo anatomy#chozo#raven beak#metroid#metroid dread#metroid dread spoilers#gotta give props to friend of the blog Ivory who reminded me of ATP synthesis and suggested a second liver of sorts
101 notes
·
View notes
Text
i feel better after ranting about glucose and cellular respiration
#AND BTW#your body has the ability to make its own sugar (gluconeogenesis) but it uses ATP (energy currency of the cell)#ATP synthesis is like. the entire point of cellular respiration so like. it's not very efficient to have your body#provide it's own glucose for glycolysis. especially with how costly it is to make new ATP#and how much ATP is used for other processes around the cell as well#so like. sugar is not your enemy. it's REALLY really not.
0 notes
Text
Lane's thesis around the emergence of eukaryotes (and why it was necessary for complex life) seems to be this:
Bacteria (both kinds, archaea and eubacteria) have pretty strict energy limits in terms of growth. One big constraint here is genome size: the more genes you have, the more energy that gets spent on protein synthesis. But the smaller your genome, the faster you replicate, which is important given how quickly bacterial generations turn over; replicating fast is a big advantage. You can pick up any new genes you need from the environment, thanks to lateral gene transfer. This is why bacteria don't have a lot of junk DNA.
You could try to scale up the size of the cell to increase the size of the membrane, which would allow you to produce more energy. But the volume of the cell scales with the cube of its size and the surface area with the square; and, for obscure reasons, it seems like there is a strong evolutionary pressure to keep the genome near the energy-producing membrane. So what you effectively get is a big dead area inside the cell (a vacuole, in some species of huge bacteria), lots of extra copies of the genome, and no extra energy for your trouble (each copy of the genome is a copy of every gene, which requires more energy to support).
Compare chloroplasts, which often have complicated, folded up membranes to facilitate photosynthesis--and also lots of copies of their genome floating around.
You could imagine breaking the genome up into plasmids, rather than copying every gene, and only putting extra copies of the genes needed for respiration near the cell membrane. But then you need complex transport systems (which require energy), and there's no evolutionary pressure for size for its own sake that would encourage you to develop such a complex transport system to support a large bacterial cell, in aid of maybe in the future developing an ATP surplus.
Eukaryotes didn't evolve to produce extra ATP, they evolved to get a consistent source of hydrogen, for cell growth. The endosymbiont provides the host cell with all the hydrogen it needs, and in return the host cell provides a stable environment for the endosymbiont.
In this safe environment, the endosymbiont's genome is free to shrink without limit, down to the absolute minimum number of genes needed to support respiration. Many genes will move to the nucleus of the host cell; others will simply be lost. But this produces an ATP surplus! All of this ATP needs to be used--if the whole pool of ADP gets converted to ATP, respiration stops (bad!), and free radicals accumulate which fuck up proteins and DNA and can kill the cell. So this excess of ATP encourages the host cell to find uses for it--like a cytoskeleton and internal transport mechanisms and new organelles.
Unlike the plasmid scenario, every step of this evolutionary process confers some benefit on both the host cell and the endosymbiont.
You might wonder, given the benefits, why this only happened once with mitochondria! This is sort of related to the fact that all apparent intermediate cells, like the archaezoa, are actually subsequent, reduced forms of the true eukaryote rather than being offshoots of the process of eukaryote development: the early eukaryote would have been genetically unstable, and small in population size. There was strong selection pressure to quickly converge on a single form (the eukaryote LUCA), and intermediate forms would have been quickly outcompeted and driven to extinction. Likewise, this was a feat not soon to be repeated: eukaryotes got lucky.
Endosymbiosis dumped a ton of introns--self-replicating genetic parasites--into the host cell. Some of these could have been from endosymbionts that failed to thrive in the host cell, and when they died dumped their genetic material into the host. We still sometimes see mitochondrial DNA segments invading and disrupting nuclear DNA.
The eukaryotic genome was able to tolerate the invasion of bacterial introns because of the benefit the endosymbionts provided; but initially, the result was a genetically unstable cell. The nucleus evolved to separate the nuclear DNA from ribosomes, giving time and space for spliceosomes to remove introns from transcribed RNA. Excess lipid synthesis from having both bacterial and archaeal enzymes for membrane formation helped promote the creation of the nucleus (and other membrane-bound organelles).
Because of the intron situation and overall genetic instability, the mutation rate of early eukaryotes was high, selection pressure was immense, and there was a lot of variation in the population--just the circumstances which, in mathematical models, favors the emergence of sexual reproduction.
Lateral gene transfer is of limited use as genomes get bigger, and sexual reproduction, with the genetic recombination that occurs during meiosis, makes individual genes much more salient to evolution, as opposed to just whole genomes.
The individual steps for the evolution of sexual reproduction are not so clear, but the mechanical precursors of both the segregation of genetic material and the fusion of cells are attested in prokaryotes. Various effects arising from natural selection also tend to favor two sexes, anisogamy, and the inheritance of mitochondria from only one parent. Handling mutations and the specialization of tissue, and the separation of cells into somatic cells and germ line cells, also led to the evolution of senescence.
the evolution of sex, incidentally, also favors moving as many mitochondrial genes to the nucleus as possible, so more beneficial versions can be selected for. there's no way to repair the mutational load of genes which remain in the mitochondria, and do not recombine.
66 notes
·
View notes
Text
i can't tell if it's a good thing or a bad thing that s5 is gonna come out as i start my first year of med school. on one hand it'll be a great way to cope if i need it. on the other, what if i become mentally incapable of thinking about pulmonary embolisms or atp synthesis because BYLER
32 notes
·
View notes
Text
Human Cell Tournament Round 2
Propaganda!


Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer. When consumed in a metabolic process, ATP converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. It is also a precursor to DNA and RNA, and is used as a coenzyme. An average human adult processes around 50 kilograms daily.
Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries.
#Adenosine triphosphate#atp#astrocytes#poll#polls#tumblr poll#tumblr polls#tournament poll#wikipedia#cells of the human body#science tournament#biochemistry
35 notes
·
View notes
Text
Some clone trooper ocs/names I've been thinking about lately - feel free to use them!
Haywire (501st or Commando)
Tax (212th Medic, or another battalion) (from Taxidermy)
Rain (Rancor battalion)
Bramble (Corrie guard)
Hijinx (fits in with Hevy, Hardcase, and Wrecker)
Headstone - just a bamf
Bow
Indigo/Indi (transfem clone)
Zeroes (nonbinary clone)
Synth (Synthesis - rather than named after destruction like many Clones)
Cane / Canine / Kane / K-9 (I like to think of this guy being with Alpha a lot and somehow more bamf but he has a soft spot for A-17. Maybe the origin of Coruscant's K9 unit. idk I'm just spitballing headcanons atp)
Harpy (transfem arc trooper <3333)
#gonna reblog with more later bc im always thinking of random clone names#star wars#the clone wars#clone troopers#clone oc#clone names#clone culture#sw tcw#sw fandom#star wars fandom#clone headcanons#star wars thoughts#sw meta#corrie guard#rancor#trans clone trooper#501st battalion#212th attack battalion#massiff troopers#the clones wars#clone commando#arc troopers#also love the idea of alpha and canine
33 notes
·
View notes
Text
I need to revisit the Midi-Chlorian are the Mitochondria and Anakin is a Medical Horror post because learning about how certain fungicides work by forcing fungi cells to stop the synthesis of a certain enzyme needed in their cellular membrane, essentially ending with the cells dissolving. So I thought what if some really crazy person found a way to make the midichlorian stopping synthesizing what helps the force user to handle better the force or just downright to melt them away. A normal person or even an average Jedi might end with some ATP and glycogen induced problems and need supplements or depending on the severity somewhat of a quick death, but what if someone did that to Anakin, because of course he needs more torture, and because his extreme accelerate regeneration, they melt and then get replaced in a neverending awful cycle. Because yes, I'm sure that would be fun.Melt those boy's midichlorian away–
Sorry I only had two hours of sleep and I visited four farms in a single day and is just now that I'm able to lay down, after being awake since 4 am
41 notes
·
View notes
Text
Mitochondrial Dysfunction in Type 2 Diabetes
Introduction
Mitochondria, essential for cellular energy metabolism, play a crucial role in bioenergetics and metabolic homeostasis. Mitochondrial dysfunction has been implicated as a key pathophysiological factor in Type 2 Diabetes Mellitus (T2DM), contributing to insulin resistance, metabolic inflexibility, and beta-cell dysfunction. This review explores the intricate mechanisms underlying mitochondrial impairments in T2DM, including defective oxidative phosphorylation, disrupted mitochondrial dynamics, impaired mitophagy, and excessive reactive oxygen species (ROS) generation, with a focus on potential therapeutic interventions targeting mitochondrial pathways.
Mechanistic Insights into Mitochondrial Dysfunction in T2DM
1. Defective Oxidative Phosphorylation and ATP Synthesis
Mitochondrial oxidative phosphorylation (OXPHOS) occurs through the electron transport chain (ETC), comprising Complexes I-IV and ATP synthase (Complex V). In T2DM, evidence suggests a downregulation of mitochondrial ETC activity, particularly in Complex I (NADH:ubiquinone oxidoreductase) and Complex III (cytochrome bc1 complex), leading to reduced ATP synthesis. This dysfunction is often linked to compromised NADH oxidation and inefficient proton gradient formation, resulting in cellular energy deficits and impaired insulin-stimulated glucose uptake.
2. Elevated Reactive Oxygen Species (ROS) and Oxidative Stress
Mitochondria are a primary source of ROS, predominantly generated at Complex I and Complex III during electron leakage. In T2DM, excess substrate influx due to hyperglycemia leads to mitochondrial overactivation, driving excessive ROS production. Elevated ROS induces oxidative damage to mitochondrial DNA (mtDNA), lipids, and proteins, disrupting mitochondrial integrity and function. Oxidative stress further impairs insulin signaling by activating stress-responsive kinases such as c-Jun N-terminal kinase (JNK) and IκB kinase (IKK), contributing to systemic insulin resistance.
3. Mitochondrial Biogenesis and Transcriptional Dysregulation
Mitochondrial biogenesis is regulated by the transcriptional coactivator Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α), which modulates downstream transcription factors such as Nuclear Respiratory Factors (NRF-1/NRF-2) and Mitochondrial Transcription Factor A (TFAM). In T2DM, PGC-1α expression is downregulated, impairing mitochondrial biogenesis and reducing mitochondrial density, leading to decreased oxidative capacity in metabolically active tissues like skeletal muscle and liver.
4. Disrupted Mitochondrial Dynamics and Mitophagy
Mitochondrial quality control is maintained through dynamic fission and fusion processes. Fission, mediated by Dynamin-related protein 1 (Drp1), is necessary for mitochondrial fragmentation and mitophagy, while fusion, regulated by Mitofusin 1/2 (Mfn1/2) and Optic Atrophy 1 (OPA1), maintains mitochondrial integrity. In T2DM, an imbalance favoring excessive fission leads to mitochondrial fragmentation, impairing energy metabolism and exacerbating insulin resistance. Moreover, defective mitophagy, regulated by PTEN-induced kinase 1 (PINK1) and Parkin, results in the accumulation of dysfunctional mitochondria, further aggravating metabolic dysfunction.
Implications of Mitochondrial Dysfunction in T2DM Pathophysiology
1. Skeletal Muscle Insulin Resistance
Skeletal muscle accounts for ~80% of postprandial glucose uptake, relying on mitochondrial ATP production for insulin-mediated glucose transport. Impaired mitochondrial function in muscle cells reduces oxidative phosphorylation efficiency, promoting a shift towards glycolysis and lipid accumulation, ultimately leading to insulin resistance.
2. Pancreatic Beta-Cell Dysfunction
Mitochondrial ATP production is essential for insulin secretion in pancreatic beta cells. ATP-sensitive potassium channels (K_ATP) regulate glucose-stimulated insulin secretion (GSIS), with ATP/ADP ratios dictating channel closure and depolarization-induced insulin exocytosis. In T2DM, mitochondrial dysfunction leads to inadequate ATP generation, impairing GSIS and reducing insulin secretion capacity. Additionally, oxidative stress-induced beta-cell apoptosis contributes to progressive loss of beta-cell mass.
3. Hepatic Mitochondrial Dysfunction and Lipid Dysregulation
Mitochondrial dysfunction in hepatocytes contributes to hepatic insulin resistance and non-alcoholic fatty liver disease (NAFLD). Impaired fatty acid oxidation due to dysfunctional mitochondria leads to lipid accumulation, exacerbating hepatic insulin resistance and systemic metabolic dysregulation.
Therapeutic Strategies Targeting Mitochondrial Dysfunction
1. Exercise-Induced Mitochondrial Adaptation
Physical activity upregulates PGC-1α expression, enhancing mitochondrial biogenesis and oxidative metabolism. High-intensity interval training (HIIT) and endurance exercise improve mitochondrial efficiency and reduce oxidative stress, mitigating insulin resistance in T2DM patients.
2. Pharmacological Modulation of Mitochondrial Function
Metformin: Enhances mitochondrial complex I activity, reducing hepatic gluconeogenesis and oxidative stress.
Thiazolidinediones (TZDs): Activate PPAR-γ, promoting mitochondrial biogenesis and improving insulin sensitivity.
Mitochondria-targeted Antioxidants: Agents like MitoQ, SkQ1, and SS-31 reduce mitochondrial ROS, preventing oxidative damage and preserving mitochondrial function.
3. Nutritional and Metabolic Interventions
Ketogenic and Low-Carb Diets: Enhance mitochondrial fatty acid oxidation, reducing lipid accumulation and improving metabolic flexibility.
Intermittent Fasting: Induces mitochondrial biogenesis and autophagy, improving metabolic homeostasis.
Nutraceuticals: Coenzyme Q10, resveratrol, and nicotinamide riboside (NR) enhance mitochondrial function and energy metabolism.
4. Emerging Gene and Cellular Therapies
Gene Therapy: Targeted upregulation of PGC-1α and TFAM to restore mitochondrial function.
Mitochondrial Transplantation: Direct transfer of healthy mitochondria to replace dysfunctional ones, an emerging frontier in metabolic disease management.
Conclusion
Mitochondrial dysfunction is a central determinant in the pathogenesis of T2DM, affecting insulin signaling, glucose metabolism, and lipid homeostasis. Targeting mitochondrial pathways through exercise, pharmacological agents, dietary modifications, and emerging gene therapies offers promising avenues for improving metabolic health in T2DM.

#Mitochondrial Dysfunction#Type 2 Diabetes Mellitus (T2DM)#Oxidative Phosphorylation (OXPHOS)#Electron Transport Chain (ETC)#ATP Synthesis#Reactive Oxygen Species (ROS)#Oxidative Stress#Mitochondrial DNA (mtDNA) Damage#Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 Alpha (PGC-1α)#Nuclear Respiratory Factors (NRF-1/NRF-2)#Mitochondrial Transcription Factor A (TFAM)#Mitochondrial Biogenesis#Mitochondrial Dynamics (Fission & Fusion)#Dynamin-related protein 1 (Drp1)#Mitofusin 1/2 (Mfn1/2)#Optic Atrophy 1 (OPA1)#Mitophagy#PTEN-Induced Kinase 1 (PINK1)#Parkin#Insulin Resistance#Skeletal Muscle Metabolism#Pancreatic Beta-Cell Dysfunction#Glucose-Stimulated Insulin Secretion (GSIS)#ATP-Sensitive Potassium Channels (K_ATP)#Lipid Dysregulation#Non-Alcoholic Fatty Liver Disease (NAFLD)#Exercise-Induced Mitochondrial Adaptation#High-Intensity Interval Training (HIIT)#Metformin#Thiazolidinediones (TZDs)
0 notes
Note
OMG HIJIHIHI THIS IS KINDA UNRELATED BUT I HAD A THOUGHT (surprising, I know)
I just realized Im a synthesis of the choices I was robbed off, I was never given the opportunity to develop personality or even preferences. And Im SO protective and dependent on my bf and our relationship because of it. Atp its kinda toxic (working on it, promise🫶) bc I tend to get really mad when he has to make a choice or do something I have no say in, because he’s the only person Ive had any control over all my life (not even myself)
MAYBEEE Just speculating. Maybe Azai felt that was about saran too? He just wanted to have complete control over him, even if (in his head) it was for Sarans own good? And ofc Saran wouldnt conform to that bc he’s actually gotten to make decisions abt himself and knows his individuality.
im not sure its quite the same? altho azai is someone who needs to be in control of things, yes. he also felt protective of saran but not enough to protect saran from himself. saran was no longer worthy of his protection or his mercy
azai admired saran greatly and idolized him to the point he was unsure if he wanted him or wanted to be like him. he made the mistake of applying his idea, his illusion of saran on the real him, thinking he knows him best. they have many differences and thoughts on things but also similarities/shared opinions on some matters and azai took those similarities and assumed they have the same beliefs in general, that theyre the same. he mistakenly believed what they had was something like vika and saran do, some sort of soulmate type of bond. he developed those possessive feelings for HIS idea of saran and couldnt handle it when everything spiraled out of control and his idea shattered to pieces
he believed saran needed him but it turned out he projected that onto saran; hes the one who needed, wanted saran
21 notes
·
View notes
Note
I'm so you when it comes to school. I may not ride F1 cars but I know they would lose braincell if you told them about ATP synthesis or the kreb cycle
I would pay good money to see them try to read an EKG or sit through a single pharmacology lecture 😭
26 notes
·
View notes
Text
An Interesting Fact About Chemical Equilibria
The rate of a chemical reaction is the speed at which it proceeds. It is primarily affected by the concentration of reactants. So, a chemical reaction will start out fast because the reactants are still abundant, but the reaction will slow down as it proceeds, because the concentration of reactants decreases.
A reversible chemical reaction is one that can occur in both the forwards and backwards direction. Consider the formation of carbonic acid, and the decomposition of carbonic acid.
CO2 + H2O => H2CO3
H2CO3 => CO2 + H2O
Carbon dioxide can react with water to form carbonic acid, and carbonic acid can decompose to form carbon dioxide and water. Each of these reactions have their own rates, which depend on the concentration of reactants. If there is a lot of CO2, but little H2CO3, the formation of carbonic acid will be fast, and its decomposition will be slow, so the total amount of carbonic acid will rise. If the there is much carbonic acid but little carbon dioxide, the formation will be slow and the decomposition will be fast, so the total amount of carbonic acid will fall.
But if the concentrations of carbonic acid and carbon dioxide are just right, the rate of the forward reaction will equal the rate of the backwards reaction, and the amount of carbonic acid formed will equal the amount of carbonic acid decomposed. There will be no net change in the amount of each chemical species, and the system is said to be in equilibrium.
Now I will propose a new chemical system, with hypothetical reactants A, B, and C.
A => B
B => C
C => A
If all three reactions were to proceed at the same rate, the system would be in equilibrium. A would turn into B just as fast as C turns into A, which is just as fast as B turns into C. There would be no net change in the amount of each chemical species. This is an unusual type of equilibrium because it consists not of one reversible reaction, but of three separate irreversible reactions.
But is such a chemical system possible? As it turns out, no! The reason why is thermodynamics.
Thermodynamics is the study of energy, and chemists use the concept of Gibbs free energy (G) to describe the amount of energy in a chemical system. If a chemical reaction would cause the amount of energy in system to increase (∆G > 0) it must use energy. If it causes the energy to decrease (∆G < 0), it produces energy. For a chemical system in equilibrium, there is no change in the properties of the system, and therefore no change in energy, so (∆G = 0).
An irreversible chemical reaction always has a negative ∆G, otherwise, it would not occur. Some reactions have a positive ∆G values, for example protein synthesis, but they only occur because they are combined with reactions with negative ∆G values, for example ATP hydrolysis, so they have a negative ∆G overall.
The chemical system outlined above is in equilibrium, so the overall ∆G should be zero. However, each of the reactions is irreversible, so they should each have a negative ∆G. The total ∆G is equal to the ∆G of each reaction, so this would mean that three negative numbers must add to equal zero. That's impossible. Therefore, a chemical system in equilibrium must be formed only of reversible chemical reactions in equilibrium, not of multiple irreversible reactions.
So, I introduce a fundamental law of chemistry:
In a chemical system in equilibrium, all chemical reactions are in equilibrium.
In other words, equilibrium systems don't have time directionality. Their reactions happen backwards in the same way as forwards.
49 notes
·
View notes
Text
🔬🌀Demystifying the Krebs Cycle: A Deep Dive into Cellular Respiration! 🌀🔬
Prepare for a thrilling journey into the heart of cellular metabolism! 🌟✨ Today, we unravel the intricacies of the Krebs Cycle, also known as the Citric Acid Cycle or Tricarboxylic Acid Cycle, a cornerstone of energy production in our cells. 💡🤯
The Krebs Cycle: Named after its discoverer, Sir Hans Krebs, this metabolic pathway occurs within the mitochondria and is a central hub in cellular respiration.
🔍Step 1: Acetyl-CoA Entry
Acetyl-CoA, derived from the breakdown of glucose or fatty acids, enters the cycle.
It combines with oxaloacetate to form citrate, a six-carbon compound.
🔍Step 2: Isocitrate Formation
A rearrangement converts citrate into isocitrate.
The enzyme aconitase facilitates this transformation.
🔍Step 3: Alpha-Ketoglutarate Production
Isocitrate undergoes oxidative decarboxylation, shedding a CO2 molecule and yielding alpha-ketoglutarate.
NAD+ is reduced to NADH in this step.
🔍Step 4: Succinyl-CoA Synthesis
Alpha-ketoglutarate loses CO2 and acquires a CoA group to form succinyl-CoA.
Another NAD+ is reduced to NADH.
This step is catalyzed by alpha-ketoglutarate dehydrogenase.
🔍Step 5: Succinate Formation
Succinyl-CoA releases CoA, becoming succinate.
A molecule of GTP (guanosine triphosphate) is generated as a high-energy phosphate bond.
Succinate dehydrogenase is pivotal, transferring electrons to the electron transport chain (ETC).
🔍Step 6: Fumarate Generation
Succinate is oxidized to fumarate with the help of the enzyme succinate dehydrogenase.
FADH2 (flavin adenine dinucleotide) is formed and transfers electrons to the ETC.
🔍Step 7: Malate Formation
Fumarate undergoes hydration to form malate, catalyzed by fumarase.
🔍Step 8: Regeneration of Oxaloacetate
Malate is oxidized back to oxaloacetate.
NAD+ is reduced to NADH.
Oxaloacetate is ready to initiate another round of the Krebs Cycle.
The Krebs Cycle - an intricate dance of chemical transformations fueling the cellular machinery of life. 🕺💃 Dive deeper into cellular respiration, where molecules tango to generate ATP, our cellular energy currency!

📚References for In-Depth Exploration📚
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). W. H. Freeman. Chapter 17.
Voet, D., Voet, J. G., & Pratt, C. W. (2008). Fundamentals of Biochemistry (3rd ed.). John Wiley & Sons. Chapter 17.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2008). Lehninger Principles of Biochemistry (5th ed.). W. H. Freeman. Chapter 17.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#biochemistry#cell#science nerds
116 notes
·
View notes
Note
I guess I should call you Mr. Curly then! Or Miss, Mrs..? Correct me if I'm wrong! What happens if you go bald or, worse, get a buzzcut haha
Your frustration with FibroFree... I get it. I don't have FMS myself, but my sister-in-law does. I remember how much she suffered while fighting for the right to affordable healthcare. That's the kind of thing I expect when TRPC-R finally hits the market. The system is broken. Don't tell me if it's too personal - do you have FMS?
I wasn't part of the team developing TRPC-R though I was here when the incident occured. I got the second-hand account from my late mentor in the immediate aftermath and, oh boy... she's deceased, so sharing won't put her in legal trouble, although I must be vague for the details aren't public.
Let's just say... you know how TRPC channel activation increases cardiac growth, right? And TRPC-R regulates the activation of these channels in low gravity to counteract hypotrophy in astronauts. No problems there. HOWEVER. Say one of the test subjects got their hand on sweetener from a 'small-horse delivery' company (not naming names) and, say, the sweetener happens to be cut with a stimulant that causes exponential growth in combination with TRPC-R... now, I'm not a medical professional like you, but I'm sure you'll agree that a human heart should remain INSIDE the body, and it certainly shouldn't explode out of their chest.
The examination room was on lockdown for a week. I can't fathom how the poor nurse doing the medical check felt, but they left on the next shuttle to Earth and we haven't heard from them since.
By the way - you probably know already - there's a super galactic storm heading in your direction. It might miss you. If it doesn't, please, please, PLEASE message me as soon as you can.
- xoxo, Pheobe
P.S. I'm SO glad you asked about anoxygenic photosynthesis! Here are the facts: one, AP is photosynthesis in the abscence of oxygen. Two: two domains are capable of AP - archae and bacteria. Three: all known extant Martian organisms are believed to belong to these domains. These three facts are foundational to Martian/Terran Divergent Theory. You may be thinking "Pheobe, what about subterranean Martians; how do they photosynthesize in the abscence of light?" - an excellent question! This is the crux of the matter, and a heated source of debate. You see... [read more]
Attachment: 'Review of ATP and NADH Synthesis in Sub-Terranian Martians and the Evidence for Divergence', pdf, 1.7 Tb
Please, 'Mr Curly' was my father, I assure you that 'Curly' is perfectly fine. :-) I fear to imagine the day I go bald, I guess I'll just have to change my ID.
I don't have FMS, no, but with the amount of people that was funnelled through the hospital I worked at I got to see many cases, and it's incredibly frustrating to have the treatment readily available yet to be completely barred from accessing it. I'm thinking of going rogue sometimes, but you never heard it and I never said it. I'm sorry to hear about your sister-in-law.
...Okay, I did not actually know about the stimulant in sweetener, and I studied the packaging. This is...concerning. Even if the chances of someone being on TRPC-R are already low, and even lower of them ingesting a certain brand of sweetener, even one case is one too many -- and makes me wonder what else I'm not aware of that I should be. You've given me a lot to investigate, thank you! Albeit at the cost of...erm... a cor extrathoracicum event. Yeesh. I've seen many a harrowing case, but I don't think a spontaneous heart evacuation like that was amongst them.
Wow, that is a hefty file, I'm setting it to download immediately. Many thanks! Please know that you are saving me from frankly lethal levels of boredom with so much to read.
Right, forgot to add -- thank you for the heads up on the storm too! I'm sure we'll be fine, the ship is in very capable hands. :-) See you on the other side.
--Curly
#n.n.#ask box temporarily closing soon!#ask blog#mouthwashing#curly mouthwashing#nurlysays#also anon i love you
9 notes
·
View notes
Text

In the Beginning: A Scientific Exploration of Life’s Hypothetical Origins
In the most profound of inquiries, humanity seeks to comprehend the genesis of its own existence, prompting a meticulous examination of the Earth’s primordial landscape. This quest to unravel the mysteries of life’s origins has captivated scientists and scholars for centuries, leading to a nuanced understanding of the intricate interplay between chemical, biological, and environmental factors that potentially gave rise to the first living organisms.
Approximately 4 billion years ago, the Earth’s canvas was vastly different from the one we know today, with minimalistic cells emerging amidst this alien landscape. Characterized by carboxylic acid membranes and RNA-driven heredity, these primitive entities laid the foundational blueprint for the astounding complexity that would eventually follow. The evolution of ribozymes, capable of catalyzing metabolic reactions, was a seminal moment, bridging the gap between a lifeless chemistry and the nascent biochemistry of early organisms. This development not only enhanced cellular capabilities but also underscored the symbiotic relationship between genetic innovation and environmental pressures.
The pursuit of energy, a fundamental drive in the evolution of life, led early organisms to harness the planet’s primordial power sources. Mineral catalysis and reactive phosphorus species might have played crucial roles in the synthesis of ATP, with the Wood-Ljungdahl pathway exemplifying the resourcefulness of these early life forms in exploiting available energy sources.
Our exploration of the Earth’s history leads us to Luca, the Last Universal Common Ancestor, whose characteristics offer a fascinating glimpse into the life of our most ancient shared forebear. The proposed environment of Luca, akin to the chemistry-rich settings of volcanic vents, underscores the profound connection between life’s emergence and the planet’s geochemical landscape. Furthermore, the concept of the Origin of Life Domain (OLD) invites us to contemplate the possibility of alternative life forms, unconnected to Luca’s lineage, and the uncharted scientific territories that await discovery.
From the First Organism to LUCA - The Evolution of Life's Core Processes (Wolfpack Astrobiology, March 2024)
youtube
Life Began Much Faster Than We Thought (Sabine Hossenfelder, December 2024)
youtube
Saturday, December 7, 2024
#scientific exploration#hypothetical origins#primordial landscape#biochemical pathways#life's beginnings#interdisciplinary approaches#environmental pressures#genetic innovation#presentations#ai assisted writing#machine art#Youtube
7 notes
·
View notes