#free medical journal
Text
Treatment of Pelvic Organ Prolapse with “Vaginal Laser”– A Mini-Review of the Literature

Treatment of Pelvic Organ Prolapse with “Vaginal Laser”– A Mini-Review of the Literature in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.006059.php
It is not yet possible to prevent the aging processes. Like other several risk factors (such as genetics, race/ethnicity, gender, obstetrical and gynaecological injuries, smoking, obesity) the aging also causes weakening of the pelvic floor connective tissue/ collagen and leads to pelvic organ prolapse (POP) [1]. Although the exact prevalence of the pelvic organ prolapse of women is unknown, this condition was reported up to 50 percent in some studies [1]. Most women having a POP, often complain of a pressure sensation respectively a bearing-down sensation [2]. The POP impairs further, as a consequence of its impact on bladder, bowel function and the sexual function, hence impacts the quality of life negatively [3]. The prolapse of pelvic organs can be treated either conservatively or surgically, each with its own advantages and disadvantages. Treatment with pessaries and the initiation of pelvic floor physical therapy with or without estrogens are the usual effective conservative treatment options for POP of women [4,5]. Surgical treatment is the other alternative for qualified cases. Surgery requires experience and can require more effort and it may cause more complications. The laser treatment is getting increasingly popular in the field of urogynaecology as an alternative option, following its adoption from dermatology and plastic-surgery. The motto of this procedure is the reversal of aging „rejuvenation“. In this paper we reviewed the literature for the use of laser for POP in women.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01
Follow on Blogger :https://biomedres01.blogspot.com/
Like Our Pins On : https://www.pinterest.com/biomedres/
#Free medical journal#Medical and Medicinal journal#Journals on Biomedical Imaging#Journals on Biomedical Engineering#journal of biomedical research and reviews impact factor
0 notes
Text
Biomed Grid | Assessment of Brain Metabolic Score (BMS) In Vivo Based on Mitochondrial Activity in Neuropathology
Introduction
The discovery of oxygen occurred toward the end of the 18th Century (1771-1775) by three scientists including Carl Wilhelm Scheele, Joseph Priestley and Antoine de Lavoisier. It took more than 100 years to discover the intracellular organelle, named mitochondrion by Carl Benda in 1898 [1], that utilized 90-95% of the oxygen taken up and consumed by the body of patients as summarized by Waltemath in 1970 [2].
The aim of the current review is to describe the historical R&D process of using light in order to study brain biochemical and physiological activities. I will deal with the function and regulation of oxygen in supplying energy to this unique central organ of the body. The relationship between the activities of the brain and using optical technologies are presented in (Figure 1). Most of the information on mitochondrial function has been collected using in vitro studies. Small portion of publications dealt with monitoring in vivo of brain mitochondrial function in real-time. Prof. Britton Chance was the leader in the field of using the light, seen in (Figure 1) in studying mitochondrial function (Figure 1) especially the brain under in vivo conditions. The study of mitochondrial function in vivo was expanded later by our group that developed the multiparametric monitoring system used as seen in (Figure 1) [3, 4, 5]
Historical Overview
The functional capacity of any tissue, and especially the brain, is related to its ability to perform its work. The assessment of this ability could be done by checking tissue oxygen balance, i.e. the ratio of oxygen supply to demand. As seen in (Figure 2) a similar description was made by Barcroft 105 years ago [6].
He showed the relationship between tissue activity, oxygen consumption as well as increase in blood supply serving as a compensation mechanism. This observation that was published in 1914 was and is supported by many studies published since then. Presentation of the balance between tissue oxygen supply and demand in a typical organ is shown in (Figure 2). Oxygen supply is dependent upon the microcirculatory blood flow (TBF), blood volume (TBV) and the hemoglobin saturation level (HbO2) in the small blood vessels, namely, the microcirculation. The saturation of the hemoglobin in the microcirculation is affected by 2 factors, namely, oxygen consumption by the mitochondria and the microcirculatory blood flow. The demand for oxygen is affected by the specific activities taking place in each organ as seen in the right side of the figure. The mitochondrial NADH (the reduced form) level is a parameter directly related to the oxygen balance.
Figure 1: The story of brain bio-photonics. A - Citation regarding the creation of light. B - The use of the UV part of the spectrum as a tool for the monitoring of mitochondrial function (C). D - Schematics of the elements that represent part of the brain tissue seen in part E.
Figure 2: The “hypothesis” formulated by Barcroft in 1914 regarding the connection between organ activity, oxygen consumption and blood flow (6). B - Presentation of tissue oxygen balance related to the energy supply and demand. Oxygen supply could be evaluated by measurement of tissue blood flow (TBF), blood volume (TBV) and hemoglobin saturation (HbO2). Oxygen demand varies between different tissues and include Ionic Homeostasis, Signal Conduction, Glandular Secretion, Muscle Contraction, and G-I tract and kidney function. Mitochondrial NADH serve as an indicator for tissue oxygen balance (16).
(Figure 3) shows the gradient of oxygen levels between air inspired to the lungs, heart, large arteries and small arterioles to the brain intracellular compartment and finally the mitochondria. The various points of patients’ clinical monitoring are shown. As seen the largest gradient of oxygen occur between the oxygen level in the large arteries and the microcirculation. The delivery of oxygen is done in the microcirculation, therefore the level of oxygen in large arteries is very high (about 100 mmHg). The last usual parameter, in the oxygen gradient, that is monitored clinically is the pulse oximeter that measures the saturation of hemoglobin in the systemic arteries. At this point the HbO2 is highly saturated as indicated in point 1 at part A of the figure. The saturation of the HbO2 at the microcirculation is depending on the organ that is evaluated. In the brain, heart or kidney (very active organs), the saturation will be in the range of 50%-60% and in the resting muscle it will be around 80%. Monitoring of the microcirculation and especially mitochondrial function in vivo is not a standard approach in daily clinical activities.
Figure 3: The gradient of O2 from air to the mitochondria in nervous system. Monitoring of patients include various parameters along the oxygen gradient (96). In the insert A, the dissociation curve of O2 and hemoglobin is presented.
The historical milestones in the development of mitochondrial NADH monitoring after its discovery in 1906 by Harden and Young are listed in (Table 1). Most of the milestones were achieved by Prof. Chance. The collaboration with the physiologist, Prof. Jobsis, led to many studies where various organs in vitro or in vivo were monitored. Most of the studies published in this field were expanded by the team working with Prof. Chance in Philadelphia and then moved to other universities around the world.
Table 1: The main Milestones in NADH Measurements.
(Figure 4) shows the pictures of the 5 scientists who affected significantly the development of the theoretical and experimental technology for the monitoring of mitochondrial NADH function in vitro and in vivo.
Figure 4: The scientists contributed significantly to our knowledge on mitochondrial function and NADH monitoring under in vitro and in vivo conditions.
Figure 5: A - Mitochondrial metabolic state, defined in vitro, by Chance and Williams, and opened up a new era in measurements of respiratory chain enzyme’s redox state in vitro as well as in vivo (43). B - The fluorescence emission spectrum showing the difference between an anaerobic an d an aerobic suspension of liver mitochondria) .The excitation wavelenght was 353nm (97). C - The emission spectra of rat cerebral cortex under aerobic conditions (lower trace) and under anoxic conditions (upper trace) (98).
It is now more than 60 years since the pioneering work of Chance & Williams on mitochondrial metabolic state in vitro shown in (Figure 5), was published [7, 8, 9, 10]. Harden & Young described the pyridine nucleotides almost 110 years ago [11, 12] followed by the description of its full structure by Warburg and collaborators 30 years later [13]. All those studies initiated the first detailed experiments, by Chance et al. [14], in which NADH (Nicotine amide adenine dinucleotide) fluorescence, was used as a marker of mitochondrial function of the brain and kidney in vivo in the anesthetized animals.
Principles of Monitoring NADH Fluorescence
Monitoring of NADH by the difference in the absorption spectrum of its reduced form, led to a limitation of that technique to the study of mitochondria in vitro, and in very thin tissue samples (e.g. muscle) or in cell suspension. More specific and better method is fluorescence spectrophotometry in the near-ultraviolet range (UVA).
The discovery of the optical properties of reduced Nicotineamide Adenine Dinucleotide – NADH (earlier names: DPNH - diphosphopyridine nucleotide, or PN - pyridine nucleotide), led to an intensive research activity since the early 1950’s. The reduced form of this molecule, NADH, shown in Figure 6A1 & 6A2 [15], absorbs light at 320-380 nm (Figure 6) and emits fluorescent light at 420-480 nm range (Figure 6) [16]. The oxidized form NAD+ does not absorb light in the UV range, therefore it was possible to measure the redox state of the mitochondria by monitoring the UV absorbance or Blue fluorescence of NADH. (Figure 6) present NADH fluorescence spectra monitored from the brain of anesthetized rat exposed to anoxia as described in detail [17].
Figure 6: A. The structures of NAD+. The nicotinamide group (broken ring) is the “functional” part of both molecules (A1) i.e. the portion of the molecules where oxidation and reduction take place (15,99). A2. The transition between oxidized and reduced NADH. A3. Excitation and emission spectra of NADH (16). B. Emission spectra of the brain under excitation of 366 nm light (A1, B2, B1, B2, and C1) or laser 324 nm light (C2). C1 and C2 were measured from a dead brain (23).
Figure 7: A. Apparatus for measurement of “fluorescence spectra”. The wavelength drum was driven by a synchronous motor. A sectioned disc d, mounted on another synchronous motor, modulated the incident light. The a.c. component of the current, caused by the light falling on the iP 21 multiplier, was amplified, rectified and fed into the recorder. B. Fluorescence spectra of NADH. The open circles are for excitation by wavelength 313 mμ, the black ones for excitation by 366 mμ. The spectrum for 313 mμ has been multiplied by a certain factor to make its maximum of equal height as the maximum of the spectrum excited by 366 mμ. The spectra appear to be identical; the maximum is at about 462 mμ (22).
The first model of fluorescence recorder was described by Theorell & Nygaard [18, 19] and Theorell, Nygaard and Bonnichsen [20]. Boyer & Theorell in 1956 showed that the fluorescence of DPNH was shifted and the intensity was increased upon combination of DPNH and liver alcohol dehydrogenase-ADH [21]. The 1st study using fluorescence spectrophotometry of NADH in intact Baker’s yeast cells and Algae cells, was published in 1957 [22] as seen in (Figure 7) .
The features of NADH fluorometers consist of the 4 components:
a. A light source (including appropriate filters).
b. An optical path to the monitored object and back to the detection unit.
c. Signal detection and processing units.
d. Signal recording and data storage units.
In our 1984 review, we specified the light-guide-based fluorometry used in our studies [23]. Ince et al. [24], included many other technical aspects of the methodology in their review. Duysens & Amez [22] schematized the first fluorescence spectrophotometer used for intact cells. They utilized the “classical” light source – the mercury arc – providing a very strong band at 366 nm, even though not at the maximal NADH absorption peak of NADH (340 nm). Using a monochromator, they were able to obtain the NADH fluorescence spectrum in baker’s yeast cells and photosynthesizing cells. They concluded that “the fluorescence excited by 366 nm can be used for measuring reduced pyridine nucleotide in vivo”.
Chance & Legallais [25] described a differential fluorometer that opened a new era in monitoring NADH fluorescence in vivo. They used a microscope, serving as the fluorometer basis, with two light sources: tungsten and mercury lamps with appropriate filters. Chance & Jobsis [26] and Chance [27] showed that mechanical muscle activity ends up with NADH oxidation measured in excised muscle. This study was the bridge from the subcellular (mitochondria) and cellular (intact cell) monitoring approaches toward actual in vivo applications.
Figure 8: 1 - Microspectrofluorometer developed and used in the 1960’s. In addition to the interference filter, a Wratten type 2C filter is also placed in the back aperture of the objective. The wave-length-range of the interference filter is 400-700 mμ, and the specification on its spectra interval is 30-40 mμ. Other features of the high pressure mercury arc excite the fluorescence of the specimen at 366 mμ by means of an ‘Eppendorf’ primary filter. Fluorescence excitation and emission pass through the Leitz Ultrapak objective and a ocular (98). 2 – Experimental setup for microfluorometry of brain and kidney cortex in the rat measured simultaneous. Two microfluorometers were focused on the exposed surfaces of the 2 organs. The oxidation-reduction level of the intracellular pyridine nucleotide was altered by changes in ventilation and the corresponding fluorescence changes was recorded by the two microfluorometers. For the kidney fluorometer, the water-cooled lamp housing attached to the Leitz “Ultrapak” illumination system is shown. 3 –Recordings of the kinetics of increases in fluorescence observed in oxygennitrogen transition for kidney (A) and brain (B) cortex of anesthetized rats. The increases in fluorescence are recorded as a downward deflection. The times when gas in the tracheal cannula was changed from oxygen to nitrogen and the times when breathing stopped and started again are indicated.
The in vivo NADH monitoring system was introduced during in the late 1950’s and early 1960’s. The effects of scattered light and tissue absorption due to blood were not evaluated or measured when NADH fluorescence was measured. The first results of in vivo NADH fluorescence measurements appeared in 1962 [14]. These “classical” papers described two microfluorometers that were modifications of previous designs [25, 28]. This micro fluorometer (Figure 8) type employed Leitz “Ultrapack” illumination, which had been used for many years until the development of UV transmitting optical fibers. To avoid movement artifacts, rats were deeply anesthetized, and their heads were fixated in a special holder on the operation table. The same instrumentation was used in other in vivo studies, including those of Chance’s group and other investigators cited in a previous review [17].
The effect of blood on NADH fluorescence was discussed early by Chance et al. [14]. In order to monitor NADH in vivo, it was necessary to avoid large blood vessels in the monitored area which interfere with the emission and excitation light. The monitoring of a second parameter in tissue fluorometry in vivo was reported in 1963 [29]. It was shown that “changes due to the deoxygenation of oxyhemaglobin do not interfere with measurement of the time course of fluorescence changes in the tissue studies”. The addition of a second monitoring signal, namely, tissue reflectance at the excitation wavelength was reported in 1968 [30]. It was based on a previous model described by Jobsis et al. in 1966 [31]. In another two papers [32, 33] , the measurement of 366 nm reflectance was used for the correction of the NADH fluorescence signal from the brain. The reflectance signal was subtracted from the fluorescence signal. The same type of fluorometer was used in by Gyulai et al. [34].
In studies of the cerebral cortex the skull was removed carefully in order to minimize bleeding; about 25 square millimeters of the cortex was exposed. The dura was left intact. The head was held similarly to that used in stereotaxic studies of the brain and was kept sufficiently motionless for continued observation of a small area between major blood vessels. In a few studies, measurements were made simultaneously on the cortex of the brain and on the kidney by means of two independent microfluorometers. In such cases the kidney was held in a clamp on the back of the animal. The apparatus is seen in Figure 8-1 & 8-2.
Areas of the brain and kidney were selected which showed a bright and uniform distribution of fluorescent material. Areas containing large blood vessels were avoided. On the brain cortex a field was selected in which the number of visible capillaries was minimal. Since continuous viewing is possible during photoelectric measurement of the fluorescence, these positions were monitored to make sure that mechanical artifacts were avoided. Figure 8A (lower left side) show the response of the kidney. The increase in fluorescence observed at the time respiration ceases is 11 percent of the total increase during anoxia. As soon as a plateau is reached, the lungs are ventilated with oxygen three times. Three seconds after ventilation is ended an abrupt decrease in fluorescence is observed and breathing starts as the decrease reaches its peak. Irregularities in the extent of oxidation in the fluorescence signal are observed for the next 0.5 minute, but the fluorescence remains above the initial value for 2½ minutes.
A Figure 8B illustrates the response of the brain cortex. No fluorescence changes are observed for 30 seconds after the inspired gas is changed from oxygen to nitrogen. A small but consistently observed diminution of fluorescence occurs, and 10 seconds thereafter hyperventilation was observed. After a plateau is established, ventilation with oxygen is commenced, and 3 seconds thereafter an abrupt decrease in fluorescence reaches its plateau, breathing commences.
These results indicate that similar fluorescence changes under anoxia in vivo, were observed in excised slices, and in isolated mitochondria.
Fiber Optic based Fluorometer/Reflectometer
A flexible means was needed to connect the tested brain to the fluorometer in order to monitor of Brain NADH fluorescence in intact anesthetized or unanesthetized animals. This happened in 1972, when UV transmitting quartz fibers became available (Schott Jena Glass, Germany). The light-guide-based fluorometer for in vivo monitoring of the brain [35, 36] subjected to anoxia or cortical spreading depression was developed and used.
Laboratory device
The development of light-guide-based fluorometry-reflectometry is shown in (Figure 9). The original fluorometer reflectometer was based on the time-sharing principle (Figure 9). Four filters were placed in front of a 2 arms light guide. Filters 1 and 3 enabled the measurement of NADH fluorescence, while filters 2 and 4 were used to measure tissue 366nmreflectance. The reflectance trace was used to correct the NADH signal for possible hemodynamic artifacts, and to indicate relative changes in the blood volume of the monitored tissue.
In this system, one photomultiplier tube was used to detect the two signals namely fluorescence and reflectance. Figure 9B presents the first in vivo brain monitoring time sharing setups, connected to the brain [36]. In order to simplify the system, the time-sharing approach (AC mode) was replaced by splitting the light emitted from the tissue into two unequal fractions for the measurement of fluorescence and reflectance signals. This was achieved by using 2 photomultipliers. This device, named the DC fluorometer, contained two arms light guide probe. In the two configurations, the reflectance signal was used for the correction of the fluorescence signal (see details [17]). This model was used in studying the brain [46, 47, [48, 49, 50], the kidney [41] and the heart [42].
The responses of the rat brain to anoxia using two size diameters of the fiber optic bundle are shown in (Figure 9) . When the brain was exposed to concentrated KCl solution, repeated cycles of NADH oxidation (decreased signal) as seen in (Figure 9). The MitoViewer Another type of fluorometer/reflectometer device (MitoViewer) was developed in 2007 by - Prizmatix Ltd as seen in (Figure 10) & B.
Another type of fluorometer/reflectometer device (MitoViewer) was developed in 2007 by - Prizmatix Ltd as seen in (Figure 10) & B.
The MitoViewer
Figure 9: The time sharing fluorometer reflectometer and B - The brain of a small animal connected to this time sharing device. Ex - Excitation, Em - Emission optical fibers for the monitoring of NADH redox state. H.V. - high voltage, PM-photomultiplier. C - The effect of the monitored tissue volume (the diameter of the fiber optic probe) was tested under anoxia as shown in C upper 3 traces (2 mm diameter) and C lower 3 traces (1 mm diameter). (A+C - (23), B - (47), D - The responses of NADH fluorescence and reflectance to cortical spreading depression initiated by exposing the cortex to 0.3M KCl (39).
Figure 10: A. The MitoViewer fluorometer uses 365 nm UV LED for excitation of the NADH fluorescence (Fluor signal). This excitation wavelength is also used for the correction of hemodynamic artifacts by measuring the reflection light intensity (the Refl signal). The light is transmitted from and to the device by means of a flexible fiber optic bundle. The software displays the Reflectance, Fluorescence and the NADH corrected fluorescence signal B. The view of the MitoViewer.
System overview
The MitoViewer device comprises the following subunits:
Light Source Unit: Provides UV light at 365nm for NADH excitation and tissue reflectance measurements. Also included is a reference photodiode that enables correction of signals in cases of intensity changes of the LED during measurement.
Fiber Optic bundle: Transmits the UV light from the Light Source to the measured tissue and transmits the collected light (the reflection (Refl) and the fluorescence (Fluor) from the tissue, to the Detection Unit.
Detection Unit: Provides detection to transform the Refl and Fluor light into electrical signals which are transmitted to the electronics circuit for amplification.
Detection Unit: Provides detection to transform the Refl and Fluor light into electrical signals which are transmitted to the electronics circuit for amplification.
USB-6009 module: Provides A/D conversion for the Refl and Fluor signals and D/A conversion for control signals sent from a PC to control the function of the MitoViewer.
PC: Personal Computer controlling of the MitoViewer operation using the MitoViewer software.
Power Adaptor: Provides the DC voltage for operation of the MitoViewer.
The fiber optic probe of the MitoViewer is connected to the surface of the brain via an appropriate holder cemented to the skull with acrylic cement. Rats (200-250 grams) anesthetized and operated as discussed in our various papers cited in the attached list.
Typical responses of the brain to oxygen depletion by exposing the rat to 100% nitrogen are presented in (Figure 11) . The Fluor signal (blue) is elevated due to inhibition of the respiratory chain activity. The Refl signal (green) is decreasing, as expected under this anoxic condition, due to the elevation in blood volume in the monitored tissue. The corrected NADH (black) shows a symmetrical increase and decrease signals during the anoxic cycle. The effects of the increase in energy utilization, were induced by exposure of the brain to Cortical Spreading Depression (by high level of potassium.) as seen in (Figure 11) . Since the oxygen supply is not limited, the Fluor (blue) and the NADH (red) decreased due to the oxidation of NADH. Under this stimulation the ATP turnover was dramatically increased, and the extra oxygen supply was provided by an increase in microcirculatory blood flow (not measured in this animal). The effects of hypoxia (6% oxygen) and hyperoxia (100% oxygen) are presented in(Figure 11).
Figure 11: Typical responses to metabolic perturbations measured in the rat brain using the MitoViewer. A - Responses to Anoxia; B - Effects of cortical spreading depression; C –The effect of hypoxia (6% oxygen) and hyperoxia (100% oxygen) on the measured signals.
Animal Preparation for NADH monitoring
An operation table and probe holding device was used to perform brain as well as other body organs preparation for the measurement period. The table for the operation procedure is shown in (Figure 12). The head is connected to a special head holder for the period of the brain operation (20-30 minutes) and then could be released, for the monitoring period, as shown in (Figure 12) . If needed, the other monitored organs i.e. muscle, kidney or liver, must be held by a micromanipulator during the measurement period. The cerebral cortex was the main organ monitored by other investigators as well as in our group.
Figure 12: A –The surgical system used to prepare and measured up to 4 organs simultaneously including the brain. The same system enables to perform a craniotomy while the animal is connected to a special head holder. B+C Stages in the preparation of the rat brain for NADH monitoring. B – The location of screws needed for the fixation of the light guide holder to the skull by dental cement. C - The view of the head at the end of the operation. D - The fiber optic probe is inserted to its holder and the animal is ready for monitoring (16).
The entire protocol of the preparation of the rat is given here. Adult male Wistar rats (180–250g) were anesthetized by intraperitoneal (IP) injection of Equithesin solution (each ml contains: Pentobarbital 9.72 mg, Chloral Hydrate 42.51 mg, Propylene Glycol 44.34%, Magnesium Sulfate 21.25 mg, Alcohol 11.5% water) 0.3 ml/100g body weight. A midline incision is made in the skin in order to expose the skull. Three holes were drilled in the skull and appropriate screws were inserted to the skull as shown in (Figure 12) . The craniotomy (3-5 mm in diameter) was drilled in the right or left parietal bone for the fixation of a light guide holder. The light guide holder and the 3 screws were then fixated to the skull using acrylic cement (Figure 12) . Ten minutes later the head was released from the holder and the fiber optic probe was inserted to the appropriate depth and fixed by a set screw (Figure 12) .
From Single Parameter to Multiparameter Monitoring Approach
(Figure 13) illustrates the various experimental and clinical perturbations used in our laboratory during the last 45 years. (Table 2) lists the studies published by Mayevsky and his collaborators on brain NADH fluorescence. The papers are classified according to the type of perturbation used. In all those papers published in the period of 1973 and 1978 we monitored the mitochondrial NADH as a single parameter monitoring system. For the physiological interpretation of the changes in NADH measured in vivo, it was necessary to move from a single parameter monitoring system to the multiparametric monitoring approach) MPA).
Table 2: Classification of the papers on brain NADH monitoring published by Mayevsky et al (1973-2017).
As described in (Figure 25) the redox state of NADH represent also the balance between oxygen demand and supply. Therefore, the multiparametric monitoring system results could provide better understanding of the pathophysiological processes developed. The term “Brain physiological mapping” based on the various parameters that could be monitored in vivo using a minimally invasive techniques presented in (Figure 14)(Figure 15) [5].
Figure 13: Presentation of the various perturbations used in monitoring brain NADH fluorescence in experimental animals and patients.
Figure 14: The technology developed for real time evaluation of energy metabolism at the tissue level. Part A shows the coupling between the macro-circulation measured by Pulse oximetry and the microcirculation. B-D The main monitoring technique of cellular and intracellular compartments (16).
Figure 15: Schematic presentation of the “basic building stones” of a typical cerebral cortex tissue. During an ischemic or other 3 events shown, the sequence of the 8 early responses developed are presented in numbered circles.
The aims are to monitor, a small volume of the cerebral cortex containing all the tissue elements that are part of a typical functioning brain tissue. The interest is in the microenvironment of the brain tissue containing neurons, glial cells, synapses and the microcirculatory elements (small arterioles and capillaries). During the process we pursued the goal of being minimally invasive to the cortical tissue itself. It was obvious that the various probes could not monitor the same volume of tissue due to the size of each probe used. Therefore, we minimized the diameter of the various probes placed in the multiparametric assembly (MPA) that had a 5-6 mm contact area with the cerebral cortex. In many perturbations used, (global ischemia, anoxia, hypoxia or hemorrhage), most of the areas in the cortex will respond in the same way. The development of the MPA after the establishment of the fiber-optic-based NADH monitoring system in 1972 when the first UV transmitting fibers appeared [36]. The connection of the brain to the fluorometer via optical fibers enabled us to monitor, for the first time, the monitoring unanesthetized brain. The initial data using this technology appeared in 2 papers [35,36]. All details of the technological aspects and animal preparation appear in the original relevant publications; our approach was to develop a new upgraded version of the monitoring system and present initial preliminary results. In the next step a large well-designed study on few groups of animals were done and the data was analyzed for its statistical significance.
Responses of Brain NADH Fluorescence to various Experimental Conditions
In the current section, the effects of various experimental treatments in animal models on brain NADH will be describe in detail. It is important to note that most of the published data on NADH monitoring, have been accumulated from the cerebral cortex.
Changes of Oxygen supply in vivo
Introduction: Chance and Williams [9, 43], found that the complete depletion of O2 from the mitochondria inhibits oxidative phosphorylation terminates ATP production. This situation affects the normal function of the tissue, and cell death can ensue. We defined anoxia as a complete deprivation of O2 caused by breathing 100% N2. Under Hypoxia the deprivation of O2 from the breathing mixture is partial and ranges between 21% (normal air) and 0% (anoxia). Under Ischemia the decrease in O2 supply is due to a decrease in blood flow to the monitored organ. The level of ischemia can vary from a full absence of flow (complete ischemia) to different levels of blood flow (partial ischemia). Although oxygen deficiency is the main event in each of the three experimental conditions (anoxia, hypoxia and ischemia), other physiological factors may differ as well. For example, brain microcirculatory blood flow is decreased under ischemia, but increases under brain hypoxia. Thus, changes in the tissue due to other blood flow related factors are not identical.
Hypoxia and Anoxia: The responses to hypoxia and anoxia are very similar; therefore, they will be discussed together. According to Chance & Williams [8, 9], a shift toward State 5 in the metabolic state of the mitochondria, involves an increase in NADH proportional to a decrease in O2 supply. Figure 16A shows the response of the brain NADH to anoxia in vivo [44, 45]. At those days the fluorescence was measured and displayed without correction for hemodynamic artifacts which was developed later in time. A clear increase in NADH fluorescence was recorded under the deprivation of oxygen. Similar responses of the kidney to anoxia are presented in the lower part of A.
In 1972, we used UV transmitting optical fibers and applied the quartz fibers to the in vivo monitoring of NADH fluorescence in the brain. It was assumed that the response of NADH fluorescence to hypoxia or anoxia, induced in vivo, should be very similar to the response of isolated mitochondria that were investigated until those days.
(Figure 16) presents an interesting two responses of the brain to anoxia. NADH and the electrical activity of the brain were measured [37]. In these experiments the rats were slightly anesthetized by Equithesin. The nitrogen was applied via a nasal mask. In part B1, the duration of the anoxia was 70 sec and in part B2, 100 sec. (Figure 16)1 shows the effect of N2 on the NADH fluorescence, reflectance, EEG and blood pressure. The top trace shows the reflectance which in all animals decreases during the N2 cycle. This decrease of reflectance was in two phases. The first decrease was small (in comparison to the second one) and occurred while the animal was breathing spontaneously. A second decrease occurred after the animal stopped breathing (SB). The recovery of the reflectance to the baseline occurred about 10 min after the rat started breathing again. The second trace from the top, the fluorescence, shows a large increase in NADH fluorescence during the first phase of the N2 cycle. In order to correct for hemodynamic artifacts induced by various treatments, we used the correction technique suggested by Jobsis et al. [32, 33] and Harbig et al. [46]. The reflectance signal at 366 nm was subtracted from the fluorescence signal at 450 nm at a 1:1 ratio. The difference between the fluorescence and reflectance signals is shown in the third trace, the ‘corrected’ fluorescence. After the cessation of respiration, a large decrease in reflectance occurs; an apparent decrease in fluorescence (oxidation) is observed which is almost undetectable in the corrected trace. The small decrease shown in the corrected trace is due to imperfection of the correction factor in this special animal. After the N2 administration had been discontinued (SN), artificial respiration (AR) was applied to induce spontaneous breathing. After the animal started breathing, a fast decrease of NADH is observed in the uncorrected fluorescence as well as in the corrected fluorescence signal. The recovery of the NADH level to the baseline was very fast in comparison to the recovery of the reflectance. The EEG of both hemispheres reaches low amplitude when the NADH level reaches 80-90% of the maximum increase during the N2 cycle. The response of the two hemispheres was identical. The recovery of the EEG follows the NADH recovery to the normoxic level. (Figure 16) shows the response of the same animal to a longer N2 cycle. The animal was exposed to N2 for 100 sec. The main differences between the two cycles are that after the recovery of the NADH to the normoxic level a further decrease in NADH occurred (third trace) and at this time the EEG was depressed and recovered to normal only later. This oxidation of NADH following the N2 cycle was observed in many animals after exposure to a long N2 cycle. The pattern of changes in reflectance, fluorescence and the corrected traces were similar to those observed in the cortical spreading depression (SD) elicited by KCl as shown also in (Figure 9)(Figure 11).
Figure 16: A - Simultaneous recordings of fluorescence changes in rat brain and kidney in the same cycle of anoxia. Fluorescence increases are indicated in a downward direction (45). B1+B2 - The effects of anoxia on brain NADH fluorescence, 366 nm reflectance, EEG, and blood pressure. SB, the animal stopped breathing; SN, stop nitrogen; AR, short artificial respiration (37).
(Figure 17) shows the responses of a dog/puppy brain to graded hypoxia (A-C) and to brain anoxia (D) [17]. As seen, the changes in the corrected fluorescence signals (CF), which represent the NADH redox state, were inversely correlated to the decrease in FiO2 levels (from 6% to 0% O2). In four records, the intensity of the decrease in the reflectance trace was also correlated with the level of hypoxia.
Figure 17: A - Simultaneous recordings of fluorescence changes in rat brain and kidney in the same cycle of anoxia. Fluorescence increases are indicated in a downward direction (45). B1+B2 - The effects of anoxia on brain NADH fluorescence, 366 nm reflectance, EEG, and blood pressure. SB, the animal stopped breathing; SN, stop nitrogen; AR, short artificial respiration (37).
The correlation between the NADH fluorescence and the FiO2 levels are presented in (Figure 17) . It was found that the FiO2 had a significant effect on the NADH redox state (F = 113.6, df = 6, p < 0.0001). However, age did not significantly affect the NADH response (F = 0.25, df = 4, p < 0.91). The NADH response of the puppies to various oxygen concentrations could be divided into four levels of hypoxia which are statistically different from each other:
In order to understand better the response of the mitochondrial NADH to anoxia/hypoxia it was necessary to monitor more physiological parameters from the same brain simultaneously. The results obtained when the multiparametric monitoring system was used are presented in (Figure 18) . The effects of complete deprivation of oxygen (anoxia) on the brain were detected when the animal was exposed to hypoxia as shown in Figure 18B [23]. The rat was exposed to 10%, 5% or to 100% N2. The decrease in oxygen supply resulted in a gradual decrease in brain pO2, as well as in an increase in NADH. The ECoG showed a clear response only to 100% N2. This response corresponded to a slight increase in extracellular K+.
Figure 18: A – The Multiprobe Assembly (MPA) used in hyperbaric chamber. The relative location of various probes above brain are in the lower part of A. Ref, reference; Ag-AgCl electrode; f, refill tube for Ref or DC electrode; c, Lucite cannula; s, Plexiglas sleeve; L, light guide; h, cable holder; Ek, K+ electrode; DCk, DCL, Ag-AgCl electrode; PO2, O2 electrode; ECoG, electrocorticography electrode (91). B - The effects of hypoxia (10% O2, 5% O2) and anoxia (100% N2) on the metabolic, ionic and electrical activities of the rat brain. In this animal the oxygenation of the brain responses to breathing air as compared to 95% O2 are shown (23).
Partial and complete Ischemia: Under partial or complete ischemia, blood flow to the monitored organ is decreased and, as a result, O2 delivery is limited or even abolished. The use of ischemia in animal models provides information relevant to clinical situations such as brain stroke. The primary factor starting the pathological state is the decrease in O2 supply, making the tissue energy balance negative.,
In the early 1960’s, Chance [45] tested the effect of irreversible ischemia on brain NADH using the decapitation model. As shown in (Figure 19), about 8 secs were required between the start of NAD reduction and the attainment of half-maximal reduction. In an attempt to observe the time for NAD reduction in ischemia, we have employed a decapitation technique with the mouse; the optical system is arranged so that the slight mechanical artifact occurring on decapitation would not disturb the fluorescence excitation.
Using the fiber optic based fluorometer, we measured, in 1976, the effect of decapitation on NADH and ECoG in the awake rat and typical response is shown in (Figure 19) [47].
Figure 19: A. Fluorescence changes of the mouse brain cortex in ischemia caused by decapitation. (45). B – Metabolic, reflected-light and electrical responses to decapitation (measured bilaterally). The upper four traces were measured from the right hemisphere and the lower four from the left hemisphere. R = reflectance; F = fluorescence; CF = corrected fluorescence (F –R); ECoG = electrocorticogram.
Later, we tested the effects of age on NADH redox state in the awake and anesthetized rat exposed to decapitation [48]. The NADH was monitored from the two hemispheres of the rat brain. Here we are presenting the four upper traces that were measured from the right hemisphere. The differences in the responses between the two hemispheres were insignificant in most cases. The 366 nm reflected light (R) shows a very small initial response to the decapitation (Figure 19) . However, a very large secondary reflectance increase was recorded
1-2 min after ECoG = 0 when NADH reached its maximal level. The uncorrected (F) and corrected (CF) 450 nm fluorescence signals were like those described previously. In order to analyze the effects of age on the responses to decapitation, various parameters were measured and calculated from the analog signals, as shown in Figure 19B.
The definitions for the various parameters are as follows:
T0 = Time (sec) when electrical activity was very low and close to 0.
T1 = Interval between decapitation and the point when corrected fluorescence started increasing.
T2 = Time when the maximum level of NADH was reached after decapitation.
T3 = Time when NADH reached a level which is half of its maximum increase (CFmax/2).
T4 = Time when a large increase of the reflectance was measured (SRI = secondary reflectance increase).
CFmax = Maximum percentage increase of NADH above baseline after decapitation.
CFo = Percentage increase of NADH above baseline when ECoG = 0.
= Percentage of NADH increase when ECoG = 0 in proportion to the maximum increase measured in the same rat.
CFN2 = Percentage increase of NADH above baseline in nitrogen environment (anoxia).
The same type of data analysis could be used in other models of ischemia such as blood vessel reversible occlusion. It was clear that under severe ischemia a hemodynamic response was measured after reaching the maximal level of NADH. We named it “Secondary Reflectance Increase” (SRI), which appears at time T4 shown in Figure 19B.
In the late 1980’s a new technique enables the monitoring of microcirculatory blood flow (laser Doppler flowmetry) was incorporated into our MPA monitoring system as seen in Figure 20A [49].
Figure 20: A - The experimental setup used. LG - Light guide for the monitoring the NADH redox state and CBF (LDF), ECoG - Electro Corticography electrodes, K, Ca, - Minielectrodes for Potassium and Calcium monitoring, To - Thermistor probe, DC - DC steady potential electrodes, DA - Dental acrylic cement, Ref - Reference electrode, KCI - cannula for KCI application. ), Ex, Em - Excitation and Emission fibers of the fluorometer, LDin, LDout - Fibers connected to the Laser Doppler flowmeter, C - Plexiglass probe holder, h - connectors holder, s - Plexiglass sleeve, f - feeling tube of reference electrode. B - The effects of unilateral (Roccl) and bilateral (Loccl) carotid occlusion on the metabolic, hemodynamic, ionic and electrical activities in the gerbil brain. R 366 nm reflectance F -450 nm fluorescence CF -NADH corrected fluorescence, LDF LDvol LDvel - Laser Doppler flow, volume and velocity. K+e(1), K+e(2), Ca2+e - Extracellular potassium and calcium electrodes. DCK+l DCK+2, DCCa2+ - DC steady potential measured concentric to the three electrodes, ECoG – Electrocorticogram (49).
(Figure 20) shows typical responses to unilateral (ROCCl) and bilateral (LOCCl) in CBF (LDF) and an increase in NADH levels (CF). During the period of ischemia, accumulation of K+ in the extracellular space was recorded (K1+, K2+) but the DC steady potential and the Ca2+ levels remained unchanged during the occlusion period. The ECoG reached the isoelectric level very soon after the second occlusion. During the reopening of the carotid arteries a fast reperfusion was recorded together with the oxidation of NADH. A spontaneous wave of SD was developed during the recovery phase, characterized by a large increase in K+e and a decrease in Ca2+e together with a negative shift in the DC steady potential. During the recovery from the SD wave a large increase in CBF (300%) was recorded accompanied by an oxidation wave of the NADH.
Hyperoxia (increase in FiO2): In order to expose an organ in vivo to elevated oxygenation
hyperoxia, it is possible to use one of the two options:
a. Normobaric hyperoxia the animal breathes elevated FiO2, between 21% O2 to 100% O2 at atmospheric pressure.
b. Hyperbaric hyperoxia (HBO) A hyperbaric chamber, in which oxygen pressure is elevated while the animal is located in the chamber, was used.
Providing animals or man with elevated oxygen will lead to the development of “oxygen toxicity.” The development of this toxic event is inversely proportional to the level of oxygenation, namely the higher the pO2, the shorter the time. Providing more O2 may be beneficial in conditions such as carbon monoxide toxicity, body oxygenation pathology. Therefore, it became necessary to understand the relationship between the level of oxygenation and the function of the mitochondria in vivo. Chance and collaborators [50, 51, [52, 53, 54] developed the setup that enabled the exposure of various types of mitochondria or the entire small animal to the hyperbaric chamber. They found that the NADH of the brain, liver and kidney became more oxidized under hyperbaric oxygenation, and this effect was correlated with a decrease in NADH measured by biochemical analysis of fixed tissue. Figure 21A shows the system that enabled the monitoring of NADH under in vitro or in vivo exposure to hyperbaric oxygenation. This technique was used also when various organs of the rat were exposed in vivo to HBO as seen in (Figure 21) [52].
Figure 21: A - Apparatus for the fluorometric measurement of changes in NADH redox state in the organs of an anesthetized rat. The fluorometer components are mounted on the top of the window of the hyperbaric chamber. The compensating photomultiplier is show in the left, center is the excitation lamp, and right is the measuring photomultiplier (52). B - Responses of rat liver to repetitive compression and decompression with oxygen. The sensitivity for measuring fluorescence changes is also indicated (52).
Rat Liver in vivo. As found previously, the fluorescence of reduced NADH observed at the surface of the rat liver decreased at high oxygen pressures. In further support of No distinctive plateau in the relationship between fluorescence decrease and oxygen pressure was observed up to oxygen pressures of 10 atm. When pressurization was rapid, the tank artifact mentioned earlier became apparent and was followed by the slower biochemical response of the liver. More satisfactory results were obtained with pressurization through a needle valve. With this method of pressurization, the oxidation of the nucleotides appeared to “keep pace” with the increase in pressure, so that little further effect was observed after final pressure was reached.
(Figure 21) demonstrates that the cycle of oxidation of the pyridine nucleotides is reversible and can be repeated. This pressurization technique was used in later experiments where the effects of uncoupling agents or amobarbital on the hyperbaric response were studied. Depending upon the susceptibility of the animal to irreversible oxygen poisoning and the final pressure of the exposure, two to four such reversible cycles can be obtained. Analyses of results for 14 animals in which such compression cycles were obtained gave decreases of 13±1% of the initial fluorescence level at pressures of 120-150 psi. The response to anoxia in rat livers, also shown in (Figure 21) , was transient, and the maximum fluorescence increase was approximately 60% of the anoxic response in this preparation.
Using the light-guide-based fluorometry, we exposed an awake brain to hyperbaric oxygenation as seen in (Figure 22) .
Results recorded from the unanesthetized rat brain exposed to 75 psi (6ATA) of oxygen are shown in (Figure 22) [55]. The reflectance at 366 nm increases during the pressure elevation period, and a few minutes later a large decrease of reflectance was recorded. The third trace from the top-the corrected fluorescence – represents the difference between the fluorescence emission at 450 nm and the reflectance at 366 nm. This correction technique is now used by several investigators [36, 39, [46, 56, 57]. During the compression, an oxidation of NADH of 10% of the normoxic fluorescence level is observed, which is maintained for 15 min. A series of oxidation-reduction cycles of NADH were recorded. About ten minutes before the animal stops breathing, the NADH increased by 50-60% at the end.
The fourth and fifth traces of Figure 22B present the EEG measured from the two hemispheres. The two hemispheres of the brain respond to HBO in the same way. A few minutes after compression, the EEG changes from the typical ‘awake’ pattern to the activated pattern, and then the seizures activity appear. The number of bursts of convulsive activity differs between animals. The EEG becomes flat just before the animal stops breathing, and the increase of NADH was recorded.
A quantitative analysis of the signals was made as seen in Figure 22C including the number of convulsions and oxidation cycles. The three parameters shown in Part B left side [55] are probably connected to each other and in most conditions occurred in the same order Between 30 and 60 psi the slopes of the changes of all three parameters were very sharp, whereas between 60 and 150 they were moderate. Thus, the 60-psi pressure is a breaking point of the line. The other three parameters shown in part C right side, are affected differently by the pressure. The maximum effect was observed at 60 psi, and the curves had a bell shape. The differences between the 60-psi point and the 30- or 150-psi level are statistically significant (P<0.005), as calculated by the Student’s t test.
Figure 22: A – The Time-sharing fluorometer/reflectometer is attached to the hyperbaric chamber for the measurement of NADH from the cortex of the awake rat exposed to HBO (40). B - Effects of pressure level during hyperbaric oxygenation on hemodynamic, metabolic, and electrical activity of the brain. C - Effects of pressure level on electrical activity and its concomitant phenomena of convulsions and spreading depression (55).
Responses of NADH Fluorescence to Increase in Energy Consumption
Chance and Williams showed that the activation of the mitochondria by increased ADP is coupled with oxidation of NADH (decreased NADH levels) and is known as the State 4 – State 3 transition in isolated mitochondria [15, 31]. As shown in (Figure 2) , the demand for energy (ATP) by various tissues is dependent on the specific tasks of each organ or tissue. Nevertheless, the stimulation of mitochondrial function is common in all tissues in the body. The effects of tissue activation on NADH fluorescence under normoxic conditions as well as during limitation of O2 supply (hypoxia, ischemia) is presented.
Effects of Cortical Spreading Depression (CSD): Brain Cortical Spreading Depression [58] was
Figure 23: A - The first publication described the development of cortical spreading depression. Gradual spread of depression from A to F. Electrodes arranged as shown in the inset. A. Before stimulation L. 7 min after K. Unless otherwise mentioned, the stimuli were induction shocks at “tetanizing” frequency, applied for 3 to 5 sec. through electrodes S (58). B - Repetitive responses of the cerebral cortex to spreading depression evoked by application of 0.4 M KCI on the dura. The third trace is on an expanded amplitude scale. The arrow direction shows an increase in the optical signals. (39).
discovered by Aristides Leao in 1944 (Figure 23), After cortical electrical stimulation he recorded a wave of EEG depression propagated from the stimulation point to the rest of the hemisphere. In parallel to the EEG depression, the steady electrical potential shows a negative shift [59]. I The wave of depolarization passing through the tissue increases energy consumption [58]. In another study Leao described the effects of CSD wave on the diameter of the blood vessels on the brain surface [59]. The involvement of the microcirculation in the response to CSD was described by Van Harreveld et al few years later [60, 61]. In other studies, the The PO2 in the brain had indicated that demand for energy and oxygen consumption increased during the CSD wave [62, 63, 64]. Bures and Krivanek [65] used a special approach to check ion shift in the rat cortex and showed that extracellular potassium is elevated [66]. In the early 1970s, Vyskocil et al. used a potassium-selective microelectrode and showed clearly that extracellular K+ was elevated significantly and lead to increase in oxygen consumption [67].
When dealing with CSD and hypoxia or ischemia it is important to define the situations clearly. The discovery of the CSD event happened when rabbits’ brain was exposed to electrical stimulation in order to elicit “experimental epilepsy”. Oxygen supply to the brain was normal. Under ischemia, the EEG is also depressed as soon as the oxygen supply to the brain is limited but the mechanism behind the EEG depression is different from that operated in CSD (Figure 31).
The effects of CSD on brain mitochondrial NADH was first described in 1973, by Rosenthal and Somjen, where a clear oxidation wave was recorded [68]. In the same year [36] our team showed the same NADH oxidation response to CSD in the rat brain (Figure 09). A year later, we demonstrated the coupling between the elevated extracellular K+ induced by CSD and the NADH oxidation needed to recover the normal ionic homeostasis by the mitochondria [38]. Also, we studied in detail the nature of the NADH response to CSD as seen in (Figure 23) [39]. In order to initiate a wave of spreading depression (SD), KCl solution was passed through one of the pairs of tubes located above to the Dura mater. At threshold (~0.1 M KCl) only one wave of SD developed, while at maximum (~0.4 M KCl) many cycles were recorded. (Figure 23) represents the latter condition, in which six SD waves developed by 0.4 M KCl were terminated by washing the brain surface with 0.9% NaCl. The oxidation of NADH is corresponding to states 4-3-4 transitions and are related to the changes in energy demand during the SD cycle.
In order to show that the CSD wave recovery is dependent on the availability of oxygen another experiment performed and the results appear in (Figure 24). If energy production is in the normal range, the pumping of potassium into the cells starts immediately and its level recover back to the normal value- 3 mM. When energy production is inhibited, the pumping of K+ is inhibited, as shown in (Figure 24) [69]. In this animal, waves of CSD developed spontaneously after a few hypoxic episodes and the left side of the figure shows the regular response to CSD. The efflux of K+ reached a level of 14-15 mM and was then pumped back into the cells and the NADH (CF) shows a clear oxidation cycle as shown also in Figure 23B. In the second CSD cycle shown in (Figure 24) , hypoxia of 4% oxygen was induced at the beginning of the recovery period and the recovery process was inhibited until 100% O2 was provided again to the rat.
Figure 24: A - The experimental setup used (see details in (Figure 18)). B - The effects of hypoxia (4% O2) on the metabolic, ionic and electrical responses to cortical spreading depression developed spontaneously in the rat brain. Abbreviations are as in Figure 18 (69).
Figure 25: A - Presentation of the experimental setup used to study brain cortical spreading depression (see details in (Figure 20)). B - Metabolic, hemodynamic, ionic and electrical responses to CSD. The MPA used in this study contained two different bundles of fibers for monitoring the relative CBF and NADH redox state. R, CF - reflectance and corrected fluorescence; LDF LDv - Laser Doppler flow and volume monitored from another optic fiber located in the MPA; ECoG - electrocorticogram; K+e , ECa2+, Cae2+ - corrected potassium, uncorrected and corrected calcium ion concentrations, respectively; DCK+, DCca2+ - DC steady potential around the K+ and Ca2+ electrodes (49).
(Figure 25) shows a laser Doppler flowmeter added in order to record microcirculatory blood flow [70]. Part B shows two sets of responses to CSD recorded after 0.1M KCl application 2 mm anterior to the MPA. A clear correlation between the oxidation of NADH (CF) and large increase in relative CBF can be seen during the six cycles recorded. The changes in relative CBF are calculated in relation to the control values before the waves were initiated, The cycles of CSD were not so clear on the volume trace and we suspect it was a reading problem, since in other studies a clear connection between flow and volume was found using the same laser Doppler flowmeter.
Effects of Epileptic activity: Jobsis et al. in 1971 [32] described the influence of epileptiform activity on brain mitochondrial NADH redox state. Using anesthetized cats exposed to an epileptogenic drug (Metrazol or Strychnine), a marked expected oxidation of NADH was recorded. The changes in the electrical activity, increased the demand for energy in order to restore ionic homeostasis during the epileptic activity. The connection between the concentration of K+ and the oxidation of mitochondrial NADH was shown in cat hippocampus [71]. The effect of seizures on mitochondrial NADH was investigated later by other groups, [72, 73, [74, 75, 76, 77, 78, [79, 80, 81]. Vern et al. showed that epileptic activity, induced in hypotensive cats, caused an increase in NADH instead of a decrease [80].
In 1975, we described the mitochondria NADH responses to epileptic activity measured in non-anesthetized rats [37]. The Metrazol (100 mg/ml) was applied epidurally, to the surface of the rat brain, using a special cannula [37]. The typical response to Metrazol occurs 3-5 min after the administration of the drug and the results are shown in Figure 26-part A. The Metrazol was applied to the right hemisphere and the left one served as a control. An increase in electrical activity was recorded together with an oxidation of NADH which was in the range of 5-10% of the normoxic level. After a period of a very intense activity, the EEG became isoelectric while the NADH showed a very large oxidation cycle A different response was recorded in other animals as. In these cases, the small oxidation under Metrazol effect was recorded but the recovery to the normoxic level showed no large oxidation cycle (data not shown).(Figure 26) presents the progression of seizure activity in a special strain of gerbils [[84], developing epileptic effects as a result of monotonic noise. As seen in the (Figure 26) the exposure of the awake gerbil to noise resulted in the development of epileptic activity followed by a CSD wave [17]. The coupling between the two pathological events is clear and manifested by the electrical activity (ECoG) as well as extracellular K+ levels and NADH redox state. During the epileptic stage, extra cellular potassium increased and started to recover to the baseline, but then a larger elevation was recorded. There is a clear correlation between the various parameters during epilepsy and CSD. The decrease in NADH was smaller during the first stage, followed by an oxidation cycle typical for CSD.
Figure 26: A - The effects of topical application of Metrazol on brain NADH fluorescence, reflectance and the EEG of the two hemispheres. Note that the amplitude of the EEG calibration in the right hemisphere is 1 mV (37). B - The development of epileptic activity followed by cortical spreading depression (CSD) wave in the brain of a seizure-prone gerbil. ECoG, electrocorticogram; PO2, partial pressure of O2; EK+, DCK+, extracellular potassium and DC steady potential measured around the K+ electrode; R, F, and CF, reflectance, NADH fluorescence, and corrected NADH fluorescence (17).
NADH responses to activation of the brain under restriction of oxygen supply: We used 2 ways to decrease oxygen supply to the brain while exposing it to an increase in oxygen demand by local brain ischemia or systemic hypoxia.
In order to study the effects on the metabolic response of the brain resulting from the limitation of its blood supply, the carotid artery ligation technique was used. The common carotid arteries were dissected in the neck, and loops of ligature were placed around the vessels. Measurements of NADH redox state were taken by a light guide holder located above the right hemisphere. Initiation of cortical spreading depression (CSD) cycles was initiated by flushing KCl solution through a small push-pull cannula implanted above the right hemisphere so that NADH fluorescence measurements were taken at the same time. KCl was applied topically to the cortex and NADH fluorescence and ECoG measurements were taken.
This, response represents the normal brain and served as a control. Three hours after the operation when the animals were fully awake, the carotid arteries were ligated.
Figure 27: A - The effect of acute bilateral carotids occlusion on the metabolic response to cortical spreading depression (SD). Parts B, C and D were measured 15 min, 90 min and 18h after occlusion, respectively (85). E -The metabolic responses of the normoxic and ischemic (F) brain to a wave of CSD R-366 nm reflectance, CF-NADH change(101).
(Figure 27) shows the response of an awake brain to a bilateral carotid artery ligation and CSD [85]. Before ligation, 0.5 M KCl was applied to the cortex and the normal metabolic response to CSD was recorded (Figure 27) . The bilateral ligation of the carotid arteries caused an increase in NADH, and since the KCl remained on the cortex during this period (about 3 min), the response to SD was immediately apparent. In this animal, the duration of the oxidation cycle became very long after the ligation, as a response to spreading depression. A similar long oxidation cycle was recorded again later (lower Part-B), but within 2h the metabolic response to SCD disappeared (not shown).
In this set of experiments 10 rats were involved. Figure 27 shows a typical response of the brain to acute bilateral carotid arteries ligation. Parts B, C and D show the response of NADH to spreading depression elicited 15 min, 90 min and 18h, respectively, after the ligation. In this animal the changes of the reflectance (upper trace in each part) was minimal during the CSD cycles. In part D, a very long biphasic cycle of the reflectance was recorded. The effect of the ligation on the NADH was transient, namely, that a small increase was recorded during the ligation, but within two minutes it recovered to the base line. The main effect of the ligation is on the response of the brain to CSD which increases oxygen utilization. During the first 60 min after ligation the oxidation cycle can be detected as a response to CSD, but the amplitude of the oxidation (decrease) was diminished and the duration of the cycle became much longer. The qualitative change in the response of the brain to CSD started several hours after the ligation. In part C, the response to CSD was recorded 90 min after the ligation. A biphasic response of NADH to CSD was recorded. At the beginning of the cycle an increase in NADH was recorded, while afterwards the NADH decreased below the base line level. In part D (18h after ligation) the response of the brain was completely opposite to what was measured before the ligation (part A). Instead of an ‘oxidation cycle’ as a response to CSD, we found a ‘reduction cycle’ namely an increase in NADH redox state. (Figure 27) -right side illustrate schematically the difference in response to CSD recorded in normoxic (E) and ischemic brain (F).
The effects of hypoxia on the response to epileptic activity induced by Metrazol is shown in (Figure 28) [37]. Matrazol was applied under hypoxic condition. The animal was exposed to air (A), 10% O2 (B), 7.5% O2 (C), and 5% O2 (D). The line N.L. represents the normoxic level of NADH. In all oxygen concentrations, an increase in activity followed by a flat EEG was recorded. The changes in NADH are the same as in Figure 26A, namely, that two phases in the oxidation were found. By giving the animal lower concentrations of O2 the baseline shifted to a more reduced level depending on the O2 level.
Figure 28: The effects of graded hypoxia on the response of the awake brain to local application to Metrazol. The fluorescence responses occur 3-5 min after the application of Metrazol. The levels of O2 were in A, air; B, 10%; C, 7.5%; and D, 5% (37).
In order to test the validity of the 2 types of NADH responses to CSD induced in the normoxic and ischemic brain we used a different approach. We constructed and tested a mathematical model [86], capable of simulating changes in brain energy metabolism under various pathophysiological conditions. The model incorporates the following parameters: cerebral blood flow, partial oxygen pressure, mitochondrial NADH redox state, and extracellular potassium. Accordingly, all the model variables are only time dependent (‘point-model’ approach). Numerical runs demonstrate the ability of the model to mimic pathological conditions, such as complete and partial ischemia, cortical spreading depression under normoxic or partial ischemic conditions. The pathological state of cortical spreading depression was induced by “potassium injection”. When CSD was induced, the increase in energy requirement leads to the activation of Na+-K+ ATPase in order to restore the normal potassium distribution and causes an increase in blood flow and a decrease in NADH. When the blood flow decreases mildly (mimicking the ischemic period, an infusion of potassium was modeled. Accordingly, the blood flow slightly decreases as a result of the infusion, and then increases to the new resting level.
Monitoring of the Human Brain in Neurosurgical OR and ICU
After accumulation of preliminary results using the 1st clinical multiparametric monitoring system [4], a new commercial device was developed. In the new device-the TiSpec, we monitored only three out of the 8 parameters monitored by the multiparametric monitoring system [4]. We used only the optical based probes in order to simplify the technology and to shorten the “time to market” of the new device. The TiSpec provided parameters of microcirculation (blood flow and volume) and mitochondrial NADH. The TiSpec and the various probes developed for this device are presented in (Figure 29). This device was tested in animal models as well as in neurosurgical patients. In (Figure 29) , one of the various holders of the fiber optic probe are shown. In. In Figure 29B the probe is located on the surface of the brain during operation. More details regarding the Tispec were published [87, 88].
The first attempt to monitor NADH from the human brain by our group was in 1990. We monitored few neurosurgical patients in the Hospital of the University of Pennsylvania after IRB approval. We monitored in those patients only Mitochondrial NADH and microcirculatory blood flow since extra time availability during the surgical procedure is very limited. The responses of the human brain to ischemia are presented in (Figure 29) In this patient a short brain ischemic episode was induced by the occlusion of the common carotid artery during the preparations for brain aneurysm procedure. As seen in (Figure 29) an immediate decrease of CBF was recoded simultaneously with an increase in NADH redox state with only very small changes in the reflectance recorded (R). The recovery of CBF and NADH oxidation were very fast. The time to reach 50% of the change in NADH during the recovery was less than 3 seconds. In contrary to the NADH recovery to the pre-ischemic level, the CBF signal shows a large hyperemic response (large overshoot in CBF).
Figure 29: A - The Tissue spectroscope (TiSpec) device used in monitoring patients in the neurosurgical operation room. B - The application of the standard optical probe with the floating arm during an operation for the removal of a brain tumor [136,137]. C - Responses of the human cortex to a decrease in blood flow induced by common carotid artery occlusion. R, F, CF – Reflectance, Fluorescence and corrected fluorescence. D - Effects of IV infusion of Mannitol on brain hemodynamic, metabolic and ionic activities in head injured patient (4).
In the 1980’s we developed the first multiparametric monitoring system that was adapted to experimental animals exposed to various pathological conditions [89-92]. Later on, we improved the system in order to introduce it to the neurosurgical operating room or intensive care unit (ICU) for patient monitoring. The concept behind the development was that the more parameters that are monitored, the better the diagnosis of the situation will be. The ideal system was to monitor eight parameters from the brain (right side) in addition to the various systemic parameters monitored in every patient hospitalized. The long-term vision was that all parameters monitored by the 2 monitoring systems will be integrated in the same data bank and an expert system will be developed for better diagnosis of the patients. The translation of this concept into a practical tool and monitoring device is presented in (Figure 30) [93-98].
Figure 30: A - Schematic representation of the multiprobe assembly (MPA) used for monitoring the brain of head-injured patients in the ICU. The MPA is connected to the brain via a special holder screwed into the skull. ICP, intracranial pressure probe; CBF, NADH-fiber optic light guide probe to measure local cerebral blood flow, (CBF) and mitochondrial redox state (NADH); K+, DC, extracellular K+ mini surface-electrode surrounded by a DC steady potential monitoring space; ECoG, bipolar electrocortical electrodes (4). B - The first clinical monitoring setup (“Brain Monitor”) used in the neurosurgical intensive care unit. The Multiparametric monitoring system consists of various devices installed in the same cart. The MPA shown in (Figure 30) is connecting the brain of the patient to the Brain monitor (96). C+D - Two responses of the brain to a wave of cortical spreading depression developed spontaneously in a severely head-injured patient. ICP intracranial pressure; R - 366 nm reflectance, NADH - 450 nm corrected fluorescence; CBF, CBV cerebral blood flow and volume measured by laser Doppler flowmetry; Ke, DC - extracellular potassium levels and the DC steady potential measured around the K+ electrode; ECoG electrocorticography.
Based on the well-developed MultiProbe Assembly (MPA) used for animal experiments [3] a new MPA was developed and applied to patients monitored in the neurosurgical operation rooms and ICU. This device allowed us to monitor, in real-time, the hemodynamic, metabolic ionic and electrical activities in the brain of comatose patients. All details regarding the technology and the clinical setup appear in our published paper [4]. Figure 30A shows the longitudinal section of the MPA (measuring 8 parameters) used in the neurosurgical operating room and ICU. The MPA was connected to the multiparametric monitoring system shown in Figure 30B.
The next step was to use the multiparametric monitoring system in the neurosurgical ICU. In this group, 14 patients were monitored (Figure 30). Only one of the 14 monitored patients had developed spontaneous changes in all parameters like the typical responses to SD in animals. These changes were recorded 4.5h after the beginning of monitoring, which was 7 hours after admittance to the hospital. During the measuring period, this patient was bilaterally irresponsive to pain, his pupils were dilated and non-reactive to light. He was mechanically ventilated, and his brain CT scan showed evidence of severe brain edema in the left hemisphere and right parietal hemorrhagic contusion. The measurements were taken from the right frontal lobe. As seen in (Figure 30) , the ECoG became depressed for 10-15 minutes and at the same time a cycle of elevated extracellular K+ and a small negative shift in the DC potential were recorded. These changes are typical to transient depolarization, which is a dominant part of cortical spreading depression. NADH was oxidized while blood flow and volume increased. This patient exhibited repetitive SD cycle every 20-30 minutes. However, the following SD like cycles that were recorded from this patient (after the first ones) showed different hemodynamic and metabolic responses (Figure 30) . While extracellular level of K+ and the pattern of the DC potential were very similar, NADH oxidation cycles were replaced by a biphasic cycle comprised mainly of a phase of increased NADH followed by a small oxidation phase. The compensation of blood flow and volume was also reversed at this time. The monophasic increase in CBF and CBV was replaced by an initial decrease followed by a smaller increase. Significant correlations were seen between CBF, CBV and NADH (CF) and extracellular K+ levels [95, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 131, 132, 133], 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198].
The severe head injured patient who showed high levels of ICP was treated with Mannitol infusion as seen in Figure 29D. The infused bolus led to a clear decrease in ICP associated with a large increase of CBF and a beneficial effect on tissue oxygenation state (oxidation of NADH) [99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109].
Discussion and Future perspectives
(Figure 31) [16] presents, in a schematic way, the relationship between various physiological parameters monitored in vivo under different experimental perturbations. The upper two factors (left side), namely, systemic hypoxia and cerebral ischemia affect the availability of oxygen so that energy metabolism is inhibited (blue arrows). The other two perturbations shown are cortical spreading depression and epileptic activity that induced cortical depolarization (purple arrows). All four perturbations are affecting the same type of processes in the brain such as CBF, NADH and extracellular levels but in the opposite direction as seen in the scheme. Under hypoxia and ischemia, the production of ATP by the mitochondria is decreased and becomes a limiting factor in the system. Due to the inhibition of the ionic pumps, the ionic homeostasis is disrupted, and hypoxic or ischemic cortical depolarization is developed[110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130]. The end points of this process are changes in the electrical activities recorded as a depression of the ECoG and a negative shift in the DC steady potential. In addition, due to the depolarization event the extracellular potassium is elevated. If this situation persists for more than a few minutes, irreversible damage to the cortex may develop. When the brain is activated by CSD or epileptic activity (lower left side) the events will propagate toward the activation of the brain and energy consumption is increased (purple arrows) [131-149]. The coupling between the two pathological events is clear and manifested by the electrical activity (ECoG) as well as extracellular K+ levels and NADH redox state. During the epileptic stage, extracellular potassium increased and started to recover to the baseline, but then a larger elevation was recorded. There is a clear correlation between the various parameters during epilepsy and CSD. The decrease in NADH was smaller during the first stage, followed by an oxidation cycle typical for CSD [150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198].
Figure 31: A schematic representation of the effects of various pathological conditions on the energetic-mitochondrial function, ionic and hemodynamic state of the cerebral cortex (details are in the text) (16).
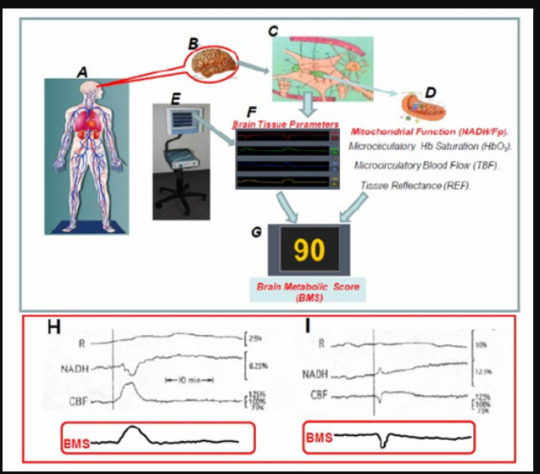
After the accumulation of huge amounts of experimental results, we decided to develop a preliminary tool named Brain Metabolic Score-BMS as seen in (Figure 32) . In the initial developmental stage, the BMS was calculated by using two parameters, namely the NADH and CBF. In the present study we are presenting the preliminary developed Brain Metabolic Score- BMS that could be used in evaluating the metabolic state of the cerebral cortex in real time. We used the brain as a typical organ for the calculation of the Score, but the same approach could be used for other organs or tissues. The goal of our study is to develop the Tissue Metabolic Score that could be monitored in the intensive care units or in the operating rooms. In those medical environments the need for objective approach for decision making processes is very important as discussed previously [183-199]
Figure 32: A-G - Schematic presentation of the elements involved in the concept “Brain Metabolic Score “(BMS). H+I - The calculated BMS of human brain during the development of two cortical spreading depression cycles in a head injured patient.
In order to develop the BMS we used the multiparametric monitoring system described in detail in (Figure 15) and the development of the score appeared in our publication [200, 201, 202, 203, 204, 205, 206, 207, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235].
Read More About this Article: https://biomedgrid.com/fulltext/volume7/assessment-of-brain-metabolic-score.001136.php
For more about: Journals on Biomedical Science :Biomed Grid | Current Issue
#biomedgrid#american journal of biomedical science & research#medical and medicinal journal#free medical journal
0 notes
Text
instagram
#nasser hospital#nasser medical complex#hospital#yemen#jerusalem#tel aviv#current events#gaza genocide#palestine#free palestine#gaza#free gaza#journalist#journalism#free palestine 🇵🇸#from the river to the sea palestine will be free#from the river to the sea 🇵🇸#end genocide#crimes against women#crimes against children#israeli crimes against humanity#no justice no peace#keep talking about palestine#keep posting about palestine#palestinian journalists#journaling#your voice matters#don't stay ignorant#don't stay silent#silence is violence
26 notes
·
View notes
Text
Why is statistics so tediousssss. Easily the most tedious part in any project is learning how to use yet another new package in Rstudio just so you can make nicer graphs. Closely followed by literature research.
I get it, statistics is easier than ever with most formulae being compactly available in a function you can just call but. ggplot2 and me? We don't get along, I can't remember your syntax. I'm going to let Github Copilot manhandle your plots, like you deserve nothing better.
#delete later#Vaxx is having a moment of weakness; apologies#My prof just told me “there's documentation on their github” and did the Tuxedo Mask gif thing#Honestly I feel like esp in sciences; if you aren't really like REALLY into your job; you won't make it far; you won't do well#What I do like though is that everyone above you is brutally honest about “Yeah I hate this part” and will teach you how to do it so#you can do it for them in the future alkjsdhakjshdasd#there's just too many frustrating things that make you want to quit like arbitrary formalia eg. which citation style you need to use#guys why does every university have its own citation guidelines whats the point in creating a “normed” style when everyone's using their ow#When a journal tells me they only take .doc files as submissions but don't have a template for the 500 format rules they came up with#Bonus if they don't accept .tex files; like why do you hate a streamlined work process; ugly pdfs from google docs is sooo 2010s#Medic was right; don't stay in academia; be free; steal a van; and start murdering people
3 notes
·
View notes
Text
I can't find that post of a shirt that says "I don't know what's wrong with me, but I have a list of medications that didn't help." But it's me!! Gonna start my new meds tonight lolol
Rexulti better fucking worth considering how fucking expensive it is 🤬
#personal#it was free bc I met my out of pocket already but SHEESH#$1700 for 30 days. Fuck this country lol#medication journal
11 notes
·
View notes
Text
I made infused (and not infused) Banana-Blondies today 🌿💨🍌 They are also gluten free and dairy free. I forgot to take a photo of the cannabutter though 🥲



#edibles#infused#medical marijuana#cannabutter#plant medicine#marijuana#cannabis#cw cannabis#cw marijuana#gluten free#dairy free#stoner#personal#online journal#digital diary#journal#personal post#diaryposting
9 notes
·
View notes
Text
oh my god I'm so angry right now
I finally decided to look up the negative reaction I had to Zofran that made the ER doc mark it down as an "allergy" last month as soon as he connected the dots.
turns out that a likely explanation for me being super, unbearably, painfully, I nearly fell off the cot multiple times, *squirmy* on top of having a bad fever, severe confusion, no ability to regulate temperature so I was shivering and sweating at once, and being at the mercy of my digestive system...
well I mean I know most of that sounds like heatstroke, but that unbearable restlessness was the part that the doctor was like "oh yeah like 1:200 have this, let me get you something for it and mark this down as don't have" (and then I failed to realize the nausea meds I had at home were the same drug until after the next hospitalization lol).
anyway yeah. seratonin syndrome. potentially. because I was also on SSRIs and they injected me with a drug that stops nausea by blocking seratonin absorption.
which I feel like really should have been a med interaction that at least one of the like 14 assorted medical professionals who saw me in the last couple months could have had some foresight on. or that at least the doc who noticed the reaction should have realized it was.
like there's a chance it's not that, it's a different side effect, but I've been given Zofran before when I *wasn't* on SSRIs and if it had any effect on me it wasn't significant enough for me to remember, because I found that restlessness to be an entirely new kind of torture to me. the closest I can come to describing it was an itch, but not in the way the sensation was, just in the way it was insatiable no matter how much I tried to move to relieve it. it didn't hurt, it wasn't itchy, just. thrash inducing and fear inducing because nothing worked to make me feel like I could stay still. the doc said "kind of like anxiety?" when I was trying to describe it and while it wasn't the same sensation exactly, it was the same persistence, so that may be my best analogy.
anyway I'm typing this up half to bitch about this having happened and half to tell you that if you also take zofran at all maybe double check that some of your symptoms aren't being caused by the supposed solution.
(this isn't saying it's universally bad; all medications have pluses and minuses and I am not a doctor, pharmacist, or anyone else qualified to speak in general terms. I know others who it works great for. but I feel like the effects here were easy enough to mistake for my nausea-related stuff getting worse that I wanted others to potentially know it)
#medical stuff#health stuff#don't mind me just journaling in public#i never know what tags to put on this stuff cuz I always assume the text itself should contain most the relevant bl words#so feel free to let me know if you need stuff like this tagged so I can
4 notes
·
View notes
Text
i get sooo worried about going off my adhd meds, but honestly i feel like i sometimes exaggerate how bad my adhd is because the last time i was unmedicated for a long period of time (right before i was diagnosed) i was also literally suicidal and had severe anxiety which uh. definitely exacerbated the lack of focus 🫠
#like. i'm unfocused and fidgety and i keep losing my phone and i'm sooo tired#but it's nowhere near as bad as 2021. i'm getting my work done (albeit slowly) & responding to emails#i used my extra free time this morning to practice piano for literally almost an hour???#idk. the amount of work i'm getting done today is not up to my normal standards but it's not NOTHING#there were soooo many days in 2021 where i literally got NOTHING done#i think i'm also better at coping lol. like i KNOW i can't focus on things for a long time so i switch tasks#today i'm interspersing my work with cutting piece for a new quilt i'm working on lol#i also did laundry!!! and picked up oat milk & treated myself to a panini from my local deli#i got an email this morning that my labwork is in so i should be able to meet with my new doctor this week and get back on meds#i have 3 pills left but i'm saving them just in case#i'm worried because i was off my meds for like 4 days before my drug test so i tested negative for amphetamines#so i'm worried they're gonna accuse me of selling them or something#but it's all good. i keep a very detailed medication log in my bullet journal. i have Evidence™#(me: i'm not that adhd / also me: writes an entire novel in the tags of this post)#m.txt
4 notes
·
View notes
Text
My ADD Medication Journey - Dec 18, 2023
12/18/2023 – Monday
1st dose taken at 7:30 am
Packed a couple of granola bars in my lunch to snack on one or two hours after taking meds
Was noticeably fatigued waking up despite getting a proper night's sleep
Don't recall being woken up in the night, felt like I slept the whole way through
About an hour after taking meds, noticed slight increase in energy
Possibly because I've completely woken up
Two hours and energy is good, focus is good
Not noticing any increase in tremor intensity
I intend to try two doses today
Friday's 2 doses and sleep suggest that acclimating to the change in chemistry is required
Insomnia might be a temporary side effect
Will note my findings tomorrow
Unsure if this is related to the medication or if it's just a point in a cycle
Noticing reduced craving for soft drinks (usually Coke)
Convenience of access still provides temptation, but it isn't as strong
Been drinking water more than anything else
Possibly just at a point where I'm less interested in the sugary taste
Possibly no longer needing the stimulation due to the medication
Unsure, but will continue to monitor
Noticing suppressed appetite, still eating my granola bars to prevent low blood sugar
Noticed uptick in tremors after eating, not enough to be a hinderance
2nd dose taken at 12:30 pm
Originally intended to take medicine at 11:30 am, but got wrapped up in work
I should try to set a regular schedule for taking my medication and put in a daily reminder on my phone
Today was the office Christmas party and lunch was provided
Unsure if there was enough citrus in the food to affect medication absorption
Not noticing any major changes in tremor intensity – though the office being so cold doesn't help
Focus is still good, mood is still good
As of 2:30 pm, still not terribly hungry and doing well
The rest of the day passed without incident
Did not notice any increase in tremors
Energy and mood remained at a good level
Appetite was not terribly strong by the time I got home, but ate dinner anyway
Interested to see if I sleep like I did on Friday, or at all
#ADD#ADHD#Attention Deficit Disorder#Attention Deficit Hyperactive Disorder#Medication#Journal#Smokey's ADD Meds Journey#Office Christmas Party#Fajitas aren't terribly Christmas-y#Hey it's free food#Insert it's free real estate meme here#They also put on Elf for everyone to watch#Not my kind of movie--no shade if you like it#I just went back to my desk and kept working after eating#Could have had a half day off but only if I was at the party#Office parties aren't really my jam
0 notes
Text
Honeyboo (Indica, dry leaf) Long duration, body high. Helps with: Mood, relaxation, fun appetite, and nause. ((Big time munchies))
☆☆☆☆
Tiger's Milk (Indica, dry leaf) Medium duration, body high. Helps with: Sleep, nausea, anxiety, and mood.
☆☆☆☆
Cheez Wiz (Hybird, dry leaf) Short duration, body high. Helps with: Relaxation, pain, and nausea.
☆☆☆☆
Golden Goat (Hybrid, dry leaf) Medium duration, mind high. Helps with mood, anxiety, and depression.
☆☆☆☆
Cookies N Chem (Hybrid, dry leaf) Medium duration, mind high. Helps with anxiety, relaxation, and motivation.
☆☆☆☆
Coffee Ice Cream (hybrid, dry leaf) Long duration, body high. Helps with: mood, anxiety, relaxation.
☆☆☆☆
Intergalactic Toad (Indica, dry leaf) Long duration, body high. Helps with: Pain, relaxation, and de-stress.
☆☆☆☆☆
Pineapple Express (Sativa, dry leaf) Medium duration, mind high. Helps with: creativity, mood, relaxation.
☆☆☆☆
WC Watermelon Z (indica, dry leaf) Medium duration, mind and body high. Helps with: Anxiety, mood, sleep, and stress. (Makes you feel euphoria.)
☆☆☆☆☆
Kush Mints x Animal Sorbet (Indica, dry leaf) Long duration, body high. Helps with: anxiety, pain.
☆☆☆☆
#Weed journal#Strains that have helped me. If they help anyone else even better. :)#I have two auto immune conditions and medical mj has helped SO MUCH.#Not having hives for years after having them really bad and almost going into anaphylaxis was a game changer. Especially when my doctor at-#the time of the hives being really bad just wanted to push experimental and expensive drugs she'd get kick back from.#I'd rather smoke / ingest weed than lose all my hair due to a shot that's been used on less than 300 patients.#Sure anaphylaxis wasn't pleasant. yes it took two shots of epinephrine and 48 hours to clear the hives that time... but it was ONCE.#My doctor wasn't actually listening or trying to target the trigger for my hives. which with my immune condition is stress.#THC / CBD building up in my system has kept me almost entirely hive free for the last 5 years!
0 notes
Text
Efficacy of IV Immunoglobulins on Psychiatric Symptoms: A Case Report
Efficacy of IV Immunoglobulins on Psychiatric Symptoms: A Case Report in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.006058.php
Borderline personality disorder (BPD) is a fairly common psychiatric illness with a prevalence between 1.8% and 2% in the general population [1]. Diagnostic Criteria in the DSM-5 [2]: BPD is a pervasive pattern of instability in interpersonal relationships, self-image, and mood and marked impulsivity that begins by early adulthood and is present in a variety of contexts, as indicated by five (or more) of the following: a) Desperate efforts to avoid real or imagined abandonment (do not include behaviours indicated in criterion 5). A pattern of unstable and intense interpersonal relationships, characterized by the alternating between extremes of hyper-idealisation and devaluation. b) Altered identity: markedly and persistently unstable selfimage or self-perception. c) Impulsivity in at least two areas that are potentially harmful to the subject (e.g. reckless spending, promiscuous sex, substance abuse, reckless driving, binge drinking,). d) Recurrent suicidal behaviour, gestures or threats, or self-mutilating behaviour (self-harm, cuts on arms and legs, cigarette burns, etc.). e) Affective instability due to marked mood reactivity (e.g., episodic intense dysphoria, irritability or anxiety, usually lasting a few hours, and only rarely more than a few days). f) Chronic feelings of emptiness. g) Inappropriate, intense anger or difficulty controlling anger (e.g., frequent bouts of anger or constant rage, recurrent physical confrontations). h) Transient paranoid ideation, associated with stress, or severe dissociative symptoms.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01
Follow on Blogger :https://biomedres01.blogspot.com/
Like Our Pins On : https://www.pinterest.com/biomedres/
#Biomedical Journal Impact Factor#Journals on Biomedical Science#medical journal in usa#Journal on medical science#Free medical journal
0 notes
Text
Biomed Grid | The Mediterranean Diet: Plant Lectins as Essential Components
Opinion
In the U.S. News and World Report annual rankings (published in 2020) we note that the Mediterranean diet (Md) has, for the third year in row, been named as the overall best healthy diet. This diet is well-known for its emphasis on a high content of fruits, nuts, vegetables and whole grains. In some meals in this class of diet one can find more than 20 individual components belonging to these groups of foodstuffs. For many years now the Mediterranean way of life has been highly looked upon for health inspiration. Based on studies of Md it is now well recognised that diets rich in fruits, vegetables, fish, and healthy fats are good for us, particularly our hearts. Numerous studies have clearly shown that eating like they do in countries such as Greece, Italy, and Turkey then there is a reduced risk of suffering from heart disease. Furthermore, according to health professionals at the Mayo Clinic, those that adhere to the Md show a reduced risk of developing cancer, Parkinson’s disease, and Alzheimer’s disease. So, what makes the Md exceptional?
In my opinion one should look for a common factor among the ingredients of the Md. A recent survey of the content of fruits, nuts, vegetables and whole grains has shown that all these commodities contain varying amounts of protein molecules termed lectins. Health authorities in Western society strongly advise members of the population to adopt the “five a day” principle i.e. one should include at least five portions of plant-based material in the diet per day (approx. 400g). In actual fact health benefactors are thus advising us that a diet rich in lectins is beneficial to health! This is in stark contrast to what can be described as anti-lectin propaganda that we read in the popular press and in opinions expressed on numerous web sites. It is indeed correct that there are reports in the scientific literature (animal experiments) that have demonstrated negative effects of diets highly enriched in certain lectins.
The amounts added to these artificial diets fed to animals are, however, in most cases so high that it would be impossible for an individual following a normal diet to attain a lectin intake likely to be detrimental to health. Furthermore, there is no evidence that individuals that keep to a vegetarian diet, i.e. daily are exposed to a diet enriched in lectins, show any signs of poor health. So what are lectins? Plant lectins were discovered by Stillmark in 1888 when he observed that castor bean extracts caused agglutination of red blood cells in vitro. The word lectin (legere; from latin, means to select) was coined by Boyd in 1954 to cover a group of hemagglutinins that were able to discriminate between blood types in the ABO system. This hemagglutination property of lectins has been exploited as a useful method to identify the presence of lectins in, for example, food extracts. This approach was used by Nachbar and Oppenheim in 1980 when they reported that lectins were present in >20 common food commodities including potatoes, carrots, tomatoes, beans and peas.
Lectins are proteins that are in general resistant to low pH in the stomach and to proteolytic enzymes in the small intestine, and for some of these molecules >90% can survive passage through the gut. It is therefore evident that biologically active lectins will be present in the intestine after a meal containing raw plant material. About 80% of our immune system is associated with the alimentary canal, such that dietary lectins will provide an activation of our immunedefense apparatus. One can surmise that a diet lacking active plant lectins would result in a weakened immune system. Plant lectins have physiological effects, such as binding to glycoproteins on the epithelial surface of the small intestine, where they may elicit local and/or systemic reactions, e.g. modulation of the immune system and the micro flora in the gastrointestinal tract.
That different lectins bind to specific regions of the small intestine has been clearly demonstrated in experiments using human biopsies. In 1996 Sharma and colleagues used a panel of 27 lectins to study binding to M-cells, enterocytes, goblet cells, lymphocytes and macrophages. They clearly established that lectins with known differences in binding properties showed individual specificity with regard to which region of the small intestine they bound. For example, only 13/27 were found to bind to the M-cells of the follicle-associated epithelium and of these 5 bound to goblet cells. Although the biological significance of this is currently not known, some lectinologists speculate that lectins which have the property of binding to different cell types associated with the small intestine will initiate or trigger, separate biological responses positive for our health/well-being. The lectin, following binding to the cell, can send information into the cell´s interior via second messengers, often modulating gene expression or the lectin itself may enter the cell through endocytosis.
A large number of animal studies have clearly shown that dietary supplementation with various lectins can reduce growth of a series of different types of tumors. In their review published in 2005 Gonzalez De Mejia and Prisecaru indicate that use of plant lectins may provide novel strategies in the future for the development of new forms of cancer treatment. Beneficial effects have been reported in cases of terminal cancer where lectin-based preparations have been utilized. As mentioned earlier lectins are protein molecules meaning that they are denatured by the high temperatures met during cooking, frying, grilling etc. resulting in loss of biological activity. Thus, the importance of emphasising the need to consume raw plant material.
Dietary lectin studies are complicated by the fact that humans have consumed lectins in their habitual diet for thousands of years, making the study of individual lectins thus virtually impossible, since many may well act in concert. Furthermore, a meal would seldom consist of e.g. a single vegetable. There is indeed information indicating that when different lectin-containing preparations are mixed separately and in various proportions, then the biological responses elicited are quite unlike. Plant lectins represent an unavoidable component of a balanced diet. During the past 10- 20 years more and more studies have been addressed to the conception that lectin molecules may have an important positive impact on our health in general. Current opinion concerns the potential that lectins may have in relation to preventing illness and should therefore be regarded as health promoting components in our diet along with vitamins, minerals and other micronutrients.
In conclusion there can be no doubt that the Md is enriched in lectins and that these protein molecules can promote both positive and beneficial effects on our health. It is therefore not difficult to voice the opinion that a balanced diet containing a rich variety of fruits, vegetables and nuts will provide the necessary diversity of lectins required to stimulate biological processes in the body, forming the basis of a healthy lifestyle. Although there is much talk of the benefits of Md there is no information readily available to the general public on why a plant-based diet is positive for our health. For good health it is not simply a matter of obtaining sufficient amounts of nutrients from our food and vitamins and minerals etc. by an intake of tablets from the local drugstore, since lectins can only be obtained, not in the form of a pill, but by ingestion of raw plant material purchased at the supermarket! In my opinion more public awareness needs to be directed to the content of Md.

Read More About this Article: https://biomedgrid.com/fulltext/volume7/the-mediterranean-diet-plant-lectins-as-essential-components.001126.php
For more about: Journals on Biomedical Science :Biomed Grid | Current Issue
#biomedgrid#american journal of biomedical science & research#free medical journal#top medical open access journal
0 notes
Text
instagram
#diseases#disease#hospital#medical needs#palestine#free palestine#gaza#free gaza#from the river to the sea palestine will be free#journalist#save palestine#genocide#journalism#end genocide#🇵🇸🇵🇸🇵🇸#free palestine 🇵🇸#فلسطين 🇵🇸#i stand with palestine 🇵🇸#from the river to the sea 🇵🇸#filistin 🇵🇸#🇵🇸#thanks to journalist we what is happening in gaza#journal#we are all palestine#we are not numbers#justice for palestinians#justice for palestine#ceasefire now#ceasefire#give palestine a ceasefire
4 notes
·
View notes
Text



Some of the angel numbers I put in my journal and my own handwriting. March 2023.
#This is over the course of like a week not just one entry#they were on varied pages in the journal#angel numbers#999#777#444#journals#journaling#handwriting#I'm currently dealing with medication changes which is what 444 was talking about but I don't feel too bad just tired sometimes#actually bipolar#my handwriting#mychatter#and it's true what I said- I stay with my treatments so my family doesn't worry about me (or they do less than they would otherwise)#also feel free to claim any of the angel numbers listed here- 999/777/444#if you believe in them or are interested in them if not that's fine too whatever works for you!
0 notes
Text
So I heard the term 'hypochondriac' thrown around throughout my life but never actually knew what it meant so I googled it and um
Obviously i'm not going to try to diagnose myself with anything after doing like 5 minutes of reading but.
Is it not normal to go to sleep kinda half expecting to just die? Like if your body is Doing Something you don't automatically assume you have stomach cancer or are having a heart attack or stroke or something??? I just attributed this to having mild paranoia bc as a kid I was so used to all my alarm bells going off literally always that I just figured I'd be dead by 25
I have! To go think about stuff!!!!!
Actually that's a lie I'm going to just keep assuming I'm dying until it's true eventually
#marble mumbles#i have spasms often which makes art and writing really hard#but i never talked to my doctor about them bc as a kid i was planning to be dead by 18#so i didn't want to bother#and now as an adult im too scared to say anything bc treatments cost money#and im not part of a medical study anymore so i can't use that to get free cat scans#i hope everything blows up tomorrow so i can be free from my job#and focus on getting my life together#i literally just thought everyone panicked every time they had a pain or nausea they couldn't identify#well! i don't have the brain power to worry about this literally at all so im just#i dunno. gonna add it to the current Stress Load i guess. nothing else for it#shrug emoticon#id tag this with negativity but this is my journal blog#ur following this blog at ur own peril
0 notes
Text
poetry outlets that support a free palestine
after finding out that the poetry foundation/POETRY magazine pulled a piece that discussed anti-zionism because they "don't want to pick a side" during the current genocide, i decided to put together a list of online outlets who are explicitly in solidarity with palestine where you can read (english-language) poetry, including, except where otherwise stated, by palestinian poets!
my criteria for this is not simply that they have published palestinian poets or pro-palestine statements in the past; i only chose outlets that, since october 7, 2023, have done one of the following:
published a solidarity statement against israeli occupation & genocide
signed onto the open letter for writers against the war on gaza and/or the open letter boycotting the poetry foundation
published content that is explicitly pro-palestine or anti-zionist, including poetry that explicitly deals with israeli occupation & genocide
shared posts that are pro-palestine on their social media accounts
fyi this is undoubtedly a very small sample. also some of these sites primarily feature nonfiction or short stories, but they do all publish poetry.
outlets that focus entirely on palestinian or SWANA (southwest asia and north africa) literature
we are not numbers, a palestinian youth-led project to write about palestinian lives
arab lit, a magazine for arabic literature in translation that is run by a crowd-funded collective
sumuo, an arab magazine, platform, and community (they appear to have a forthcoming palestine special print issue edited by leena aboutaleb and zaina alsous)
mizna, a platform for contemporary SWANA (southwest asian & north africa) lit, film, and art
the markaz review, a literary arts publication and cultural institution that curates content and programs on the greater middle east and communities in diaspora
online magazines who have published special issues of all palestinian writers (and all of them publish palestinian poets in their regular issues too)
fiyah literary magazine in december 2021, edited by nadia shammas and summer farah (if you have $6 usd to spare, proceeds from the e-book go to medical aid for palestinians)
strange horizons in march 2021, edited by rasha abdulhadi
the baffler in june 2021, curated by poet/translators fady joudah & lena khalaf tuffaha
the markaz review has two palestine-specific issues, on gaza and on palestinians in israel, currently free to download
literary hub featured palestinian poets in 2018 for the anniversary of the 1948 nakba
adi magazine, who have shifted their current (october 2023) issue to be all palestinian writers
outlets that generally seem to be pro-palestine/publish pro-palestine pieces and palestinian poetry
protean magazine (here's their solidarity statement)
poetry online (offering no-fee submissions to palestinian writers)
sundog lit (offering no-fee submissions to palestinian writers through december 1, 2023)
guernica magazine (here's a twitter thread of palestinian poetry they've published) guernica ended up publishing a zionist piece so fuck them too
split this rock (here's their solidarity statement)
the margins by the asian-american writers' workshop
the offing magazine
rusted radishes
voicemail poems
jewish currents
the drift magazine
asymptote
the poetry project
ctrl + v journal
the funambulist magazine
n+1 magazine (signed onto the open letter and they have many pro-palestine articles, but i'm not sure if they have published palestinian poets specifically)
hammer & hope (signed onto the letter but they are a new magazine only on their second issue and don't appear to have published any palestinian poets yet)
if you know others, please add them on!
4K notes
·
View notes