#histone-DNA complex
Explore tagged Tumblr posts
Text

TSRNOSS, p 622.
#vitamin D synthesis#viscosity of DNA solutions#circular DNA#scission#sludging of blood#plasmid#salivary glands of Dipteran insects#histone-DNA complex#aestivatiion
0 notes
Note
hey..
at what point do collectors opt to turn things from puppets to scrolls? I feel like turning an entire living creature into [a piece of paper] is very complicated, while turning them into simple puppets is easier because they keep all the same parts, just simplified and wood?
It is! It depends on the person's proficiency and understanding of the mechanism regarding when and how they change the creature. Once someone gets good at it, the creature can be transformed into a lifeless object without it dying in the process, and they will move on to more complex and efficient ways.
The way I see it, archiving is a form of information compression and storage—and there is A LOT of information. When looking at Earth creatures we have everything from single-cell bacteria to whales that range up to 100 quadrillion cells, all with different sizes. The smallest single-cell critter is 0.3 μm, while the largest single cell is an ostrich egg that can get to 18 cm. So it's not just noting "a cell"—there's also a lot of information about the cell content, size, the DNA, current water, and oxygen levels, what protein it contains and how much. Then there are spatial dimensions. (While we can consider there being more, especially in fiction, I’m sticking to three; trying to visualize four fills me with frustration and existential dread xD) Every cell has its place in space in relation to the others, and all the contents' relations are also important. If, suddenly, all histones materialize inside a mitochondria instead of the nucleus, we can have a problem. Additionally, physical and chemical processes gotta be considered. There's electricity powering our brains, hearts, running nerves, air in airways traveling to lungs, chemical signals traveling between synapses that also need to be accounted for. So, you have all the contents in space, their vectors, and building blocks. Thats a ton to save. This information has to be compressed to be preserved in an organized manner while also remaining lossless so that when returned to its original shape, it's as it was. Not even mentioning that in intelligent beings, there are also minds to take care of. Jellyfish might be fine after 100 years in a static void, but a human? Yhhhhh.
I think the mechanism would work by saving information in intangible magic and assigning it to a physical medium—be it a statue, doll, book, or scroll. If it is physical and can carry information, it can be used. We can argue the mind is part of the soul, or it is a biochemical process, but the fact is nobody really knows for sure what it is and Im not a theolog, so for the sake of this universe, I'll say it's something that occupies the same space magic does and is influenced by chemical processes, meeeeaning it can also be tricked by them. And the magic.
The first degree of preservation would be spells that only change the material but keep all shapes and info in place. This wouldn't require much thought while executing and could be "automated" or worse, taught to mortals (if they have enough magic to power the spell), like petrification or changing someone into wood, metal, or any other solid material. It's not perfect, if the structure is damaged, the spatial information is damaged too. Breaking is one thing, but imagine if the statue melts.
The next step would be assigning objects with some compression and change, like toys and dolls. I feel like there would need to be a system like a content library, so not every single atom is saved each time, but chemical structures like nucleotides in DNA (the ATGC thingies) would just have a shortcut. Larger repeating patterns could also be assigned their own id to save data, and it would slowly stack up. While things are written in intangible magic form and anchored to the medium, the medium can be somewhat customized, like the decorations the Collector added to the dolls. The mind, running in controlled magic, can also be affected, as we saw with Collie trying to scare them and Luz’s dream. On the spell keeping the preserved critter stable has a link to what shortcut it uses so with countless diffrent worlds and structres it wouldnt mix up.
Then we go further into compression, reducing size and dimensions until we reach a point where one axis is almost entirely removed, and we end up with a scroll. Then there are other things—creatures saved as amber miniatures, snow globes, scrolls, or drawings, sometimes purely to annoy the sibling that has to deal with the creature in unhandy form. A more permanent binding would be in a book that can contain a bunch of different animals. Rebinding for long-term preservation is the Curator’s job.
Looking at Earth creatures, eucariotic life shares ancestry with some ancient bacteria that decided to rebel and started to cooperate, so we share similarities even with distant organisms in some strutures since they come from each other. So when it comes to preserving whole populations with relations, the library of compression doesn’t have to be separate for every single animal or plant. For each section of the archive, there would be a common library of building blocks, and scrolls being somewhat separate carrying the exact instructions for body arrangement and the soul/mind/the part that makes them alive attached.
Next is unpacking the information. I think this requires the ability to interpret and recreate what was saved that mortals lack. While they couldn't really unpetrify others, a collector could (assuming the mind hadn’t deteriorated into a husk). In the case of an automated spell, I think it would result in a very lossy transmutation—like a jpg losing pixels, the creature might lose like heart funtion. The Collector's spell also looked temporary or incomplete since an influx of other types of magic (like in Amity or Raine’s case) was able to push back on it. That might also be why they were conscious in the form they were in. Not meant for long just enough to take them to archive in normal conditions. When a creature is heavily compressed, it needs external force to rebuild, as it's essentially written fully in magic. That’s what I think happened to the Owl Beast. Lilith released it from the medium, but since it wasn’t fully rebuilt, it being a magic form attached itself to a magic source.
SO YEAH, its a process that takes quite a while for them to master and it comes with experience. But when experience is based on life it often makes it hard to practice so those with less empathetic approach master it faster. Thanks for the ask! I was dying to talk about that for such a long time and that was a perfect thing to organise thoughts
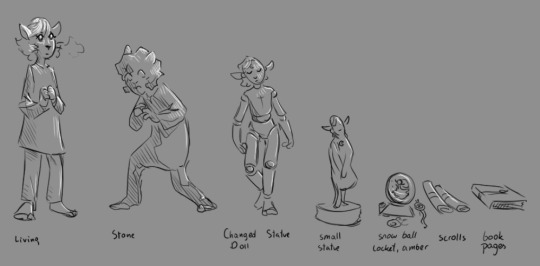
#and consider the absolute body horror that is transmutation#imagine how it has to feel on the border of skin that is being turned to stone when nerve endings cant send what is happening#but can send the numbness of “there is something super wrong” like in severe frostbite#both must feel like tissue dying#tw body horror#i did not use that one in a moment#In the begining i had a concept that it all saves the same way like a doll so diffrent archivists would have diffrent methods#like Anatomist using scrolls Wayfarer drawings and so on but then realised that would be super unhandy when a book carries more info#and its easier to fix a doll than a scroll so settled on this#thats also why in the comic where Way damaged creature they were turned into a doll Way was just very unexperienced with archiving spells#Collection Incomplete au#the owl house#owl house#toh#the collector#toh collector#toh archivists#the archivists#toh collectors#ask#i took sleeping meds before writing this safe to say they didnt work
59 notes
·
View notes
Text
Human Cell Tournament Round 1
Propaganda!
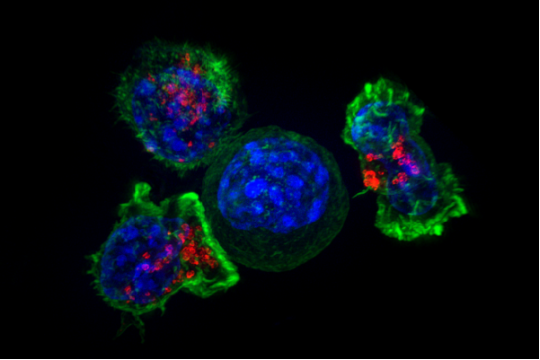

A killer T cell is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens (such as viruses or bacteria), or cells that are damaged in other ways. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigen. An antigen is a molecule capable of stimulating an immune response and is often produced by cancer cells, viruses, bacteria or intracellular signals. Antigens inside a cell are bound to class I MHC molecules, and brought to the surface of the cell by the class I MHC molecule, where they can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecule and the antigen, and the T cell destroys the cell.
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei and in most Archaeal phyla. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers.
#t4 cells#killer t cells#Hisotones#poll#polls#tumblr poll#tumblr polls#tournament poll#wikipedia#cells of the human body#science tournament#biochemistry
50 notes
·
View notes
Text

Epigenetics: A Journey Through Inheritance Beyond Genes
For centuries, scientists have been fascinated by the mysteries of heredity and how traits are passed down from generation to generation. DNA, the molecule that stores our genetic code, was once thought to be the sole determinant of our characteristics. However, a new frontier in biology, revealing a captivating layer of complexity beyond the DNA sequence itself: Epigenetics.
What is Epigenetics?
The term "epigenetics" was first coined in the 1940s by British biologist Conrad Waddington, but it wasn't until the late 20th century that its significance truly blossomed. Epigenetics, literally meaning "above genetics," refers to the study of heritable changes in gene expression that occur without alterations to the DNA sequence itself. Imagine DNA as the musical score, but epigenetics are the conductor and musicians who determine how the music is played. Through chemical modifications and adjustments to the proteins around DNA, epigenetics dictates which genes are turned on or off, influencing how cells function and ultimately shaping our health, development, and even behavior. Think of your DNA as the hardware: it contains the basic instructions for building and running your body. But epigenetics acts like the software, fine-tuning those instructions and determining which genes get turned on or off at specific times and in specific cells. These modifications, like chemical tags or changes in the packaging of DNA, don't alter the underlying code itself, but they can have a profound impact on how it's read and interpreted.
The Key Players:
DNA methylation: This process involves adding a methyl group to DNA, essentially silencing the gene it's attached to. Imagine it like putting a dimmer switch on a light bulb.
Histone modifications: Histones are proteins that package DNA, and changes in their structure can make genes more or less accessible to the cellular machinery needed for expression. Think of it like adjusting the curtains around a window - open wide for full light, slightly closed for filtered light.
Non-coding RNAs: These are molecules that don't code for proteins but can regulate gene expression in various ways. They're like the backstage crew in a play, ensuring everything runs smoothly.
The Power of Epigenetic Regulation
Epigenetic regulation plays a crucial role in various biological processes, including:
Development: During embryonic development, different cell types emerge from the same DNA blueprint by activating or silencing specific gene sets through epigenetic modifications.
Cellular differentiation: Specialized cells like muscle or nerve cells have unique functions due to differences in their active genes, controlled by epigenetic mechanisms.
Learning and memory: Epigenetic changes in brain cells are thought to be essential for learning and forming memories.
Aging: As we age, our epigenome accumulates changes that can contribute to age-related decline and disease.
Environmental influences: Diet, exercise, stress, and exposure to toxins can leave epigenetic marks on our genes, potentially impacting our health and even the health of future generations.
Epigenetics reminds us that we are not simply products of our genes. Our environment, choices, and experiences leave their mark, shaping who we are and potentially influencing our children's health. This deeper understanding of ourselves opens doors for self-awareness, empowerment, and potentially reshaping our narratives – not just as individuals, but as a species with the potential to leave a healthier legacy for generations to come.
#life science#biology#science sculpt#molecular biology#biotechnology#epigenetics#daily dose of science#dna#genetic inheritance#genetics#decoding dna#genetic code#science#double helix
128 notes
·
View notes
Text
Exploring the Marvels of Biological Macromolecules: The Molecular Machinery of Life (Part 3)
Nucleotide Structure: The Building Blocks
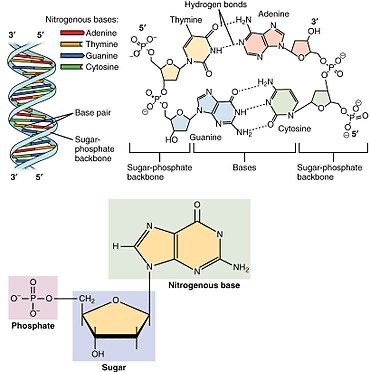
Nucleotides, the monomers of nucleic acids, consist of three fundamental components:
1. Phosphate Group (PO4): Provides a negatively charged backbone for the nucleic acid strand.
2. Pentose Sugar: In DNA, it's deoxyribose; in RNA, it's ribose. The sugar moiety forms the framework of the nucleotide.
3. Nitrogenous Base: Adenine (A), Guanine (G), Cytosine (C), Thymine (T) in DNA, and Uracil (U) in RNA. These bases are responsible for the genetic code.
DNA (Deoxyribonucleic Acid): The Repository of Genes
DNA is a double-stranded helical molecule, with each strand composed of a linear sequence of nucleotides. It encodes the genetic information necessary for an organism's development, growth, and functioning. The Watson-Crick base pairing rules—A with T and C with G
DNA (Deoxyribonucleic Acid): The Repository of Genes
DNA is a double-stranded helical molecule, with each strand composed of a linear sequence of nucleotides. It encodes the genetic information necessary for an organism's development, growth, and functioning. The Watson-Crick base pairing rules—A with T and G with C—ensure DNA's complementary and faithful replication.
RNA (Ribonucleic Acid): From DNA's Blueprint to Protein Synthesis
RNA plays diverse roles in the cell, including serving as a messenger (mRNA) for protein synthesis, a structural component of ribosomes (rRNA), and an adapter molecule (tRNA) that brings amino acids to the ribosome during translation. Unlike DNA, RNA is often single-stranded and contains uracil (U) instead of thymine (T).
Genome Organization and Chromosomes
Genomic DNA is organized into chromosomes within the cell nucleus. These structures enable efficient storage, replication, and transmission of genetic information during cell division and reproduction.
Replication and Transcription
DNA replication ensures the faithful duplication of genetic material during cell division, while transcription converts DNA into RNA, providing a template for protein synthesis.
Translation
The cellular machinery, composed of ribosomes and tRNA, reads the mRNA code and assembles amino acids into polypeptides during translation, ultimately forming functional proteins.
Genetic Code
The genetic code, a triplet code of nucleotide sequences (codons), dictates a protein's sequence of amino acids. It is nearly universal, with only minor variations across species.
Epigenetics
Epigenetic modifications, such as DNA methylation and histone modifications, regulate gene expression without altering the underlying DNA sequence, pivotal in development and cell differentiation.
Macromolecular interactions are the essence of cellular life. Within the complex microcosm of a cell, countless molecules engage in precise and choreographed dances, forming intricate networks that govern every facet of biology. These interactions, governed by the principles of biochemistry, are the foundation upon which life's processes are built.
Amino Acids: The Building Blocks
Proteins are composed of amino acids organic molecules that contain an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a distinctive side chain (R group). There are 20 different amino acids, each with a unique side chain that confers specific properties to the amino acid.
Primary Structure: Amino Acid Sequence
The primary structure of a protein refers to the linear sequence of amino acids in the polypeptide chain. The genetic information in DNA encodes the precise arrangement of amino acids.
Secondary Structure: Folding Patterns
Proteins don't remain linear; they fold into specific three-dimensional shapes. Secondary structures, such as α-helices and β-sheets, result from hydrogen bonding between nearby amino acids along the polypeptide chain.
Tertiary Structure: Spatial Arrangement
The tertiary structure is the overall three-dimensional shape of a protein, determined by interactions between amino acid side chains. These interactions include hydrogen bonds, disulfide bridges, ionic bonds, and hydrophobic interactions.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins, known as quaternary structures, comprise multiple polypeptide chains. These subunits come together to form a functional protein complex. Hemoglobin, with its four subunits, is an example.
Protein Functions: Diverse and Essential
Proteins are involved in an astounding array of functions:
1. Enzymes: Proteins catalyze chemical reactions, increasing the speed at which reactions occur.
2. Structural Proteins: Proteins like collagen provide structural support to tissues and cells.
3. Transport Proteins: Hemoglobin transports oxygen in red blood cells, and membrane transport proteins move molecules across cell membranes.
4. Hormones: Hormonal proteins, such as insulin, regulate various physiological processes.
5. Immune Function: Antibodies are proteins that play a crucial role in the immune system's defense against pathogens.
6. Signaling: Proteins are critical in cell signaling pathways, transmitting information within cells.
Protein Denaturation and Folding
Protein Diversity: The vast diversity of proteins arises from the combinatorial possibilities of amino acid sequences, secondary structure arrangements, and three-dimensional conformations.
Nucleic acids, the remarkable macromolecules that govern all living organisms' genetic information, are life's quintessential molecules. These complex polymers of nucleotides play an unparalleled role in the storage, replication, and expression of genetic information, shaping the development, characteristics, and functions of every living entity on Earth. Let's embark on an exploration of the intricate world of nucleic acids.
Nucleotide Structure: The Building Blocks
Nucleotides, the monomers of nucleic acids, consist of three fundamental components:
1. Phosphate Group (PO4): Provides a negatively charged backbone for the nucleic acid strand.
2. Pentose Sugar: In DNA, it's deoxyribose; in RNA, it's ribose. The sugar moiety forms the framework of the nucleotide.
3. Nitrogenous Base: Adenine (A), Guanine (G), Cytosine (C), Thymine (T) in DNA, and Uracil (U) in RNA. These bases are responsible for the genetic code.
DNA (Deoxyribonucleic Acid): The Repository of Genes
DNA is a double-stranded helical molecule, with each strand composed of a linear sequence of nucleotides. It encodes the genetic information necessary for an organism's development, growth, and functioning. The Watson-Crick base pairing rules—A with T and G with C—ensure DNA's complementary and faithful replication.
RNA (Ribonucleic Acid): From DNA's Blueprint to Protein Synthesis
RNA plays diverse roles in the cell, including serving as a messenger (mRNA) for protein synthesis, a structural component of ribosomes (rRNA), and an adapter molecule (tRNA) that brings amino acids to the ribosome during translation. Unlike DNA, RNA is often single-stranded and contains uracil (U) instead of thymine (T).
Genome Organization and Chromosomes:
Replication and Transcription: DNA replication ensures the faithful duplication of genetic material during cell division, while transcription converts DNA into RNA, providing a template for protein synthesis.
Translation: The cellular machinery, composed of ribosomes and tRNA, reads the mRNA code and assembles amino acids into polypeptides during translation, ultimately forming functional proteins.
Genetic Code: The genetic code, a triplet code of nucleotide sequences (codons), dictates the sequence of amino acids in a protein. It is nearly universal, with only minor variations across species.
Epigenetics: Epigenetic modifications, such as DNA methylation and histone modifications, regulate gene expression without altering the underlying DNA sequence, pivotal in development and cell differentiation.
Macromolecular interactions are the essence of cellular life. Within the complex microcosm of a cell, countless molecules engage in precise and choreographed dances, forming intricate networks that govern every facet of biology. These interactions, governed by the principles of biochemistry, are the foundation upon which life's processes are built.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#genetics#genetic engineering#science nerds#dna activation#new dna
24 notes
·
View notes
Text
RNAs, introns and introduction to recombinant DNA.
The genome is a vault of genetic information, but on its own its kind of useless as the information cannot be used in the cell. Utilization of this information requires a coordinated effort from enzymes and proteins, this coordination of chemical reactions is known as genome expression. The initial step of genome expression is known as the transcriptome, a collection of RNA molecules which is made up of the genes active in the cell at the time. (its constantly changing to the environment that the cell is in) the transcriptome is maintained by transcription a simple process in which individual genes are copied and pasted into RNA molecules known as mRNA. The second product within gene expression is known as proteome which you can think of as the cells library of proteins at hand. This gives the cell its unique and individual characteristics that it can express. The proteins in this library known as the proteome are synthesized by the translation of some RNA molecules.
I think it is of utmost importance to start with the most simple and important step…
Transcription:
The first step in transcription is initiation,
Transcription begins with the binding of RNA polymerase 2 to the promoter region of a gene, the most common promotor contains a conserved gene sequence we call the TATA box, but there are many others that exist which are show by the different TF families.
The next step we have the formation of transcription initiation complex, this is when transcription factors such as TATA-binding protein also known as TBP, bind to the promoter, forming a pre-initiation complex. Other general transcription factors join, and RNA polymerase 2 is recruited to form the transcription initiation complex.
Next, we have initiation of transcription where RNA polymerase 2 unwinds the DNA helix at the transcription “start site”. The enzyme catalyses the synthesis of a short RNA primer (which is about 10 nucleotides in length) complementary to the template DNA strand.
The second step is Elongation:
Once the RNA polymerase has synthesized the initial RNA primer, it proceeds to elongate the RNA chain, The RNA polymerase adds ribonucleotide complementary to the template DNA strand. This process is known as RNA chain Elongation.
After this, we have nucleosome remodelling where the DNA in eukaryotic cells is wrapped around histone proteins to form nucleosomes. As transcription proceeds, nucleosomes are temporarily disrupted and then reassembled, allowing RNA polymerase to access the DNA template.
The third step is Termination:
Eukaryotic mRNA molecules undergo post-transcriptional modification, this includes polyadenylation which involves the addition of a poly-A tail to the 3’ end of the RNA. Polyadenylation is then accompanied by cleavage of the RNA precursor at the specific site.
During this process transcription termination signals are recognized by RNA polymerase 2, leading to its dissociation from the DNA template. The termination signal will most likely include the poly-A signal.
The fourth step being RNA processing:
The first step of RNA processing is known as “capping”. Capping the 5’ end of the newly synthesized mRNA is modified with a 7-methylguanosine cap, the whole point of this cap is just to protect the mRNA as it leaves the nucleus and helps in the transportation process.
The second step is splicing, Eukaryotic genes often contain introns (which are kind of gaps or non-coding regions) and exons (which are the important bits, coding regions). In a process called splicing the annoying useless introns are cut and the exons are then pinched together with each other. This is all catalysed by a complex of RNA and proteins known as spliceosomes.
The fifth step being transport and translation:
This is when mRNA is transported out of the nucleus to the cytoplasm where it serves as a template for translation.
The sixth step is regulation:
Gene expression if tightly regulated at the transcriptional level by various elements, including transcriptional factors and chromatin modification, there is also enhancers and silencers that help regulate elements that influence transcription.
We also have post-transcriptional regulation, which is processes such as alternative splicing and RNA stability which play roles in determining the final mRNA products.
It is important to acknowledge that Eukaryotic transcription is more complex than prokaryotic transcription due to the presence of introns, the involvement of multiple RNA polymerases, and the necessity for additional processing steps.
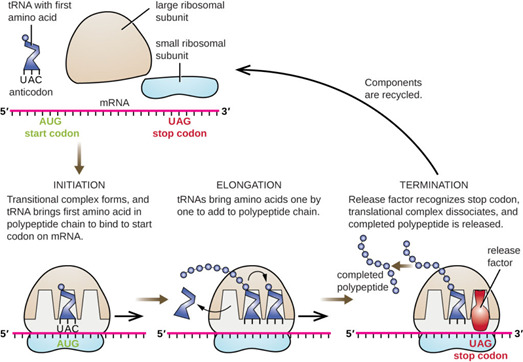
This diagram helps visualise the main steps more simply.
Hope this was clear excuse any mistakes in grammar :)
Referencing:
Picture link above^
2 notes
·
View notes
Text
Epigenetics Diagnostics Market Analysis & Industry Growth Analysis 2025 to 2037
The global Epigenetics Diagnostics Market is experiencing rapid growth, driven by increased awareness of personalized medicine and the role of epigenetic alterations in complex diseases. The market is projected to expand significantly, rising from a value of USD 2.9 billion in 2024 to USD 15.8 billion by 2037, reflecting a Compound Annual Growth Rate (CAGR) of 19.9%. This growth trajectory underscores the transformative potential of epigenetic tools in diagnostics, offering non-invasive, early-detection solutions for diseases that were previously difficult to identify at an early stage.
Epigenetics Diagnostics Industry Demand
The Epigenetics Diagnostics Market focuses on tools and technologies designed to detect inheritable alterations in gene activity that occur without altering the underlying DNA sequence. These epigenetic changes are largely driven by processes like DNA methylation, histone modification, and the regulation of gene expression through non-coding RNAs.
Key Demand Drivers:
Cost-Effectiveness: Epigenetic tests often allow for early diagnosis, which can reduce the need for expensive treatments down the line, improving patient outcomes while cutting healthcare costs.
Ease of Use and Minimally Invasive Techniques: Many epigenetic diagnostics can be conducted using blood, saliva, or other non-invasive samples, making them attractive for routine screening.
Long Shelf Life of Reagents and Kits: Modern epigenetic diagnostic kits are designed with extended stability, reducing waste and simplifying logistics in research and clinical settings.
Growing interest in precision medicine, increased funding for biomarker research, and advancements in next-generation sequencing (NGS) technologies are all accelerating the adoption of epigenetic diagnostics in both clinical and research settings.
Epigenetics Diagnostics Market: Growth Drivers & Key Restraint
Growth Drivers –
Rising Prevalence of Chronic Diseases The increasing global burden of cancer, cardiovascular disorders, and neurological diseases is driving demand for diagnostic methods that can detect and monitor disease progression at a molecular level.
Technological Advancements in Epigenetic Platforms Innovations in high-throughput sequencing, single-cell epigenomics, and AI-assisted diagnostic algorithms are making epigenetic testing faster, more accurate, and scalable for clinical use.
Expansion of Outsourcing and CRO Collaborations Pharmaceutical and biotech firms are increasingly partnering with Contract Research Organizations (CROs) to conduct large-scale epigenetic studies, reducing internal development costs and accelerating timelines.
Restraint –
High Cost of Diagnostic Equipment and Skilled Personnel
Despite long-term cost benefits, the initial investment required for setting up epigenetic testing facilities—along with a need for trained molecular biologists and bioinformaticians
Epigenetics Diagnostics Market: Segment Analysis
Segment Analysis by Product Type (Enzymes, Instruments and Consumables, Reagents, Software, Services):
Enzymes: Critical for modifying DNA or histones in diagnostic assays; demand is rising with the development of targeted diagnostic platforms.
Instruments and Consumables: Includes sequencing systems, PCR machines, and related consumables; instrumental in clinical and high-throughput research settings.
Reagents: Reagents for methylation analysis, chromatin accessibility, and histone modification are widely used in diagnostics and research.
Software: Bioinformatics tools that help analyze large epigenetic datasets; increasingly integrated with AI for disease classification and prediction.
Services: Outsourced diagnostic services, such as methylation profiling and biomarker discovery, are gaining popularity among companies without in-house capabilities.
Request Report Sample@ https://www.kennethresearch.com/sample-request-10352538
Segment Analysis by Application (Oncology Applications, Metabolic Diseases, Developmental Biology, Immunology, Cardiovascular Diseases, Neurodegenerative Disorders):
Oncology Applications: A dominant segment, as epigenetic alterations are hallmarks of many cancers. Diagnostic tests help in early detection, treatment stratification, and monitoring.
Metabolic Diseases: Epigenetic diagnostics aid in understanding the underlying gene-environment interactions in conditions like diabetes and obesity.
Developmental Biology: Used for studying congenital anomalies and inherited disorders through epigenetic screening.
Immunology: Helps decipher immune system dysfunctions and autoimmune conditions by assessing epigenetic markers.
Cardiovascular Diseases: Epigenetic biomarkers are being studied for their predictive potential in atherosclerosis and heart failure.
Neurodegenerative Disorders: Alzheimer’s, Parkinson’s, and other cognitive diseases show distinct epigenetic signatures, opening new diagnostic avenues.
Segment Analysis by End‑User (Academic and Research Institutes, Pharmaceutical Companies, Biotechnology Companies, Contract Research Organizations (CROs), Hospitals and Clinics):
Academic and Research Institutes: Drive innovation through basic research and clinical studies involving epigenetic biomarkers.
Pharmaceutical Companies: Use epigenetic diagnostics for patient stratification, clinical trial optimization, and companion diagnostics.
Biotechnology Companies: Focus on product development, kit manufacturing, and commercializing epigenetic technologies.
Contract Research Organizations (CROs): Serve a critical function in the outsourcing of diagnostic innovation, offering expertise in biomarker analysis, clinical validation, and adherence to regulatory standards.
Hospitals and Clinics: Are progressively adopting epigenetic diagnostics as part of standard medical evaluations, aiding in early disease detection and personalized treatment strategies.
Epigenetics Diagnostics Market: Regional Insights
North America:
North America, led by the United States, dominates the epigenetics diagnostics market due to high healthcare spending, extensive R&D activities, and early adoption of precision medicine technologies. Strong government support for cancer research, coupled with a robust presence of key market players and academic centers, contributes to market maturity and consistent innovation.
Europe:
Europe holds a strong position driven by significant investments in genomics research and national healthcare programs supporting early disease detection. Countries like Germany, the UK, and France are particularly active in integrating epigenetic diagnostics into oncology and rare disease programs. Regulatory harmonization and public-private partnerships further enhance market expansion.
Asia-Pacific (APAC):
APAC is emerging as a fast-growing region, fueled by expanding biotechnology infrastructure, rising healthcare awareness, and government-backed genomics initiatives in countries like China, Japan, South Korea, and India. Cost-sensitive innovations and increasing CRO presence are making epigenetic diagnostics more accessible and scalable in this region.
Access our detailed report link:https://www.kennethresearch.com/report-details/epigenetics-diagnostics-market/10352538
Top Players in the Epigenetics Diagnostics Market
Illumina, Inc.,Thermo Fisher Scientific,QIAGEN N.V.,F. Hoffmann-La Roche Ltd,Merck KGaA,Eisai Co., Ltd.,Takara Bio Inc.,Bioneer Corporation,Cell Signaling Technology,Zymo Research Corporation,Bio-Rad Laboratories,Agilent Technologies,PerkinElmer, Inc.,Novogene Co., Ltd.,BGI Genomics,Syngene International,GeneCare Research Institute,Australian Genome Research Facility,Pacific Biosciences,Epigentek Group Inc.
0 notes
Text
Epigenetic Biomarkers: Revolutionizing Disease Diagnosis and Treatment
U.S. Epigenetics Market Growth & Trends
The U.S. Epigenetics Market size is anticipated to reach USD 13.10 billion by 2030, growing at a CAGR of 14.5% from 2024 to 2030, according to a new report by Grand View Research, Inc. The growing prevalence of cancer and chronic diseases, advancements in epigenetic research and technologies, rising investment in biomedical research, and increasing adoption of personalized medicine are anticipated to increase the demand for epigenetics over the forecast period.
The COVID-19 pandemic has had a positive impact on the market. The pandemic spurred a significant increase in research activities related to understanding the virus and its effects on human health. This included epigenetic research to study how COVID-19 affects gene expression and immune responses. Consequently, there was increased funding from both government and private sectors for epigenetic studies related to COVID-19.

Moreover, companies have increased investments in the development of epigenetics products. Many biotechnology and pharmaceutical companies have increased their research and development budgets to explore the role of epigenetics in disease mechanisms and treatment responses. This influx of funding has accelerated the pace of discoveries and innovations in the field. The transition from external funding to domestic investment in health systems underscores the need for innovative solutions. Epigenetics, embedded in advancing healthcare, is projected to transform the phase by offering solutions for improving health outcomes.
However, the growth of the market is constrained by a scarcity of skilled professionals. The complex nature of epigenetics demands a deep understanding of genetics, molecular biology, and data analysis. This shortage can decelerate research & technological progress, hinder complex data analysis crucial for insights, slow clinical translation, and hamper educational initiatives. This is anticipated to hamper the growth of the market to a certain extent.
Curious about the U.S. Epigenetics Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
U.S. Epigenetics Market Report Highlights
Reagents dominated the product segment with the largest revenue share of 32.5% in 2023. Reagents are versatile and used in a wide range of applications, including basic research, clinical diagnostics, and drug development. On the other hand, services are expected to grow at the fastest CAGR over the forecast period.
The DNA methylation held the largest market share in 2023 for the technology segment. DNA methylation is one of the key epigenetic modifications that plays a vital role in regulating gene expression. However, on the other hand, histone acetylation is expected to grow at the fastest CAGR over the forecast period.
The oncology held the largest market share in 2023 for the application segment. This is attributed to the growing prevalence of cancer. However, on the other hand, non-oncology is expected to grow at the fastest CAGR over the forecast period.
Based on end-use, academic research dominated the segment with the largest revenue share in 2023. This is attributed to the increasing number of clinical studies which are being conducted in academic institutes and the growing funding for research activities. On the other hand, clinical research is anticipated to grow at the fastest CAGR over the forecast period.
U.S. Epigenetics Market Segmentation
Grand View Research has segmented the U.S. epigenetics market based on product, technology, application, and end-use:
U.S. Epigenetics Product Outlook (Revenue, USD Million, 2018 - 2030)
Reagents
Kits
ChIP Sequencing Kit
Whole Genomic Amplification Kit
Bisulfite Conversion Kit
RNA Sequencing Kit
Others
Instruments
Enzymes
Services
U.S. Epigenetics Technology Outlook (Revenue, USD Million, 2018 - 2030)
DNA Methylation
Histone Methylation
Histone Acetylation
Large Non - coding RNA
MicroRNA Modification
Chromatin Structures
U.S. Epigenetics Application Outlook (Revenue, USD Million, 2018 - 2030)
Oncology
Solid Tumors
Liquid Tumors
Non – oncology
Inflammatory Diseases
Metabolic Diseases
Infectious Diseases
Cardiovascular Diseases
Others
U.S. Epigenetics End-use Outlook (Revenue, USD Million, 2018 - 2030)
Academic Research
Clinical Research
Hospitals & Clinics
Pharmaceutical & Biotechnology Companies
Others
Download your FREE sample PDF copy of the U.S. Epigenetics Market today and explore key data and trends.
0 notes
Text
An Immune-Based Memory Mechanism: The Memory Circuit "Created" by TLR9 Inflammatory Signals and DNA Damage!
1. “Brain Inflammation” and DNA Damage: The Surprising Keys to Long-Term Memory
When you hear “inflammation” and “DNA damage,” you might immediately think of disease or injury. However, in brains, these two processes are key steps in forming long-term memories, particularly related to specialized cells in our brain called hippocampal neurons.
These neurons act like architects, assembling information into microcircuits to store life’s moments. However, this process comes at a “cost”: hippocampal neurons undergo an energy-intensive phase where their DNA temporarily experiences double-strand breaks (DNA double-strand breaks, DSBs), alongside activation of a signaling molecule called TLR9. This mechanism resembles a small-scale “inflammation” within neurons, laying the foundation for memory storage[1].

Fig. 1 Simplified model of the interaction between neural plasticity and DNA damage and repair[2].
Activation of NMDA/AMPA receptors at synapses induces single-strand (SSB) or double-strand DNA breaks (DSB), and promotes their repair through base excision repair (BER) or non-homologous end joining (NHEJ), respectively. Conversely, DNA damage and repair alter the expression and activity of these receptors, thereby regulating neuronal gene expression, leading to changes in plasticity.
2. Nature: Formation of Memory Components Through DNA-Sensing TLR9 Signaling Pathway
A recent study published in Nature found that after prolonged learning, excitatory hippocampal CA1 neurons exhibited a series of events, including double-strand DNA breaks, rupture of the nuclear membrane, and the release of histones and double-strand DNA fragments. Following these early events, some neurons began to exhibit an inflammatory phenotype, involving the activation of the TLR9 signaling pathway, along with the accumulation of DNA damage repair complexes. If TLR9 function is impaired, basic memory mechanisms may become the beginning of genomic instability and cognitive impairment, associated with accelerated aging, mental illness, and neurodegenerative diseases.
The critical role of TLR9: Responsible for DNA damage repair, involved in the production of cilia and the establishment of surrounding neuron networks.
2.1 The Key Role of Immune Response Gene (TLR9) in Memory Formation
The authors first conducted an in-depth analysis of the transcriptional profile of dorsal hippocampal neurons and found significant differences in gene expression profiles related to remote memory at 96 hours or 21 days after Contextual Fear Conditioning (CFC). The researchers observed that most differentially expressed genes are immune response genes involved in nucleic acid sensing and cytokine release (Figure 2A).

Fig. 2 Gene expression profiles and TLR9 protein levels after contextual fear conditioning (CFC), and co-localization of TLR9/LAMP2 at different times[3].
2.2 CFC Triggers dsDNA Breaks and DNA Damage Repair (DDR)
The researchers used a specific antibody against phosphorylated histone γH2AX for immunofluorescence labeling and found that within 1 hour and 3 hours after CFC, the number of dsDNA breaks significantly increased in some neurons, exhibiting neuron-specificity. Although the number of nuclear foci subsequently decreased, larger γH2AX-labeled foci appeared within individual neurons (Figure 3A-B), and there was colocalization of perinuclear γH2AX signals with TLR9 signals (Figure 3C).

Fig. 3 DNA damage and DNA damage repair (DDR) after CFC[3].
2.3 TLR9 in CA1 Neurons is Essential for Contextual Memory

Fig. 4 Contextual memory impairment after neuron-specific deletion of hippocampal TLR9[3].
In addition, the authors observed the hippocampal regions of WT and TLR9fl/fl mice through experiments and found that the number of double-strand DNA breaks in neurons was significantly increased. The deletion of TLR9 disrupted nuclear and centrosomal DDR, making it impossible for CA1 neurons to recruit DDR complexes or form cilia and perineuronal nets (PNNs) during CFC, confirming the role of TLR9 in DDR, cilia formation, and PNN accumulation.
3. Conclusion
Learning-induced TLR9 signaling links DNA damage to DDR in neurons, which plays a key role in the stability and persistence of memory. TLR9 activation may be triggered by γH2AX and double-strand DNA fragments. TLR9 participates in DNA sensing, rather than the more traditional cGAS-STING pathway in CFC, suggesting that neurons employ an immune-based memory mechanism. Therefore, maintaining the integrity of TLR9 inflammatory signaling is a promising preventive strategy for neurocognitive deficits and may provide new insights into diseases such as aging, mental illness, and neurodegeneration.
Product Recommendation
PLX5622
Oral CSF1R inhibitor, BBB-penetratable, microglial scavenger
ODN 2088
TLR3,TLR7 and TLR9 inhibitor
RU.521
cGAS inhibitor
H-151
STING antagonist
γH2AX antibody
For WB, ICC/IF assay
References
[1] Madabhushi R, et al. Cell. 2015 Jun 18;161(7):1592–605. [2] Konopka A, et al. Front Cell Neurosci. 2022 Jun 23;16:836885. [3] Jovasevic V, et al. Nature. 2024 Apr;628(8006):145–153.
1 note
·
View note
Text
Epigenetics Market Analysis: Future Trends, Forecasts, and Growth Potential through 2032

Epigenetics is the study of changes in gene expression or cellular phenotype that do not involve alterations to the underlying DNA sequence. Over the past decade, this field has gained considerable traction due to its potential to provide insights into various diseases, developmental biology, and aging processes. As a result, the epigenetics market has grown rapidly and is expected to continue expanding significantly in the coming years. In this report, we will explore the size, share, and growth prospects of the epigenetics market, along with the driving factors and emerging trends that are shaping the industry.
The global epigenetics market has experienced remarkable growth over the past few years, with advancements in technology, increasing research funding, and a growing understanding of epigenetic mechanisms driving this trend. According to a report, the global epigenetics market size was valued at USD 1.84 billion in 2024 to USD 5.54 billion by 2032, growing at a CAGR of 14.8% during the forecast period (2025-2032).
To Learn More About This Report, Request a Free Sample Copy - https://www.skyquestt.com/sample-request/epigenetics-market
Key Market Drivers
Several factors are contributing to the growth of the epigenetics market:
1. Rising Prevalence of Chronic Diseases: The increasing incidence of chronic diseases such as cancer, neurological disorders, and cardiovascular diseases has spurred research into epigenetics. Studies show that epigenetic changes play a pivotal role in the development and progression of these diseases, thereby driving demand for epigenetic therapies and diagnostics.
2. Technological Advancements in Epigenetic Research: The rapid progress in next-generation sequencing (NGS), CRISPR-based gene editing, and DNA methylation technologies has enhanced the understanding of epigenetic modifications. These advancements enable more accurate and high-throughput analysis, which is vital for both clinical applications and research purposes.
3. Growing Demand for Personalized Medicine: As personalized medicine gains momentum, the role of epigenetics in tailoring therapies to individuals’ genetic makeup has become crucial. Epigenetic modifications influence how individuals respond to drugs, paving the way for the development of precision treatments, especially in oncology.
4. Government Funding and Research Initiatives: Governments across the world are investing in research programs aimed at understanding the role of epigenetics in human health. The National Institutes of Health (NIH), for example, have allocated substantial funding toward epigenetic research projects, which in turn drives market growth.
5. Increased Awareness and Focus on Aging and Genetic Disorders: Epigenetic modifications are known to influence aging processes, including the onset of age-related diseases. As the global population ages, there is a growing interest in epigenetics to explore potential interventions for extending lifespan and improving health during aging.
Make an Inquiry to Address your Specific Business Needs - https://www.skyquestt.com/speak-with-analyst/epigenetics-market
Market Segmentation
The epigenetics market can be broadly segmented based on product type, application, end-user, and region.
1. By Product Type:
- Reagents and Kits: Reagents and kits for DNA methylation analysis, histone modification analysis, and chromatin immunoprecipitation (ChIP) are widely used in research and clinical settings.
- Instruments and Equipment: These include DNA sequencers, PCR machines, and microarrays that are essential for studying epigenetic modifications.
- Software and Databases: Bioinformatics tools for analyzing complex epigenetic data play a key role in processing and interpreting large datasets generated from research.
2. By Application:
- Cancer Research: Epigenetic changes are crucial in the development of various cancers, making cancer research a significant driver of market growth.
- Neurological Diseases: Research into the role of epigenetics in neurological disorders like Alzheimer’s, Parkinson’s, and autism is increasing.
- Cardiovascular Diseases: The link between epigenetic modifications and heart diseases has led to growing research investments in this area.
�� - Genetic Disorders and Aging: Epigenetic mechanisms are critical in understanding the onset of genetic disorders and aging processes.
3. By End-User:
- Academic and Research Institutes: These institutions lead the research efforts into the molecular mechanisms of epigenetics and its potential therapeutic applications.
- Pharmaceutical and Biotechnology Companies: Companies are increasingly investing in epigenetic research to develop novel drugs and therapies, especially in oncology and other chronic diseases.
- Hospitals and Diagnostic Laboratories: Hospitals and diagnostic labs use epigenetic tests to diagnose and monitor diseases, particularly cancers and genetic disorders.
Regional Analysis
North America: North America is the dominant market for epigenetics, accounting for the largest market share. The U.S. is a key player, driven by strong government funding, well-established pharmaceutical and biotechnology sectors, and the presence of advanced research facilities.
Europe: Europe is another significant market, with countries such as Germany, France, and the UK leading in epigenetic research. The European Union has also supported various initiatives to enhance research in epigenetics.
Asia-Pacific: The Asia-Pacific region is anticipated to witness the highest growth rate due to increasing investments in healthcare and life sciences research, growing awareness of epigenetics, and advancements in research infrastructure in countries like China, Japan, and India.
Latin America and Middle East & Africa: Although these regions are currently smaller markets, they are expected to grow steadily due to rising awareness, improvements in healthcare infrastructure, and increased research funding.
Take Action Now: Secure Your Epigenetics Market Today - https://www.skyquestt.com/buy-now/epigenetics-market
Top Player’s Company Profiles in Epigenetics Market
AbbVie Inc.
AstraZeneca PLC
Bayer AG
BeiGene Ltd.
Bristol Myers Squibb Company
Epizyme, Inc.
GlaxoSmithKline PLC
Incyte Corporation
Johnson & Johnson
Merck & Co., Inc.
Novartis AG
Pfizer Inc.
Regeneron Pharmaceuticals, Inc.
Roche Holding AG
Seattle Genetics, Inc.
Takeda Pharmaceutical Company Limited
Targovax, Inc.
Vertex Pharmaceuticals Incorporated
Vivid Biosciences Inc.
Zymeworks Inc.
Emerging Trends
1. Integration of Artificial Intelligence (AI) in Epigenetics: AI and machine learning algorithms are increasingly being employed to analyze epigenetic data, enabling researchers to uncover patterns and predict disease outcomes more efficiently.
2. Epigenetic Biomarkers in Diagnostics: The use of epigenetic markers for early detection and prognosis of diseases such as cancer is a growing trend. These biomarkers can offer non-invasive diagnostic alternatives to traditional methods.
3. Epigenetic Therapeutics: The development of drugs targeting specific epigenetic modifications, such as DNA methylation and histone modifications, is opening new avenues for treating diseases that were previously difficult to manage with conventional therapies.
4. Clinical Trials and Drug Development: The expansion of clinical trials focused on epigenetic therapies, particularly in cancer treatment, is likely to increase during the forecast period. Pharmaceutical companies are keen to develop drugs that can modify the epigenome to treat genetic disorders and cancers.
Challenges
Despite its promising growth, the epigenetics market faces several challenges:
- High Research Costs: The costs involved in conducting epigenetic research and developing related therapies are considerable, which may limit the accessibility of these technologies.
- Regulatory Hurdles: The regulatory framework surrounding epigenetic-based therapies and diagnostics is still evolving, which may create delays in product development and approval.
- Ethical Concerns: The manipulation of the epigenome raises ethical concerns, particularly regarding gene editing and its implications for human health and future generations.
Read Epigenetics Market Report Today - https://www.skyquestt.com/report/epigenetics-market
The epigenetics market is poised for robust growth, driven by technological advancements, increased research funding, and the rising prevalence of chronic diseases. With the potential to revolutionize personalized medicine, diagnostics, and therapeutics, epigenetics is becoming an increasingly integral part of the biomedical landscape. However, challenges such as high research costs and ethical concerns need to be addressed for the market to reach its full potential. As the field continues to mature, the global epigenetics market is expected to experience continued innovation and expansion through 2032 and beyond.
#Epigenetics#EpigeneticsMarket#Genomics#Biotechnology#PrecisionMedicine#MolecularBiology#GeneExpression#DNAResearch#MedicalResearch#EpigeneticsTherapies#PharmaInnovation#CancerResearch#GeneTherapy#BiotechIndustry#LifeSciences#EpigeneticRegulation#GeneEditing#HealthTech#PersonalizedMedicine#BiotechInnovation
0 notes
Text
With generative AI, MIT chemists quickly calculate 3D genomic structures
New Post has been published on https://sunalei.org/news/with-generative-ai-mit-chemists-quickly-calculate-3d-genomic-structures/
With generative AI, MIT chemists quickly calculate 3D genomic structures

Every cell in your body contains the same genetic sequence, yet each cell expresses only a subset of those genes. These cell-specific gene expression patterns, which ensure that a brain cell is different from a skin cell, are partly determined by the three-dimensional structure of the genetic material, which controls the accessibility of each gene.
MIT chemists have now come up with a new way to determine those 3D genome structures, using generative artificial intelligence. Their technique can predict thousands of structures in just minutes, making it much speedier than existing experimental methods for analyzing the structures.
Using this technique, researchers could more easily study how the 3D organization of the genome affects individual cells’ gene expression patterns and functions.
“Our goal was to try to predict the three-dimensional genome structure from the underlying DNA sequence,” says Bin Zhang, an associate professor of chemistry and the senior author of the study. “Now that we can do that, which puts this technique on par with the cutting-edge experimental techniques, it can really open up a lot of interesting opportunities.”
MIT graduate students Greg Schuette and Zhuohan Lao are the lead authors of the paper, which appears today in Science Advances.
From sequence to structure
Inside the cell nucleus, DNA and proteins form a complex called chromatin, which has several levels of organization, allowing cells to cram 2 meters of DNA into a nucleus that is only one-hundredth of a millimeter in diameter. Long strands of DNA wind around proteins called histones, giving rise to a structure somewhat like beads on a string.
Chemical tags known as epigenetic modifications can be attached to DNA at specific locations, and these tags, which vary by cell type, affect the folding of the chromatin and the accessibility of nearby genes. These differences in chromatin conformation help determine which genes are expressed in different cell types, or at different times within a given cell.
Over the past 20 years, scientists have developed experimental techniques for determining chromatin structures. One widely used technique, known as Hi-C, works by linking together neighboring DNA strands in the cell’s nucleus. Researchers can then determine which segments are located near each other by shredding the DNA into many tiny pieces and sequencing it.
This method can be used on large populations of cells to calculate an average structure for a section of chromatin, or on single cells to determine structures within that specific cell. However, Hi-C and similar techniques are labor-intensive, and it can take about a week to generate data from one cell.
To overcome those limitations, Zhang and his students developed a model that takes advantage of recent advances in generative AI to create a fast, accurate way to predict chromatin structures in single cells. The AI model that they designed can quickly analyze DNA sequences and predict the chromatin structures that those sequences might produce in a cell.
“Deep learning is really good at pattern recognition,” Zhang says. “It allows us to analyze very long DNA segments, thousands of base pairs, and figure out what is the important information encoded in those DNA base pairs.”
ChromoGen, the model that the researchers created, has two components. The first component, a deep learning model taught to “read” the genome, analyzes the information encoded in the underlying DNA sequence and chromatin accessibility data, the latter of which is widely available and cell type-specific.
The second component is a generative AI model that predicts physically accurate chromatin conformations, having been trained on more than 11 million chromatin conformations. These data were generated from experiments using Dip-C (a variant of Hi-C) on 16 cells from a line of human B lymphocytes.
When integrated, the first component informs the generative model how the cell type-specific environment influences the formation of different chromatin structures, and this scheme effectively captures sequence-structure relationships. For each sequence, the researchers use their model to generate many possible structures. That’s because DNA is a very disordered molecule, so a single DNA sequence can give rise to many different possible conformations.
“A major complicating factor of predicting the structure of the genome is that there isn’t a single solution that we’re aiming for. There’s a distribution of structures, no matter what portion of the genome you’re looking at. Predicting that very complicated, high-dimensional statistical distribution is something that is incredibly challenging to do,” Schuette says.
Rapid analysis
Once trained, the model can generate predictions on a much faster timescale than Hi-C or other experimental techniques.
“Whereas you might spend six months running experiments to get a few dozen structures in a given cell type, you can generate a thousand structures in a particular region with our model in 20 minutes on just one GPU,” Schuette says.
After training their model, the researchers used it to generate structure predictions for more than 2,000 DNA sequences, then compared them to the experimentally determined structures for those sequences. They found that the structures generated by the model were the same or very similar to those seen in the experimental data.
“We typically look at hundreds or thousands of conformations for each sequence, and that gives you a reasonable representation of the diversity of the structures that a particular region can have,” Zhang says. “If you repeat your experiment multiple times, in different cells, you will very likely end up with a very different conformation. That’s what our model is trying to predict.”
The researchers also found that the model could make accurate predictions for data from cell types other than the one it was trained on. This suggests that the model could be useful for analyzing how chromatin structures differ between cell types, and how those differences affect their function. The model could also be used to explore different chromatin states that can exist within a single cell, and how those changes affect gene expression.
Another possible application would be to explore how mutations in a particular DNA sequence change the chromatin conformation, which could shed light on how such mutations may cause disease.
“There are a lot of interesting questions that I think we can address with this type of model,” Zhang says.
The researchers have made all of their data and the model available to others who wish to use it.
The research was funded by the National Institutes of Health.
0 notes
Text
Human Cell Tournament Round 2
Propaganda!
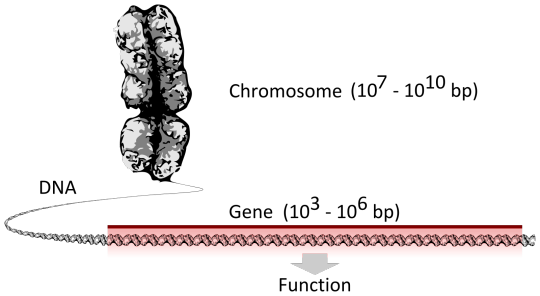
A chromosome is a package of DNA with part or all of the genetic material of an organism. In most chromosomes, the very long thin DNA fibers are coated with nucleosome-forming packaging proteins; in eukaryotic cells the most important of these proteins are the histones. These proteins, aided by chaperone proteins, bind to and condense the DNA molecule to maintain its integrity.[1][2] These chromosomes display a complex three-dimensional structure, which plays a significant role in transcriptional regulation.
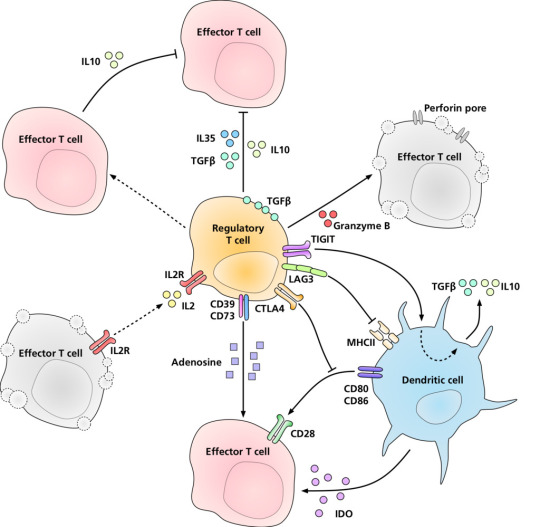
The regulatory T cells (Tregs), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. T regulatory cells are a component of the immune system that suppress immune responses of other cells. This is an important "self-check" built into the immune system to prevent excessive reactions. Mouse models have suggested that modulation of Treg cells can treat autoimmune disease and cancer and can facilitate organ transplantation and wound healing.
#Chromosomes#Regulatory T cell#Tregs#poll#polls#tumblr poll#tumblr polls#tournament poll#wikipedia#cells of the human body#science tournament#biochemistry
3 notes
·
View notes
Text

The Nucleosome: DNA's Fancy Packaging and Party Trick!
Imagine cramming two meters of yarn into a pea-sized box. Sounds impossible, right? Well, that's the impressive feat that cells pull off every single day with DNA! They use a clever structure called the nucleosome to pack this massive genetic blueprint into the tiny nucleus.
The journey began in 1974 when Don and Ada Olins, peering through an electron microscope, spotted repeating beads – the first glimpse of nucleosomes. Roger Kornberg, building upon this observation, proposed the now-iconic "subunit theory," envisioning DNA wrapped around histone protein cores. This theory, later solidified by Pierre Oudet's term "nucleosome," laid the groundwork for further exploration. The 1980s witnessed a flurry of activity, with Aaron Klug's group using X-ray crystallography to reveal the left-handed superhelical twist of DNA around the histone octamer. But the true masterpiece arrived in 1997 when the Richmond group, armed with advanced techniques, unveiled the first near-atomic resolution crystal structure of the nucleosome. This intricate map, showcasing the precise interactions between DNA and histones, remains a cornerstone of our understanding.
The Players:
DNA: The star of the show, carrying our genetic code in the form of a double helix.
Histones: Protein spools around which DNA tightly winds. Imagine eight of them forming a core, like a mini-protein drum set.
Linker DNA: Short stretches of DNA connecting the spools, like the spaces between beads on a necklace.
The Steps:
Wrap and Roll: Picture DNA gracefully wrapping around the histone core, like thread around a spool. Each nucleosome holds about 146 base pairs of DNA, making about 1.67 turns.
Connect and Repeat: Linker DNA bridges the gap between nucleosomes, forming a "beads-on-a-string" structure. Think of it as pearls strung between the spools.
Compact and Condense: This repetitive unit folds further, creating intricate 30-nanometer fibers. Imagine these as twisted strands of pearls!
Here's the coolest part: histones aren't static. They can be chemically modified, like adding or removing phosphate groups. These modifications act like tiny flags that tell the cell how tightly to wrap the DNA, essentially throwing a "party" for specific genes by making them more accessible. This fine-tuning allows cells to respond to their environment and express the right genes at the right time. Understanding the nucleosome model is crucial for unraveling the mysteries of gene regulation and diseases like cancer. By studying how modifications affect nucleosome structure and gene access, scientists can develop new therapies to target specific genes and potentially treat diseases at the root cause.
While the nucleosome model is the foundation, the story gets even more intriguing. Different histone types and modifications create variations, influencing chromatin structure and function. Think of it as different music genres influencing the dance moves! Additionally, other proteins interact with the nucleosome, adding another layer of complexity to this fascinating choreography.
The nucleosome model is more than just a neat way to package DNA. It's a testament to the intricate dance between molecules that orchestrates life's processes. By understanding this fundamental structure, we gain deeper insights into cellular function, paving the way for advancements in medicine and beyond.
Remember, this is just the beginning! The world of nucleosomes and chromatin is vast and ever-evolving. So, keep exploring, keep questioning, and keep dancing to the rhythm of DNA!
#molecular biology#biology#science sculpt#life science#science#dna#biotechnology#genetics#Histone#Nucleosome#chromatin
10 notes
·
View notes
Text
Exploring the Epigenetics Market: Trends, Growth, and Future Prospects
The epigenetics market is gaining significant momentum in the life sciences and healthcare sectors. This field, which studies heritable changes in gene expression without altering the DNA sequence, is instrumental in understanding complex biological processes and diseases. From drug discovery to personalized medicine, epigenetics offers transformative potential, making it a crucial area of research and development.
In this blog, we’ll delve into the key trends, market dynamics, applications, and growth drivers shaping the epigenetics market.
Understanding Epigenetics
Epigenetics refers to modifications on DNA or associated proteins that regulate gene activity without changing the underlying sequence. These modifications include:
DNA Methylation – The addition of methyl groups to DNA, often silencing gene expression.
Histone Modification – Changes in proteins around which DNA is wrapped, affecting gene accessibility.
Non-Coding RNAs – Molecules that influence gene expression post-transcriptionally.
Epigenetic mechanisms are reversible, making them attractive therapeutic targets for diseases like cancer, neurodegenerative disorders, and autoimmune conditions.
Market Overview
Market Size and Growth
The global epigenetics market was valued at approximately $1.4 billion in 2023 and is projected to grow at a CAGR of 15-18% over the next decade. This growth is driven by increasing research in gene therapy, rising cancer prevalence, and advancements in epigenetic technologies.
Key Market Segments
The market can be categorized into the following:
Products:
Reagents
Kits
Instruments (e.g., sequencers, microarrays)
Software
Applications:
Oncology
Developmental Biology
Metabolic Disorders
Neurology
End Users:
Academic Research Institutions
Pharmaceutical and Biotechnology Companies
Contract Research Organizations (CROs)
Drivers of Market Growth
1. Rising Prevalence of Cancer
Cancer is a leading application area for epigenetic research. Abnormal epigenetic modifications are closely linked to tumorigenesis. Epigenetic therapies, such as DNA methylation inhibitors and histone deacetylase (HDAC) inhibitors, are showing promising results in cancer treatment.
2. Advances in Epigenomics Technologies
The development of high-throughput sequencing and microarray platforms has made it possible to study epigenetic changes on a genome-wide scale. Tools like CRISPR-based epigenome editing are expanding research possibilities.
3. Increasing Focus on Personalized Medicine
Epigenetics plays a critical role in tailoring therapies based on individual genetic and epigenetic profiles. This approach is gaining traction, especially in oncology and chronic disease management.
4. Government and Private Funding
Governments worldwide are investing heavily in genomics and epigenetics research. For instance, the National Institutes of Health (NIH) in the U.S. allocates substantial grants for epigenetics projects. Private investments and collaborations are also fueling market growth.
Challenges in the Epigenetics Market
1. High Costs of Research and Equipment
Epigenetic research requires advanced instruments and reagents, which can be cost-prohibitive for smaller organizations.
2. Complexity of Epigenetic Mechanisms
The dynamic and reversible nature of epigenetic changes makes it challenging to pinpoint causal relationships between modifications and diseases.
3. Regulatory and Ethical Issues
Using epigenetic data in personalized medicine raises concerns about data privacy and ethical implications.
Emerging Trends in the Epigenetics Market
1. Integration of AI and Big Data
Artificial Intelligence (AI) and machine learning algorithms are being used to analyze complex epigenomic datasets, accelerating discoveries.
2. Focus on Epitranscriptomics
This subfield studies modifications in RNA rather than DNA, opening new avenues for understanding gene regulation.
3. Development of Epigenetic Biomarkers
Biomarkers are being developed for early diagnosis, prognosis, and treatment monitoring in diseases like cancer, Alzheimer’s, and diabetes.
4. Expansion of Non-Oncology Applications
While oncology dominates the market, epigenetics is increasingly applied in neurodegenerative diseases, cardiovascular disorders, and metabolic syndromes.
Competitive Landscape
Key players in the epigenetics market include:
Illumina, Inc. – Leading in sequencing technologies.
Thermo Fisher Scientific, Inc. – Offering comprehensive epigenetics solutions.
Abcam plc – Specializing in antibodies and kits for epigenetic research.
Qiagen – Providing tools for epigenomic studies.
Merck KGaA – Known for its advanced reagents and inhibitors.
Collaborations, acquisitions, and product launches are common strategies adopted by these players to strengthen their market position.
Applications of Epigenetics
1. Cancer Research and Therapy
Epigenetic drugs are used to reprogram cancer cells, making them more susceptible to traditional therapies.
2. Developmental Biology
Epigenetics helps unravel how environmental factors influence gene expression during development.
3. Neurology
Research in conditions like Alzheimer’s and Parkinson’s diseases focuses on epigenetic mechanisms underlying neuronal dysfunction.
4. Agriculture and Veterinary Science
Epigenetic studies in plants and animals aim to enhance productivity and disease resistance.
Future Prospects
The future of the epigenetics market is promising, with continued advancements in technology and an expanding scope of applications. Personalized medicine and precision oncology are expected to be major growth areas. Moreover, the rise of epigenome editing tools and novel biomarkers will drive innovation in diagnostics and therapeutics.
Conclusion
The epigenetics market represents a dynamic and rapidly evolving field with immense potential to transform healthcare and research. As we continue to uncover the intricacies of the epigenome, the applications of this science will expand, offering solutions to some of the most challenging medical and scientific problems.
For stakeholders, the key to success lies in leveraging technological advancements, fostering collaborations, and addressing ethical challenges. With sustained investment and innovation, epigenetics is poised to become a cornerstone of modern medicine.
0 notes
Text
The Connection Between Damaged Mitochondria and Arthritis

Mitochondria are integral organelles responsible for various critical cellular functions, primarily energy production through oxidative phosphorylation. They are involved in maintaining cellular homeostasis, regulating metabolism, modulating calcium levels, and controlling apoptosis. Emerging evidence has highlighted mitochondrial dysfunction as a key contributor to a variety of diseases, including arthritis. This formal overview aims to explore the complex relationship between damaged mitochondria and arthritis, focusing on the molecular mechanisms that link mitochondrial dysfunction to the pathogenesis of inflammatory joint diseases, particularly rheumatoid arthritis (RA) and osteoarthritis (OA).
Mitochondrial Structure and Function
Mitochondria are double-membraned organelles found in eukaryotic cells, and they are crucial for cellular energy metabolism. Their primary role is the production of adenosine triphosphate (ATP) via oxidative phosphorylation, a process that takes place in the inner mitochondrial membrane. During this process, the electron transport chain (ETC) generates a proton gradient across the inner membrane, which drives ATP synthesis through ATP synthase. However, this process also generates reactive oxygen species (ROS) as byproducts, primarily from complexes I and III of the ETC. Under normal physiological conditions, ROS are neutralized by antioxidants, including superoxide dismutase (SOD), catalase, and glutathione. However, under pathological conditions, excessive ROS production can lead to oxidative stress, contributing to cellular damage and dysfunction.
In addition to ATP production, mitochondria have essential roles in calcium buffering, apoptosis regulation, and the maintenance of cellular integrity. Damage to these organelles disrupts these functions, contributing to various diseases, including arthritis.
Mitochondrial Dysfunction in Arthritis
Arthritis is a group of diseases characterized by inflammation and degeneration of the joints. It includes conditions like rheumatoid arthritis (RA), an autoimmune disease, and osteoarthritis (OA), a degenerative disease. In both types of arthritis, mitochondrial dysfunction has been identified as a critical factor that exacerbates disease progression through several mechanisms, including increased oxidative stress, immune activation, and tissue damage.
1. Oxidative Stress and Mitochondrial Damage
Oxidative stress is a hallmark of both RA and OA, and mitochondria are central to its production. In these conditions, mitochondrial dysfunction results in an increase in ROS production, overwhelming the cell’s antioxidant defenses. This oxidative stress leads to the modification of cellular structures, including proteins, lipids, and DNA, causing further mitochondrial damage. In RA, pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) stimulate immune cells like macrophages and neutrophils to release large amounts of ROS. These ROS contribute to the local inflammatory environment and accelerate joint destruction by damaging mitochondria and amplifying oxidative stress.
Mitochondrial damage results in a feedback loop where impaired mitochondrial function generates more ROS, further promoting inflammation. For instance, in RA, markers of oxidative damage such as 8-hydroxy-2'-deoxyguanosine (8-OHdG) and malondialdehyde (MDA) have been found to correlate with disease activity, suggesting a direct relationship between mitochondrial dysfunction and disease severity.
2. Mitochondrial DNA Damage and Inflammatory Signaling
Mitochondrial DNA (mtDNA) is particularly vulnerable to oxidative damage due to its proximity to the ETC, where ROS are produced during ATP synthesis. Unlike nuclear DNA, mtDNA is not protected by histones and has limited repair mechanisms, making it prone to mutations. Damage to mtDNA impairs mitochondrial function and can lead to the release of mtDNA fragments into the cytoplasm or extracellular space.
In the context of arthritis, mtDNA damage has been implicated in immune activation. When damaged mtDNA is released into the cytoplasm, it is recognized by pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), on immune cells. TLRs, particularly TLR9, activate downstream inflammatory signaling pathways that lead to the production of pro-inflammatory cytokines such as TNF-α and IL-6. This further exacerbates the inflammatory response in joints and contributes to the progression of arthritis. Studies have shown that the presence of mtDNA fragments in the serum of RA patients correlates with disease activity, indicating the role of mtDNA in driving inflammation.
3. Mitochondrial Dynamics and Arthritis Pathogenesis
Mitochondrial dynamics refer to the continuous processes of mitochondrial fission (division) and fusion (joining), which maintain mitochondrial function and integrity. Fission allows for the removal of damaged mitochondria, while fusion helps to integrate mitochondrial contents and maintain a healthy mitochondrial pool. Imbalance between fission and fusion is associated with several diseases, including arthritis.
In the case of RA, excessive mitochondrial fission and reduced fusion have been observed. This imbalance results in mitochondrial fragmentation, which impairs mitochondrial function, increases ROS production, and contributes to cellular stress. Fission is regulated by proteins such as dynamin-related protein 1 (Drp1) and fission 1 protein (Fis1), while fusion is controlled by mitofusins (Mfn1 and Mfn2) and optic atrophy 1 (OPA1). Dysregulation of these proteins in RA leads to a fragmented mitochondrial network, which exacerbates oxidative stress and inflammation in synovial tissues.
4. Mitochondrial-Dependent Cell Death
Mitochondria are also central regulators of programmed cell death, particularly apoptosis. In the pathogenesis of arthritis, excessive or dysregulated apoptosis contributes to joint destruction. Mitochondrial dysfunction plays a critical role in the intrinsic apoptotic pathway by releasing pro-apoptotic factors such as cytochrome c and apoptosis-inducing factor (AIF). These factors activate caspase-dependent and caspase-independent pathways, leading to the death of synovial cells and cartilage cells, which contributes to the progressive tissue damage observed in both RA and OA.
Furthermore, mitochondrial permeability transition pore (mPTP) opening, which is induced by oxidative stress, can lead to necrosis, a form of uncontrolled cell death. Necrotic cell death in the joints increases inflammation and tissue degradation, particularly in OA, where cartilage breakdown is a hallmark feature.
Therapeutic Approaches Targeting Mitochondrial Dysfunction in Arthritis
Given the significant role of mitochondrial dysfunction in the pathogenesis of arthritis, various therapeutic strategies aimed at improving mitochondrial function are under investigation.
1. Mitochondrial Antioxidants
Mitochondrial-targeted antioxidants, such as MitoQ and MitoTEMPO, have been developed to selectively accumulate in mitochondria, where they can neutralize ROS and reduce oxidative stress. These compounds have shown promise in preclinical models of arthritis, where they help to reduce inflammation, protect mitochondrial function, and limit joint damage. The use of mitochondrial antioxidants could be an effective strategy to mitigate oxidative stress in arthritic conditions.
2. Mitochondrial Biogenesis Enhancement
Another potential therapeutic approach is the activation of mitochondrial biogenesis, the process by which new mitochondria are formed to compensate for damaged mitochondria. Agents that activate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a key regulator of mitochondrial biogenesis, could help restore mitochondrial function in arthritic tissues. Compounds such as resveratrol and NAD+ precursors are under investigation for their ability to promote mitochondrial biogenesis and improve cellular metabolism in arthritis.
3. Mitochondrial Dynamics Modulation
Restoring the balance between mitochondrial fission and fusion is another therapeutic strategy. Inhibiting excessive mitochondrial fission or promoting mitochondrial fusion may help maintain mitochondrial integrity and reduce inflammation in arthritis. Drugs targeting Drp1 or enhancing Mfn1/Mfn2 activity are potential candidates for modulating mitochondrial dynamics in arthritic diseases.
4. Mitophagy Enhancement
Mitophagy, the selective autophagic degradation of damaged mitochondria, is essential for maintaining mitochondrial quality. Enhancing mitophagy through the use of compounds like spermidine or activators of the PINK1/PARK2 pathway could help eliminate dysfunctional mitochondria and reduce inflammation, making it a promising therapeutic approach in arthritis.
Conclusion
Mitochondrial dysfunction plays a critical role in the pathogenesis of arthritis, contributing to oxidative stress, inflammation, and joint damage. The intricate relationship between damaged mitochondria and immune activation highlights the importance of targeting mitochondrial health in the treatment of arthritis. Emerging therapeutic strategies aimed at restoring mitochondrial function, reducing oxidative stress, and modulating mitochondrial dynamics hold promise for improving the management of arthritis and preventing joint destruction. Further research into mitochondrial biology and its role in arthritis is essential for the development of more effective, targeted therapies for these debilitating conditions.
#Mitochondrial dysfunction#Autoimmune disorders#Oxidative stress#Reactive oxygen species (ROS)#Mitochondrial dynamics#Mitochondrial fission#Mitochondrial fusion#Mitophagy#Apoptosis#Mitochondrial DNA (mtDNA)#Damage-associated molecular patterns (DAMPs)#Immune cell activation#Systemic lupus erythematosus (SLE)#Rheumatoid arthritis (RA)#Multiple sclerosis (MS)#Pattern recognition receptors (PRRs)#Toll-like receptors (TLRs)#Pro-inflammatory cytokines#Cytochrome c#NF-κB signaling#MitoQ#MitoTEMPO#Spermidine#PINK1/PARK2 pathway#Mitochondrial-targeted antioxidants#Immune dysregulation#Chronic inflammation#Mitochondrial fragmentation#Mitochondrial permeability transition pore (mPTP)#Autoantibodies
0 notes
Text
Corona from hydrogen
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
hydrogen atom proton electron
Oxygen atom fuses and becomes stable
Chromosomes which are XX XY and YY
female male impotent like whatever in life
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
hydrogen atom proton electron
Oxygen atom fuses and becomes stable
Chromosomes which are XX XY and YY
female male impotent like whatever in life
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Just like proton electron is very mobile and active but neutron is patient and stable. Similarly, egg having XX and XY chromosomes is very mobile and active. But YY which is not there at all, which is an error, is actually inactive. Do not think that just like hydrogen, the atom itself is active. In the same way, perhaps the egg also gets fertilized by the sperm. Fertilization does not happen without ovary. Sperm is not hydrogen, friends, yes, but hydrogen is a part of it. Perhaps that is why, in ovary having XX chromosomes, sperm having XY chromosomes and sperm having YY chromosomes may get fertilized. It is said that embryo having XY chromosomes is male. Embryo having XX chromosomes is female. YY chromosomes are not mobile. If it happens, then it is due to some error. Perhaps it is called eunuch. What is this chromosome? Chromosome is a package of DNA which contains some or the whole part of the genetic material of an organism. In most chromosomes, very long thin DNA fibers are covered by packaging proteins that form nucleosomes; the most important of these proteins in eukaryotic cells are histones. Wikipedia
Why are chromosomes identified as XY?
In most cases, females are XX and males are XY. Each individual must have at least one X chromosome. Since the female is XX, each egg she ovulates has an X chromosome. The male, being XY, can produce two kinds of sperm: half have an X chromosome, half have a Y.
What is this electron doing in hydrogen?
The electron in the hydrogen atom revolves around the nucleus in a circular orbit. The energy of the electron in the orbit is proportional to its distance from the nucleus. The farther the electron is from the nucleus, the greater its energy. Only a limited number of orbitals with certain energies are allowed.
We understand protons, electrons and neutrons
But why is it called XY chromosome instead of AB or 12 chromosome in the chromosome?
Human X and Y chromosomes determine the biological sex of a person, with XX specifying female and XY specifying male. Although the Y chromosome contains a small region of similarity to the X chromosome so that they can pair during meiosis, the Y chromosome is much shorter and contains many fewer genes.
Chromosome Abnormalities Fact Sheet
National Human Genome Research Institute (.gov)
https://www.genome.gov › about-genomics › Chromos...
15 Aug 2020 — Chromosome abnormalities can either be numerical or structural and usually occur when there is an error in cell division.
Genes and Chromosomes - MSD Manual Consumer Version
MSD Manuals
https://www.msdmanuals.com › ... ��� Genetics
A chromosome is made of a very long strand of DNA and contains many genes (hundreds to thousands). The genes on each chromosome are arranged in a particular sequence, and each gene has a particular location on the chromosome (called its locus). The form of the gene that occupies the same locus on each chromosome of a pair (one inherited from the mother and one from the father) is called an allele. In addition to DNA, chromosomes contain other chemical components that influence gene function.
Pairing
Except for certain cells (for example, sperm and egg cells or red blood cells), the nucleus of every normal human cell contains 23 pairs of chromosomes, for a total of 46 chromosomes. Normally, each pair consists of one chromosome from the mother and one from the father.
There are 22 pairs of nonsex (autosomal) chromosomes and one pair of sex chromosomes. Paired nonsex chromosomes are, for practical purposes, identical in size, shape, and position and number of genes. Because each member of a pair of nonsex chromosomes contains one of each corresponding gene, there is in a sense a backup for the genes on those chromosomes.
The 23rd pair is the sex chromosomes (X and Y).
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
hydrogen atom proton electron
Oxygen atom fuses and becomes stable
Chromosomes which are XX XY and YY
female male impotent like whatever in life
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
hydrogen atom proton electron
Oxygen atom fuses and becomes stable
Chromosomes which are XX XY and YY
female male impotent like whatever in life
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Hydrogen's action in water, in clouds
Similarly, X chromosome is impatient in X and Y
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Corona from hydrogen
Small water grown from outside
This is the story
Rocky gas, icy and smoky
Animals and humans are also complex but
A miracle similar to life
Animals and humans are also complex but
A miracle similar to life
Translate Hindi
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन की एटम प्रोटोन इलेक्ट्रान
ऑक्सीजन की एटम में संगम और स्थिर
क्रोमोसोम जो एक्सएक्स एक्सवाई वाईवाई
नारी पुरुष नामर्द जैसे जीवन में जो भीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की एटम प्रोटोन इलेक्ट्रान
ऑक्सीजन की एटम में संगम और स्थिर
क्रोमोसोम जो एक्सएक्स एक्सवाई वाईवाई
नारी पुरुष नामर्द जैसे जीवन में जो भीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
जैसे प्रोटोन इलेक्ट्रान काफी गतिशील और क्रियाशील होता है लेकिन निउट्रान धैर्यपूर्ण और स्थिर होता है
उसी तरह एक्सएक्स और एक्सवाई क्रोमोसोम वाली डिम्बाणु शु्राणु काफी गतिशील और क्रियाशील होता है
मगर वाईवाई जो होता ही नहीं है जो त्रुटि है वास्तव में क्रियाहिन है
आप यह न समझ लेना दोस्तों हाइड्रोजन जैसे खुद ही एटोमिक क्रियाशील है
उसी तरह शायद इंपोर्टेंट भी शुक्राणु द्वारा निषेचित होते होंगे
निषेचन बिना ओवरी होता नहीं है
शुक्राणु हाइड्रोजन नहीं है दोस्तों हाँ मगर हाइड्रोजन जैसी की ही एक अंश है
शायद इसीलिए एक्स एक्स क्रोमोसोम वाली ओवरी में एक्स वाई क्रोमोसोम वाले शुक्राणु की वाई वाई क्रोमोसोम वाली एरर निषेचन बन जाते होंगे
कहते है
एक्स वाई क्रोमोसोम वाली भ्रूण मेल होता है
एक्स एक्स क्रोमोसोम वाली भ्रूण फिमेल
वाई वाई क्रोमोसोम सचराचर होते नहीं है अगर होता है तो कुछ त्रूटि के कारण होता है शायद इसे हिजड़ा कहा जाता होगा
यह क्रोमोसोम क्या है
गुणसूत्र डीएनए का एक पैकेज है जिसमें किसी जीव की आनुवंशिक सामग्री का कुछ या पूरा भाग होता है। अधिकांश गुणसूत्रों में, बहुत लंबे पतले डीएनए फाइबर न्यूक्लियोसोम बनाने वाले पैकेजिंग प्रोटीन से ढके होते हैं; यूकेरियोटिक कोशिकाओं में इन प्रोटीनों में सबसे महत्वपूर्ण हिस्टोन होते हैं। विकिपीडिया
क्रोमोसोम को एक्स वाई में ही क्यों पहचाना जाता है
ज़्यादातर मामलों में, मादा XX होती है और नर XY होता है। हर व्यक्ति में कम से कम एक X गुणसूत्र होना चाहिए। चूँकि मादा XX होती है, इसलिए उसके हर अंडे में एक X गुणसूत्र होता है। नर, XY होने के कारण, दो तरह के शुक्राणु उत्पन्न कर सकता है: आधे में X गुणसूत्र होता है, आधे में Y।
हाइड्रोजन में यह इलेक्ट्रान का कैरेक्टर क्या कर रहा है
हाइड्रोजन परमाणु में इलेक्ट्रॉन नाभिक के चारों ओर एक गोलाकार कक्षा में घूमता है। कक्षा में इलेक्ट्रॉन की ऊर्जा नाभिक से उसकी दूरी के समानुपाती होती है। इलेक्ट्रॉन नाभिक से जितना दूर होगा, उसकी ऊर्जा उतनी ही अधिक होगी। केवल कुछ निश्चित ऊर्जा वाली सीमित संख्या में कक्षाओं की अनुमति है।
प्रोटोन इलेक्ट्रॉन निउट्रान हमें समझ आता है
मगर यह क्रोमोसोम में ए बी या 1 2 गुणसुत्र न होकर एक्स वाई गुणसुत्र क्यों कहा जाता है
मानव X और Y गुणसूत्र व्यक्ति के जैविक लिंग का निर्धारण करते हैं, जिसमें XX महिला और XY पुरुष को निर्दिष्ट करता है। हालाँकि Y गुणसूत्र में X गुणसूत्र के साथ समानता का एक छोटा सा क्षेत्र होता है ताकि वे अर्धसूत्रीविभाजन के दौरान जोड़ी बना सकें, Y गुणसूत्र बहुत छोटा होता है और इसमें बहुत कम जीन होते हैं।
गुणसूत्र असामान्यताएँ तथ्य पत्रक
राष्ट्रीय मानव जीनोम अनुसंधान संस्थान (.gov)
https://www.genome.gov › about-genomics › Chromos...
15 अगस्त 2020 — गुणसूत्र असामान्यताएँ संख्यात्मक या संरचनात्मक हो सकती हैं और आमतौर पर तब होती हैं जब कोशिका विभाजन में कोई त्रुटि होती है।
जीन और गुणसूत्र - MSD मैनुअल उपभोक्ता संस्करण
MSD मैनुअल
https://www.msdmanuals.com › ... › आनुवंशि��ी
एक गुणसूत्र DNA के बहुत लंबे स्ट्रैंड से बना होता है और इसमें कई जीन (सैकड़ों से हज़ारों) होते हैं। प्रत्येक गुणसूत्र पर जीन एक विशेष क्रम में व्यवस्थित होते हैं, और प्रत्येक जीन का गुणसूत्र पर एक विशेष स्थान होता है (जिसे उसका स्थान कहा जाता है)। जीन का वह रूप जो एक जोड़ी के प्रत्येक गुणसूत्र (एक माता से विरासत में मिला और एक पिता से) पर एक ही स्थान पर रहता है, उसे एलील कहा जाता है। डीएनए के अलावा, गुणसूत्रों में अन्य रासायनिक घटक होते हैं जो जीन फ़ंक्शन को प्रभावित करते हैं।
जोड़ी बनाना
कुछ कोशिकाओं (उदाहरण के लिए, शुक्राणु और अंडाणु कोशिकाएँ या लाल रक्त कोशिकाएँ) को छोड़कर, प्रत्येक सामान्य मानव कोशिका के नाभिक में 23 जोड़े गुणसूत्र होते हैं, कुल 46 गुणसूत्र होते हैं। आम तौर पर, प्रत्येक जोड़ी में माँ से एक गुणसूत्र और पिता से एक गुणसूत्र होता है।
गैर-लिंग (ऑटोसोमल) गुणसूत्रों के 22 जोड़े और सेक्स गुणसूत्रों का एक जोड़ा होता है। जोड़े गए गैर-लिंग गुणसूत्र, व्यावहारिक उद्देश्यों के लिए, आकार, आकृति और स्थिति और जीन की संख्या में समान होते हैं। क्योंकि गैर-लिंग गुणसूत्रों की एक जोड़ी के प्रत्येक सदस्य में प्रत्येक संगत जीन में से एक होता है, इसलिए एक तरह से उन गुणसूत्रों पर जीन के लिए एक बैकअप होता है।
23वीं जोड़ी सेक्स गुणसूत्र (X और Y) है।
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन की एटम प्रोटोन इलेक्ट्रान
ऑक्सीजन की एटम में संगम और स्थिर
क्रोमोसोम जो एक्सएक्स एक्सवाई वाईवाई
नारी पुरुष नामर्द जैसे जीवन में जो भीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की एटम प्रोटोन इलेक्ट्रान
ऑक्सीजन की एटम में संगम और स्थिर
क्रोमोसोम जो एक्सएक्स एक्सवाई वाईवाई
नारी पुरुष नामर्द जैसे जीवन में जो भीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन की क्रिया पानी में भी बादल में भी
वैसे ही एक्स क्रोमोसोम एक्स और वाई में अधीर
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
हाइड्रोजन में से कोरोना
बाहरी ओर से उगाए स्मलवटर
इसी में ही है कहानी
चट्टानी गैस बर्फीले और धुंआधार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
पशु इंसान भी जटिल मगर
जीवन जैसी कुछ ऐसी ही चमत्कार
0 notes