#Rituxan
Text
A Jamie update
I have so much to catch you up on, I’m not really sure where to start. I seem to have the UTI’s under control. I’ve made it two months without needing antibiotics. I am talking a prophylactic antibiotic nightly as a precaution. Hopefully I am through the cycle I was living inside. I was also given a round of IVIG therapy to boost my immune system. That is an IV of immunoglobulin. By boosting my…

View On WordPress
#daily life with ms#diagnosed with MS#difficulties with ms#ivig#living with a chronic disease#living with MS#living with multiple sclerosis#MS#ms challenges#ms fatigue#ms life#Ms progression#ms treatments#Multiple sclerosis#Rituxan#steroids
2 notes
·
View notes
Text
How does B cell therapy for multiple sclerosis work?
How does B cell therapy for multiple sclerosis work?
After Cherie Binn’s breakthrough symptoms of multiple sclerosis developed while being treated with an interferon drug, she weighed her options carefully.
Finally, her neurologist prescribed rituximab. It’s a type of B cell therapy, which gets its name because it targets the B cells that cause nerve damage when you have MS.
Binns, a 69-year-old nurse who works with MS patients in Wakefield, RI,…
View On WordPress
0 notes
Text
I haven’t touched on my illness on here in a very looooong time but I’m a lil anxious to start my new medication, rituxan. It’s more aggressive than any other medication but that’s not what’s giving me anxiety. Like yes it’s scary what it could do to you, like death is listed as a side effect, hello, but that’s not particularly worrisome for me. I’m more concerned with the fact that this will be my 9th medication for RA, and it will basically be my Hail Mary pass(sports!). If this doesn’t work then I have no idea where my treatment will go and that frustrates me. I see so many stories of people who have been on medication for 8 years+ and the longest I’ve been on medication was 2 years and it barely did anything for me. And if I do find a medication that helps a lot it will stop working after a few months 😩.
Anyways… my current doctor seems to be willing to try many things; she even was the first doctor to say that maybe a lupus diagnosis isn’t as far fetched as other doctors made it seem. Other doctors were so quick to say “it’s not lupus!” even with a positive ANA and RNP antibodies. So maybe she will explore treating me for lupus if this doesn’t pan out the way we hope it will. IDK!
I’m trying to stay positive because I’ve heard this medication works well for others who have RA but it would be cool if y’all could send me love and light or prayers or any positive thoughts. I’d appreciate it, truly.
#maybe writing this down will help idk!#I haven’t felt this anxious over my illness in a while so yeah#rheumatoid arthritis#rituxan#chronic illness
1 note
·
View note
Text
Christ, I hate being sick.
I've spent the last month or so waiting for today. Insurance approved it easily, I caught it before I needed too much prednisone.
Any then Redd got COVID. He's doing better, though I worry about him and long COVID. I don't think he gave it to me (I continuously tested negative at home) but it meant treatment had to be pushed back a week and a half.
I am fairly certain what happened overall is that I went from getting a 500 ml dose to 1000 ml dose and my body was not ready for that. I'll likely talk to my doctor about only doing 500 ml next time.
They couldn't get my veins to work. The first time or two was likely dehydration, then the rest was a panic attack. The super nice nurses told me I wasn't being the worst, gave me oxygen and let me blast Taylor Swift while tried to calm down. Once the IV was in, I fell asleep almost instantly.
At some point I woke up, and the nurse confirmed she would turn up the speed on the IV now that I had been in and out for 90 minutes. It was uncomfortable, but not the worse.
Until it was the worse. I nearly passed out, needing oxygen again. It doesn't help that I have this cough and sinus whatever. I felt like I Was going to throw up, pass out, jump out of my skin....I dunno.
I ended up throwing up towards the end. I am exhausted, achy, sick, though I am keeping food down.
Fuck my body so much.
#Granulomatosis with polyangiitis#churg strauss syndrome#chronic illness#fucking sucks#maybe next time I just curl up in the woods and expire#Jesus now I remember why this used to be such a harder debate#wegener's granulomatosis#rituxan#rituximab
1 note
·
View note
Text
Every single person I tested for flu or covid today came back positive and half of them were not wearing masks. I am very worried I am going to get sick. I cannot get sick. I've got no sick time left because I've had covid and flu multiple times this past fall and winter. There are many, many downsides to having an impaired ability to gain immunological memory.
#plus they'll move my rituxan if i get sick and i really need it#i needed it like a month ago honestly
27 notes
·
View notes
Note
Thinking about your son- how is he doing?
Oh thank you for thinking of him ❤️…he’s hanging in there. He had an infusion of rituxan in late Oct that apparently might be working, or at least isn’t actively detrimental according to his labs, so we have another infusion of Rituxan scheduled for February (with two more to come every 12 weeks). His symptoms are definitely still present (lots of tics, still some compulsive behaviors but not as all-consuming, fatigue & mood swings, a lot of anxiety) and he still can’t go to school but 🤷🏻♀️ we’re just kinda keeping on keeping on at this point. TL,DR: kinda the same but that’s a win for now. Here’s a pic of him before he got sick, just because he’s so cute.

#he got sick around thanksgiving and his PANDAS symptoms flared back up very badly#but I think the Rituxan infusion helped the flare not be as intense as his last one#his flares had been getting longer and worse each time but this one is not as bad as the summer so yeah#his specialist was telling me how hard it is to get new neurologists/rheumatologists to specialize in treating PANDAS#because it’s such a frustrating disease to treat and the families are so desperate for help#that it feels extra awful when you can’t offer clear answers#his specialist (Dr Latimer) is one of the best in the world for this#but even she can’t say for sure what will help or how long recovery might take#ANYWAY sorry lol thank you for keeping my sweet boy in your thoughts!
25 notes
·
View notes
Text
Hey guys so I'm about to get a little personal here.
My name is Camila and I am 25 years old. I recently went to urgent care for what I thought was a rash. Instead, I was immediately sent to the ER and then admitted in the same night. I was immediatly seen by an hematologist/oncologist.
First time I was in the hospital I got diagnosed with a rare blood disease called ITP. (Immune Thrombocytopenia Purpura) It's a rare disease that targets young women and children and at my age considered chronic.
Just to explain a little, normal platelets in a healthy person is 150,000 and up. The first time I came in with 1,000 plaletes and now now I have been admitted for the second time with zero (0) plaletes.
Just a little medical background--Plaletes help the blood clot and tipically it's what separates red blood cells from plasma.
I am at a very high risk for bleeding out. It is a life long rare disease and 70% who are diagnosed don't come back to the hospital. I am the 30% that did!
Protocol for my condition are steroids, IV IG line and spleen removal.
However, my oncologist wants to try an Injection (Rituxan) and/or the pills (Promacta). If not successful, we will have major surgery to remove my spleen.
I am in good spirits, I am very optimistic that this medication will work, but I need help financially. I have no insurance and I still need to take care for my medical bills and medication. If it's your heart please donate, every little helps. Share with your friends and family, the world is full of strangers rooting for other people. Thank you ❤ Also, dont feel pressured to donate please, a share will be fine too, thank you guys. Links below!
Cashapp:
49 notes
·
View notes
Text
I got my Rituxan infusion today and it went well ☺️ there was only one moment where my throat got really itchy but he just paused the meds and upped my fluids and I was fine after!! I was wearing my cutie lil pink tracksuit because I have to be a bad bitch while getting my meds of course
2 notes
·
View notes
Text
Top Pharmaceutical Manufacturing Companies in India
There are numerous pharmaceutical manufacturing companies worldwide, ranging from large multinational corporations to smaller, specialized firms. Here are some well-known Pharmaceutical manufacturers Companies in India :
Pfizer Inc. - Based in the United States, Pfizer is one of the largest pharmaceutical companies globally, known for products like Viagra, Lipitor, and Prevnar.
Novartis International AG - Headquartered in Switzerland, Novartis is a major player in the pharmaceutical industry, producing drugs such as Diovan, Gilenya, and Cosentyx.
Roche Holding AG - Another Swiss-based company, Roche is renowned for its innovations in cancer treatments, including drugs like Herceptin, Avastin, and Rituxan.
Johnson & Johnson - Based in the United States, Johnson & Johnson operates in various sectors, including pharmaceuticals, consumer health, and medical devices. Some of its pharmaceutical products include Remicade, Stelara, and Zytiga.
Merck & Co., Inc. (known as MSD outside the U.S. and Canada) - Merck is a leading global healthcare company, producing drugs like Keytruda, Januvia, and Gardasil.
Sanofi - Headquartered in France, Sanofi develops pharmaceuticals across various therapeutic areas, such as diabetes, oncology, and vaccines. Popular products include Lantus, Dupixent, and Toujeo.
AstraZeneca - A British-Swedish multinational, AstraZeneca focuses on treatments for cardiovascular, respiratory, and oncology conditions. Notable products include Crestor, Symbicort, and Tagrisso.
GlaxoSmithKline plc (GSK) - Based in the United Kingdom, GSK produces a wide range of pharmaceuticals, vaccines, and consumer healthcare products. Some of its well-known brands include Advair, Augmentin, and Tivicay.
These are just a few examples of prominent pharmaceutical manufacturers, but there are many others across the globe, each contributing to the development and production of vital medications for various health conditions.
Contact US:-
2nd Floor Plot No-5, Nidhi Plaza-II, LSC Gulabi Bagh,
Near Shakti Nagar Railway Bridge Delhi-52 India
24X7 Customer Care:
+91-9315951001
Phone:
+91-11-23653537, 23653404
Email:
[email protected]
0 notes
Text
Targeted therapies for brain tumors are treatments that aim to specifically target certain molecules or pathways involved in the growth and spread of the tumor.
These therapies are designed to be more precise and selective than traditional chemotherapy, potentially resulting in fewer side effects and improved outcomes.
Here are some targeted therapies commonly used for brain tumors:
Tyrosine Kinase Inhibitors (TKIs): These drugs target specific tyrosine kinases, enzymes involved in signaling pathways that regulate cell growth and division. Examples include drugs like imatinib (Gleevec), which targets the BCR-ABL fusion protein in chronic myeloid leukemia, and erlotinib (Tarceva), which targets the epidermal growth factor receptor (EGFR).
Angiogenesis Inhibitors: Angiogenesis is the process by which new blood vessels are formed, and it plays a critical role in tumor growth and spread by supplying nutrients and oxygen to the tumor. Angiogenesis inhibitors like bevacizumab (Avastin) can block the formation of new blood vessels, thereby starving the tumor of its blood supply.
Monoclonal Antibodies: Monoclonal antibodies are laboratory-made molecules that can target specific proteins on the surface of cancer cells. For example, rituximab (Rituxan) targets CD20, a protein found on B-cell lymphomas, and trastuzumab (Herceptin) targets HER2, a protein overexpressed in certain breast cancers.
Immunotherapy: Immunotherapy works by harnessing the body's immune system to recognize and attack cancer cells. Checkpoint inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo) can block inhibitory signals on immune cells, allowing them to more effectively target and destroy tumor cells.
Targeted Radiation Therapy: Techniques such as stereotactic radiosurgery and proton therapy allow for precise delivery of radiation to the tumor, minimizing damage to surrounding healthy tissue.
Gene Therapy: Gene therapy involves introducing genetic material into cells to replace or supplement faulty genes. This approach holds promise for treating brain tumors by targeting specific genetic mutations driving tumor growth.
Signal Transduction Inhibitors: These drugs interfere with signaling pathways involved in cell proliferation and survival. For example, inhibitors of the PI3K/AKT/mTOR pathway, such as everolimus (Afinitor), can suppress tumor growth in certain types of brain tumors.
The cost of targeted therapy for brain tumors in India can vary depending on several factors, including the specific type of therapy, the duration of treatment, the dosage required, the brand of medication, the healthcare facility where treatment is received, and any additional supportive care or monitoring needed during the course of treatment.
Generally, targeted therapies tend to be more expensive than conventional chemotherapy or radiation therapy due to their specialized nature and often involve ongoing treatment over an extended period. The cost of targeted therapy may also include fees for medical consultations, diagnostic tests, imaging studies, and hospitalization if required.
In India, the cost of targeted therapy can range from several thousand to several lakh rupees per month, depending on the factors mentioned above. Some targeted therapies may be available at lower costs through government healthcare facilities or subsidized programs, while others may be more expensive and only accessible at private hospitals or specialty clinics.
Get the best treatments for brain tumor at the best hospitals in Mumbai like H N Reliance Hospital Mumbai.
#targeted therapy#health#surgery#brain health#brain tumors#diseases#parkinson's disease#deep brain stimulation#epilepsy#depression
0 notes
Text
Researching the Future of Orphan Drugs Market Services
Market Overview –
The market for orphan pharmaceuticals was estimated to be worth USD 160.78 billion in 2021 and is expected to increase to USD 355.00 billion by 2030, showing a 9.20% compound annual growth rate (CAGR) between 2022 and 2035.
The Orphan Drugs Market is a niche segment of the pharmaceutical industry dedicated to treating rare diseases. These diseases, often affecting a small number of people, have historically been overlooked by drug manufacturers due to limited commercial viability. However, with increasing awareness and regulatory incentives, the orphan drugs market has gained traction.
The market's growth is primarily driven by regulatory support, including extended market exclusivity, tax credits, and research grants provided to pharmaceutical companies developing orphan drugs. Additionally, advancements in biotechnology and genomic research have facilitated the discovery and development of treatments for rare diseases.
The Orphan Medicine market is experiencing notable growth, propelled by advancements in rare disease treatment and supportive regulatory initiatives. Orphan drugs target rare conditions, offering hope to patients previously overlooked by mainstream pharmaceuticals. With increased investment and research focus, the market for orphan medicines is poised for further expansion in the coming years.
Patient advocacy groups and nonprofit organizations play a significant role in raising awareness about rare diseases and advocating for the development of orphan drugs. These efforts have contributed to increased funding for research and development in this field.
Despite these positive trends, challenges such as high development costs, limited patient populations, and complex regulatory processes remain significant barriers for companies operating in the orphan drugs market. Nevertheless, the potential for high returns on investment and the opportunity to make a meaningful impact on patients' lives continue to attract pharmaceutical companies to this segment.
Overall, the orphan drugs market presents opportunities for innovation and growth, driven by a combination of regulatory support, scientific advancements, and increased awareness of rare diseases.
Segmentation –
The drug type, sale, drug, therapy class, and geography are the segments that make up the global orphan drug market.
The global orphan drug market is divided into biologics and non-biologics based on the kind of drug. In the global market for orphan pharmaceuticals, the biologics segment has the biggest market share. In 2017, the segment brought in $75,103.32 million USD.
The global orphan medicine market is divided into prescription and generic categories based on sales.
The medications that make up the global orphan drugs market are: Adcetris, Jakaf, Pomalyst, Darzalex, Spinraza, Imbruvica, Opdivo, Revlimid, and Rituxan.
The global market for orphan pharmaceuticals is divided by treatment classes, which include respiratory, hematological, oncology, endocrine, central nervous system, and cardiovascular.
Regional Analysis –
The Orphan Drugs Market showcases distinctive regional dynamics influenced by factors like regulatory frameworks, healthcare infrastructure, and disease prevalence. North America leads the market, propelled by favorable orphan drug policies, robust research and development (R&D) infrastructure, and high healthcare spending.
The region also benefits from strong collaborations between pharmaceutical companies, research institutions, and patient advocacy groups. Similarly, Europe holds a significant market share, supported by initiatives like the European Medicines Agency's orphan drug designation and incentives for development. In Asia Pacific, the market is poised for rapid growth due to improving healthcare access, rising awareness about rare diseases, and government initiatives to address unmet medical needs. Latin America and the Middle East & Africa regions present opportunities for market expansion, driven by increasing healthcare investments and growing recognition of rare diseases. However, challenges such as limited healthcare resources and reimbursement issues may affect market penetration in these regions. Overall, the Orphan Drugs Market demonstrates a dynamic landscape across different regions, with varying opportunities and challenges shaped by regional healthcare ecosystems and regulatory environments.
Key Players –
Orphan Drugs companies include F. Hoffmann-La Roche AG (Switzerland), Mylan (US), Celgene Corporation (US), Novartis AG (Switzerland), Biogen (US), Takeda Pharmaceutical Company Limited (Japan), Merck KGaA (Germany), Eli Lilly And Company (US), Sanofi (France), and Janssen Services LLC (US).
Related Reports –
Oral Cancer Diagnostics
Automatic Pill Dispenser
Lancet
Medical Oxygen Concentrators
For more information visit at MarketResearchFuture
#Orphan Drugs Market#Orphan Drugs Market Size#Orphan Drugs Market Share#Orphan Drugs Market Outlook#Orphan Drugs Market Report
0 notes
Text
I’m writing a blog
I keep meaning to write. I have every intention. Then at the end of the day, I realize I didn’t write again. Normally I actually write my blog the day before but since WordPress had an update, I schedule the blog but it never posts. I finally got annoyed and stopped writing the day before and then stopped writing on the day. So here I am. I would love to say so much has happened since I last…

View On WordPress
#daily life with ms#diagnosed with MS#difficulties with ms#living with a chronic disease#living with MS#living with multiple sclerosis#MS#ms accommodation#ms challenges#ms difficulties#ms life#ms over 10 years#ms over 20 years#Ms progression#ms symptoms#Multiple sclerosis#Rituxan
3 notes
·
View notes
Text
FDA Oncology Approval
The world of oncology continues to advance at a rapid pace, as multiple new drugs have been approved for various cancer types — both solid and hematologic malignancies — over the last few months. There are also new therapies in the pipeline, too, as ongoing clinical trials are leading to New Drug Applications, Priority Reviews and more from the Food and Drug Administration (FDA).
In March, the FDA approved Zynyz (retifanlimab-dlwr) for patients with metastatic or locally recurrent Merkel cell carcinoma — a rare type of skin cancer. The approval, which was based off findings from the PODIUM-201 trial, marks the first PD-1 inhibitor (a type of drug that blocks the PD-1 protein on cancer cells, thereby preventing them from hiding from the immune system) to be approved for this patient population.
Lynparza plus abiraterone for BRCA-mutant prostate cancer.
The FDA approved Lynparza (Olaparib) plus abiraterone and prednisone for patients with deleterious or suspected deleterious BRCA-mutant castration-resistant prostate cancer, based on findings from the PROpel clinical trial, which showed that adding Lynparza to abiraterone significantly improved the time patients lived before their disease got worse (an endpoint known as progression-free survival) compared with placebo plus abiraterone.
Polivy combination for diffuse large B-cell lymphoma.
Polivy (polatuzumabvedotin-piiq) plus Rituxan (rituximab), cyclophosphamide, doxorubicin and prednisone was approved in April for patients with previously untreated diffuse large B-cell lymphoma (DLBCL), ending a nearly 20-year period where no new options were approved for this patient population. The approval was based on findings from the POLARIX trial, which showed that the newly approved regimen led to a 27% reduction in the risk of disease progression or death compared with the current standard of care.
Padcev plus Keytruda for advanced bladder cancer.
Padcev (enfortumab vedotin-ejfv) plus Keytruda (pembrolizumab) was granted an accelerated approval for the treatment of patients with locally advanced or metastatic urothelial carcinoma (a type of bladder cancer) who are not eligible to receive cisplatin-based chemotherapy, a group that, according to one expert, is comprised of approximately 8,000 to 9,000 patients in the United States. Two clinical trials — EV-103 and KEYNOTE-869 — led to the regimen’s approval, after they showed that the majority of patients given Padcev plus Keytruda experienced a response, while 12% had their disease completely disappear.
Epkinly for relapsed/refractory diffuse large B-cell lymphoma.
In May, the FDA approved another regimen — Epkinly (epcoritamab-bysp) — for DLBCL, this time for patients with relapsed or refractory disease or for those with disease that is the result of indolent lymphoma and high-grade B-cell lymphoma that has been treated with two or more lines of therapy. The FDA made the approval decision based on findings from the EPCORE NHL-1 trial, which showed that 61% of patients responded to the treatment (meaning that their cancer shrank or disappeared), with 38% of patients experiencing a complete response, meaning that there were no signs of cancer after treatment.
New therapies are constantly being investigated in the cancer space.
0 notes
Text
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
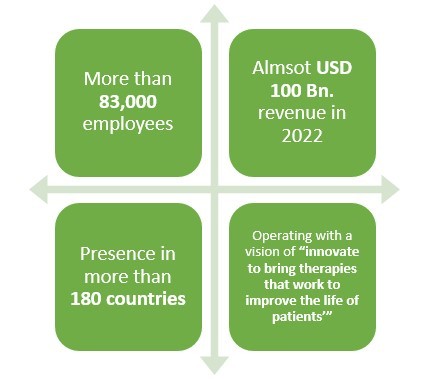
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
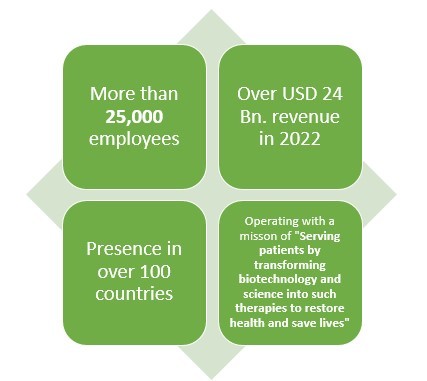
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
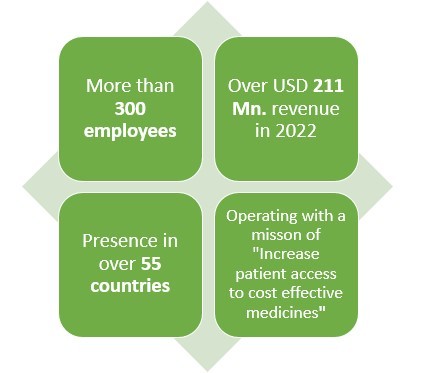
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
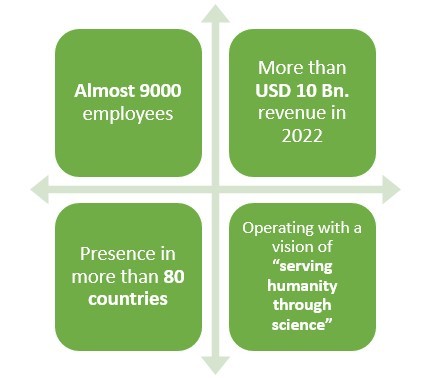
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Good news: depressive state is finally improved! It coincided with both my Rituxan and DST, forcing me to admit that I have possibly spontaneously developed seasonal affective disorder after a lifetime of never dealing with it. But yeah. I feel a LOT better.
26 notes
·
View notes