#chaotic energy of a student
Text
Me *proceeds to eat garbage*
Me *eats a salad one (1) time*: Oh, I feel so healthy now
My horrified internal organs: What… what is she doing? What is she talking about?
My brain:

#I don’t even know what to put in the tags#that’s like my whole recent life summed up#chaotic energy of a student#slash workaholic#I live on instant ramen#jesus christ#my internal organs are going to leave me
2 notes
·
View notes
Text
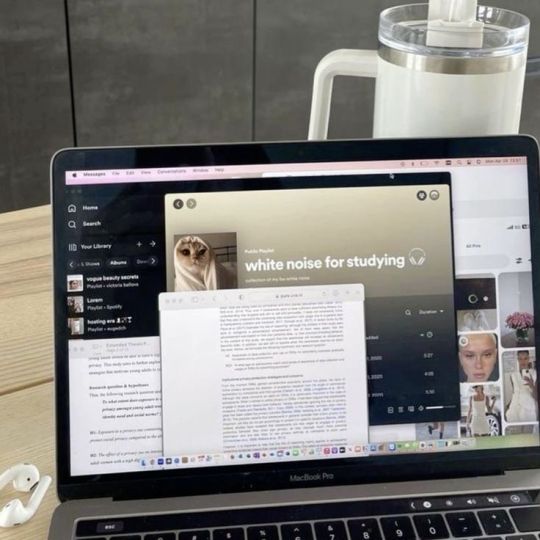


how to stay motivated long-term
trust, me i know that long-term motivation and consistency is hard. long-term motivation might be difficult to maintain, but there are effective techniques to help you stay focused and determined. whether you're seeking personal ambitions, academic achievements, or professional success, here are some strategies to help encourage motivation:
understand the reason behind your goal
☆ does your goal contribute to personal growth or meaningful relationships?
☆ how does your goal impact others?
☆ is your goal meaningful to you?
if your goal lacks meaning, it may be hard to maintain motivation.
positive and negative motivation
motivation can come from different places
☆ positive motivation: the desire to experience pleasure
☆ negative motivation: the desire to avoid something (an outcome)
both types of motivation have their place, so learn to recognise what type fits in where. (post on this coming soon)
set up systems
use your initial motivation to set up structures:
☆ create routines, systems and habits that help you towards your goal even when your motivation fluctuates
☆ when your emotions wane, rely on these systems and disciplines to maintain momentum
break down goals
☆ tackle one goal at a time to avoid feeling overwhelmed
☆ set achievable milestones and celebrate each step forward
☆ keep the momentum going by focusing on manageable tasks
validate good work
☆ give yourself a little reward, or thank yourself, for completing hard tasks
☆ this reinforces motivation and encourages effort
remember--motivation isn't in a constant state, it ebbs and flows. these small tips will help to stay motivated. i'm going to provide more information in upcoming posts, and i will link them here once they are published.
luck on your journey ❤️
#student#student life#study blog#elonomh#studyblr#elonomhblog#study motivation#academia#chaotic academia#productivity#100dop#100 days of productivity#100 days of studying#100 dop#100 days challenge#100 days of self discipline#that girl#it girl#pink pilates princess#pilates#it girl energy#pinterest girl#glow up#dream girl#level up journey#becoming that girl#levelling up
2K notes
·
View notes
Text
Getting My (Sick 🤕) Life Together
*TV-Ad announcer voice* Do YOU like seeing a nice long to-done list BUT you ALSO love being lazy?? Well, I got JUST THE THING for you! ✨ Zesty's Life ✨ where you can have BOTH! justnotallinonedayandyouneverreallyknowwhatkindofdayit'sgonnabesoyouknowgoodluckiguess



In Order of Appearance 📺
Called in sick from work due to waking up before the sun with severe hip pains 🥺
Went back to sleep until almost noon 💤
*cue sleeping intermission* 🧸
Morning pages (because technically it was still morning) and future-self affirmation journaling while listening to music
Completed Chinese lesson (talking about time is so difficult for me 🥺 always 我错了)
Tried using my Mandarin Pinyin keyboard for the first time since I set it up (see feeble attempt above)
Read for Clinical Psych — we're learning about schizophrenia and I find it very interesting so far! (I didn't get very far, but as Lisa Loeb said, "at least I'm excited" 🥺)
Afternoon nap 🛌
*cue sleeping intermission pt. 2* 🧸
Wore a cute outfit to make me feel better 🥺
Went to physio appointment — I always feel like a new woman after ☺️
Did my physio exercises (3x)
Watched Cognitive Psych lectures
Reviewed Cognitive Psych notes
Completed Japanese lesson (2x)
Started reading Mother-Daughter Murder Night for the first time since I got it and I AM OBSESSED 📚
Binge-watched The Nanny (for educational motivational purposes, of course)
Finished chores while listening to music



💌: When life happens to you, I believe you have to happen right back and whoever gets more happenings in before the day ends wins 😂 Also, yes, it is a 🥺 kind of time. Thank you for your patience 🥺
#studyblr#workblr#studystudystudy#student life#study motivation#study inspiration#psychology student#studyspo#getting my life together#becoming that girl#dream girl#pink pilates girl#it girl energy#that girl#clean girl#chaotic academia#chaotic academic aesthetic#light academia#langblr#zesty's life#pink pilates princess#pink academia#self improvement#self empowerment#self care#self love#university#chinese langblr#japanese langblr#astudentslifebuoy
26 notes
·
View notes
Text
taking your notes in glitter pens >>>>>>
#chaotic studyblr#chaotic academia#main character#main character energy#academicsunite#academia aesthetic#dark academia blog#dark academia#light academia#light acadamia aesthetic#college student#study tips#study notes#studying#college life#college advice#college studyblr#social work studyblr#studyblr community#studyblr
80 notes
·
View notes
Text
Randomly remembered all those times someone on a forum or wherever would ask, like, "if your comic was ever turned into a movie or a tv-show, what would you want it to be like", and I was always spewing something about obvious answers like Fortiche or whatever
BUT ACTUALLY. That's wrong.
The correct answer is that I would want it to be filmed as one of those film-student-project fan movies. Where no one has any money and their camera isn't stabilized and the 'sets' are all local buildings but everyone is very committed to making it work with what they have. And all the actors are friends or classmates or someone's parents and therefore look especially real and human.
#wanna see some film student whose drive hasn't yet been muted by the industry choreograph some insane fighting#if it looks campy and the blood is obviously fake but the energy is kept 100% then nothing can be better#and I'm not talking polished cosplay music videos with wigs and perfect outfit replicas no-no#get the essence of the characters and if they don't look like they do on pages then that's GOOD#get an actual butch femme couple in their thirties for Jo and Leah#whom you met at a local queer bar and they were down to help you with the film#get the scrawniest most chaotic girl for Lyoka and who cares if her eyebrows are bushy don't fucking pluck or draw them#'cause she as a character sure doesn't (just lacks in that area) and that's her human characteristic#which is more important than the consistency with the picture#get a black girl with glasses to play Nia and just let her straight up keep her own glasses instead of looking for a round frame#Life is Strange's fan films What If and Dawn are honestly my main examples of what I generally mean#pof posting
13 notes
·
View notes
Text









Femininity in STEM Moodboard | The Feminine Urge to be Hot and Intelligent
#women in stem#stem aesthetic#stem academia#stem moodboard#stem student#stem major#stem studyblr#dark academia#studyblr#dark acadamia aesthetic#dark academia vibes#chaotic academia#chaotic academia moodboard#study motivation#femininity#the feminine urge#dark feminine energy#feminine moodboard#femininity in stem
40 notes
·
View notes
Text

Me right now on tumblr when I should be studying for my oncology exams
#dark feminine energy#my thoughts#dark academia#dark aesthetic#chaotic academia#alt girl#random thoughts#medblr#med student#medical school#medicine#student#i need to study#med studyblr#studying#i should be studying#funny image#funny memes#hot girl semester
4 notes
·
View notes
Text
my chaotic academia things🎧⏳✒️
the girl next to me pretending to be happy she remembered her vocab then said “i’m dead inside”
worrying about failing my quiz even though i had the answers pulled up
my stats teacher likes charlie puth
trying to memorize questions and answers to compare with my friends
getting laughed at by my chemistry teacher for messing up our lab
making sure people see my airpods so they don’t talk to me when i work
my teacher congratulated me and my friend for being on time for once
goggle marks
submitting an application at 11:30 and mentioning that i’m great with deadlines
every pen i own has some kind of problem
old spanish teacher: do u have a 100 in spanish?
me: i think i have a 99
i checked later i had a 100
”where’s my pencil??? WHERES MY PENoh there it is”
shoving papers in my bag’s computer pocket bcs i don’t want to get out my binder
#classic academia#light academia#dark academia#chaotic academia#dark acadamia aesthetic#dark acadamia quotes#academia aesthetic#chaotic energy#introverts#relatable#high school#chaotic academic aesthetic#chaotic academia quotes#chaotic academia aesthetic#chaotic academia moodboard#chaotic academia things#romantic academia#dark academia aesthetic#school#student life#student#high school quotes#romanticise everything#romanticizing school#romanticize#romanticizing#romanticism#romanticise school#romanticise your life#romanticizing life
49 notes
·
View notes
Text
'A girl and her everyday headaches'- A book about me.
#chaotic academia#chaotic thoughts#headache#migraine#anxious mess#musings#alone and stressed#dead poets society#chaotic energy#dark academia#student life
10 notes
·
View notes
Text

Guess who just ordered a shit ton of monster energy off grubhub. Making some cool and sexy financial decisions right now because gotta finish this book by morning. Wish me luck 🙃
#chaotic academic aesthetic#gothic academia#chaotic academia#dark academia#dark academia aesthetic#darkest academia#stem academia#green academia#light academia#studyspiration#stem aesthetic#stem student#stemeducation#study aesthetic#study inspiration#study motivation#study tips#studyblr#studyspo#monster energy#goth aesthetic#gothic#goth#goth girl#gothcore#alt#romantic goth#engineering#stemblr#women in stem
10 notes
·
View notes
Text




2023-10-25
If only we had more time —
#just did a chem test today#literally have no energy#picture are from my panic studying yesterday#I’m so tired#studyblr#studyspo#dark academia#study aesthetic#chaotic academia#student#my posts
4 notes
·
View notes
Text


what do we think?? i was playing around with formatting,, this is one of my older posts
#elonomh#elonomhblog#student#student life#that girl#academia#chaotic academia#productivity#becoming that girl#study blog#newspaper#news#it girl mentality#it girl#it girl aesthetic#it girl energy#pinterest girl#pink pilates princess#girlblogging#girl blogger#girlblog aesthetic#this is a girlblog#live laugh girlblog#gaslight gatekeep girlblog#hell is a teenage girl#im just a girl#vanilla girl#wonyoungism#wellness girl#clean girl
1K notes
·
View notes
Text
Now Playing: ENERGY by Beyonce
You know that Kacey Musgraves song that goes "I'm the kind of person who starts getting kind of nervous when I'm having the time of my life"? Yeah,,, that's me 😅



Now Playing: 고맙다 by Seventeen
I woke up well-rested!!
I did painfully average on one of my psych tests and let the negative thoughts go in an instant ✨
Nominated my team members for a recognition award 🥺
Read Portrait of an Unnamed Woman
Completed Japanese lesson
Completed Korean lesson
My physiotherapy exercises and rest have taken immediate effect, so I've been pain-free for the first time in about a week 🙏
My future-self affirmations lately:
I am overflowing with positive energy!
My positive energy is contagious to those around me 🤗
I am energized by finding positivity in every situation 😎
I create positive energy by taking care of my physical condition ❤️
I build up positive energy to spend on what is truly important by pacing myself 😌



💌: I keep thinking I'll run out of different ways to say the same affirmation/intention but every day comes out different and I always know in the moment what area I need to focus on to gain that positive energy 😇 Still, there are only so many times one can say "positive energy" before one gets absolutely sick of it 😜
#studyblr#psychblr#zesty's life#studyspo#workblr#software engineering#coding#mittonstudies#student life#study motivation#study inspiration#diaryofastemstudent#chaotic academia#psychology student#astudentslifebuoy#light academia#dark academia#becoming that girl#it girl energy#100 days of productivity#100 days of studying#100 days of code#100dop#productivity#codeblr#progblr#programming#software engineer#heydilli#heyfrithams
15 notes
·
View notes
Text
valence bond (VB) theory
today is a lazy day but i really need to clean up these notes for future me...so here i am, pt. 1 🙃
So there's two different models that we use to describe bonding in molecules: VB theory (this one) and molecular orbital (MO) theory (which is actually more accurate). They are similar but NOT the same, don't mix them up. The model we choose to use depends on the purpose.
The reason we need these 2 theories is because while Lewis theory is easily applied, it doesn't explain odd-electron species (i.e. radicals) and resonance very well (no, the double/triple bond does NOT move around the molecule, and it doesn't exist for like, half the time and then not exist the other half) and while both Lewis and VSEPR theories tell us what molecular shapes are pretty accurately, neither tell us quantitatively what the bond energies or bond lengths are for molecules, i.e. covalent compounds, i.e. compounds whose atoms share electrons. (Bond energy and bond length are intrinsically related: the higher the bond energy, the more the bond pulls two atoms together, the shorter the bond length, i.e. internuclear distance.) And bond energies are the most important factor in many chemical reactions and chemical properties, so we need smth better to explain what actually happens during bonding. If we know what happens during bonding we can figure out how to quantify things to do with bonding. And we know that quantum mechanics explains what's actually happening with electrons in atoms, and since covalent bonds are made of electrons pairing up, we can use the notion of orbitals to explain covalent bonding. VB and MO theories both say that bonds are created by orbital overlap but the difference is in WHICH orbitals they use and HOW they use them.
VB theory is like the Lewis theory of bonding theories: it's pretty rigid. It says that a bond is made by overlapping only 2 atomic orbitals (orbitals associated with a single atom) and those orbitals are always going to be valence shell orbitals and those orbitals are always localized, i.e. it assumes that each orbital is fixed in position.
So there's 4 different kinds of basic atomic orbitals, right? s, p, d, and f. And remember that each of these have specific shapes and specific angles at which they exist?
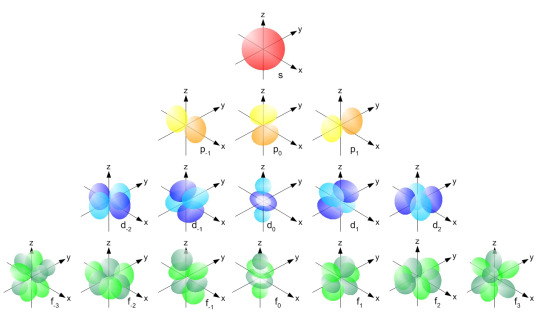
If each of these are localized and these are the only orbitals we use, we won't always get the right bond angles for compounds. In these cases, we need to use hybridized orbitals - new atomic orbitals created by mixing certain basic atomic orbitals in the valence shell.
The square of the electron wave function is a probability distribution telling us where electrons are most likely to be. The shape of that wave function is a spherical harmonic, which is the shape of orbitals. So the orbitals are wave functions aka probability distributions. They aren't actually electrons. So when we hybridize orbitals, we're just changing where electrons are most likely to be.
Like, say we look at methane, which has a tetrahedral shape and therefore a bond angle of ~109.5 degrees:
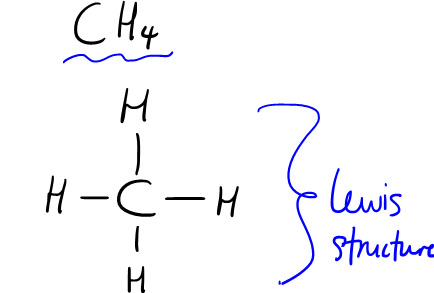
We know that unpaired valence electrons are used in bonding, so let's look at the ground-state electron configurations for these elements: H's only electron is in the 1s orbital, so only its 1s orbital is involved in bonding. Meanwhile, C's ground-state electron configuration is:
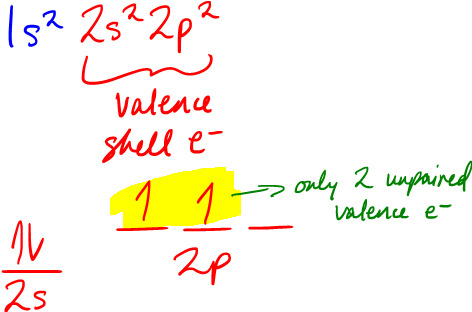
So you'd think you need to promote 1 of the 2s electrons into the 2p orbital like this to create 4 unpaired electrons:
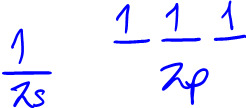
But that poses a problem when you do the bonding because when you overlap the H's 1s orbital with the C's 2s or 2p orbital, you don't get the correct bond angle of 109.5 degrees.

So C must not be using 2s and 2p orbitals to make bonds. It must be combining its 2s and 2p orbitals. Remember, orbitals are wave functions, mathematical functions with a spherical harmonic shape. So when you combine them, you get a new shape with a different point of destructive interference (node) and different areas of constructive interference (electrons are likely to be here where there's constructive interference). These new shapes could be sp, sp2, or sp3 hybridized orbitals. When you combine wave functions, the number of wave functions you combine = the number of different ways they can be combined, e.g. combining 4 orbitals - s and 3 p orbitals - creates 4 wave functions - 4 sp3 hybridized orbitals, subscript 3 b/c you used 3 p orbitals. And when C uses sp3 hybridized orbitals to bond with H's 1s orbitals, you get the correct bond angle of 109.5 degrees. So you have to promote a 2s electron but then hybridize the orbitals to get the right molecular geometry and shape.


Similarly, for trigonal bipyramidal molecules, a hybrid orbital made of and s orbital, 3 p orbitals, and 1 d orbital leads to the correct bond angles for that molecular shape. And for octahedral molecules, a hybrid orbital made of an s orbital, 3 p orbitals, and 2 d orbitals leads to the correct bond angles for that molecular shape. All these orbitals will be valence orbitals, btw.
Here's a cheatsheet so you don't have to think about how hybridization comes about in order to figure out what hybrid orbitals a particular molecule is going to use:

So when we hybridize orbitals, sometimes we're left with unhybridized p orbitals:

Those unhybridized p orbitals form pi bonds, which form the 2nd and 3rd bonds in double and triple bonds. There's 2 kinds of bonds. There's sigma bonds - sigma, which is the Greek letter 's', because sigma bonds are always single bonds. They result from orbitals that overlap along the internuclear axis:
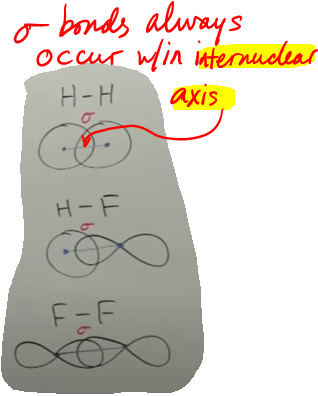
Then there's pi bonds - pi, which is the Greek letter 'p', because they're always only made of unhybridized p orbitals overlapping above and below the internuclear axis:

So in summary, VB theory says that a bond is made by overlapping only 2 atomic orbitals - s, p, d, or f, OR hybridized orbitals. Those orbitals are always going to be valence shell orbitals and those orbitals are always localized, i.e. fixed in position. And the specific atomic orbitals used are whatever atomic orbitals would lead to the correct bond angles for that molecule as determined by VSEPR. These orbital overlaps can form sigma (single) bonds or pi bonds (double or triple bonds). But what about other molecules that can't be simply described by VB theory, like molecules that display resonance (fractional bond orders)? VB theory only explains single, double, and triple bonds... What about things in between? Enter MO theory...

Any annotated screenshots originally came from here.
#chaotic academia#chaotic academia aesthetic#if aesthetic can be words then maybe this falls under that tag?#idk how to tag things#studyblr#chemblr#stemblr#stem student#valence bond theory#molecular orbital theory#chemistry#chemical bonding#bond energies#my unsolicited notes#if you view this is dark mode then is it#dark academia#? XD#i hope these notes will still make sense for future me...
3 notes
·
View notes
Note
Hi,
I don't know who you are, what you do irl or even what is your gender.
All I know is from the moment I started following your posts, your chaotic energy gave me hope.
Not hope for the world to be a better place, but hope that at least it is still a funny dumpster on fire.
Have a good day
what do you mean. i am just normal men

#do i have chaotic energy ? im just some guy#if you want to know tho: hi im dani im an anthropology student and my gender is ehhhh *does a hand wavey gesture*#op#ask
2 notes
·
View notes
Text
haha yeah babe i have that bad girl energy (*watching it's gonna be me performances when i have an incomplete assignment due in 45 minutes*)
#chaotic academia#this isn't even chaotic academia this is just me being a bad student#bad student energy#smth smth lower your expectations bo burnham#uhhhhhhhhhhhh look jc is hot i don't make the rules#hotter than my assignment at least
2 notes
·
View notes